Autotrophs bridge the nonliving (CO2) and living (organic carbon) realms by fixing carbon through photosynthesis. All life is based on multiple functions that differ in their elemental requirements, and homeostasis of cellular composition is one of the hallmarks of life. Nevertheless, autotrophs on land and in water have the biological potential to adjust their nutrient content in response to nutrient and light availability, as well as other factors (1). These adjustments arise because there are separate cellular pathways for incorporation of carbon vs. nutrient elements. Carbon enters the cell via light-driven photosynthetic processes, whereas nutrients such as N and P enter the cell via more or less-specific transporters coupled to cellular energy use. Within the cell standing-stock inventory, adjustments of macromolecular composition occur, in some cases using specific nutrient storage compounds. This flexibility of autotroph nutrient composition (often thought of as the C:N:P ratio), some of which is represented as species-to-species differences and some of which represents physiological plasticity, generates a host of ecological dynamics, from element limitation of herbivores to unstable community dynamics and others (1). Nevertheless, until very recently the thinking about autotrophs in the open sea has almost exclusively considered them as having fixed, not variable, stoichiometry. The report by Galbraith and Martiny (2) in PNAS is an important step away from that paradigm and into another.
Since the pioneering work of Redfield (3, 4), the reigning paradigm regarding carbon and nutrient cycling in the ocean at the large scale has been based on a fixed stoichiometry of C, N, and P in marine particles (having a strong influence of autotrophs) (5). By that thinking, a biotic potential for flexibility in cellular C:N:P was not expressed. Reasons for this constraint have been discussed and debated over the years (e.g., refs. 6 and 7) and relative to autotrophs on land or in lakes, marine plankton do exhibit much more constrained C:N:P composition (8). In Redfield’s time observations of marine C:N:P were scarce, and perhaps because theoretical emphasis was on lack of variation, additional data—especially of particulate P—were slow to accumulate.
It happens occasionally in science, however, that accumulation of data and increases in precision and accuracy add texture and nuance to ideas, at some point overthrowing old paradigms. Something like that is now occurring in marine biogeochemistry. Our knowledge of global cycling of carbon and nutrients in the ocean is hardly complete, being based on a dataset consisting of ∼600 direct station observations of surface C:P along with a more substantive set of ∼4,000 observations of C:N. Still, that information shows clearly that surface particulate C:N:P ratios are not constant, instead varying for example with the scale of observation (9) and with latitude (10). Autotrophs in low-nutrient, low-latitude ocean surface waters do what autotrophs on land and in lakes do when light is abundant and nutrients are scarce (11): they adjust cellular C:N:P and become relatively nutrient-poor in their biomass.
This set of findings now challenges us to work out how much significance to attach to this “muted” amount of variation in C:N:P at the base of the marine pelagic food web. In PNAS, Galbraith and Martiny (2) first create a predictive model that allows a highly spatially nonrandom and somewhat limited set of data on marine particle composition to be extrapolated and interpolated worldwide, and then they use that information in a simple but proven four-box global biogeochemical model of the atmosphere and oceans. The results are eye-opening; this information applied to this model indicates that the observable variable phytoplankton stoichiometry redistributes global carbon pools, shifting steady-state CO2 out of the atmosphere and into the deep ocean.
The new predictive model links particulate N:C and P:C ratios to concentrations of nitrate and phosphate. Particles are slightly N-depleted at the lowest range of observed nitrate concentrations. A wider dynamic range for P:C is seen, however, with a linear model linking particulate P:C and dissolved phosphate. There are many more observations of dissolved nitrate and phosphate than particulate N and P, so that a spatially explicit, global image of marine C:N:P could be generated (figure 2 in ref. 2). P:C ranges about fourfold from ∼5‰ (low latitudes) to ∼20‰ (high latitudes) (mass:mass). N:C meanwhile varies from ∼130 (low latitudes) to ∼160 (high latitudes). The predictive model has a common-sense mechanistic basis: where a specific nutrient is scarce, organisms are generally more efficient in using it (12).
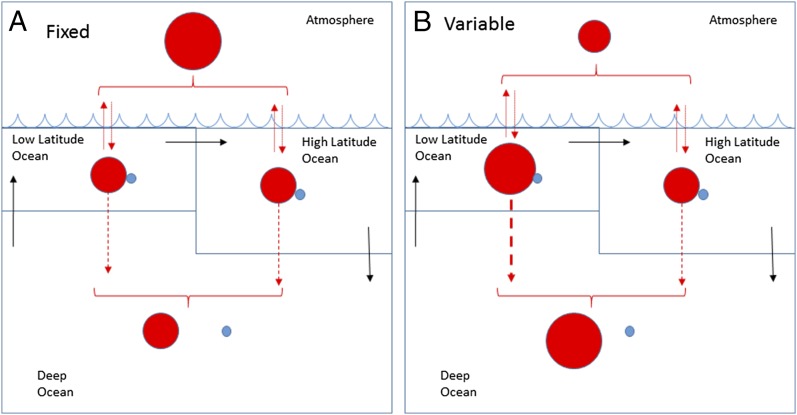
The comparatively little-studied P:C has large significance in the model; a dynamic coupling between low-latitude PO4 and particulate P:C was incorporated. To visualize the most salient features of the global model, Fig. 1 represents C in red and P in blue and the relative sizes of circles show the defining contrasts between fixed (Fig. 1A) and variable (Fig. 1B) particulate composition. Where stoichiometry was allowed to vary according to biological principles and statistically observable relationships, low-latitude particles associate more organic carbon with each unit of phosphorus and by normal sinking processes deliver more carbon to the deep ocean. With fixed inventories of elements in the model, this downward movement of C moves carbon from the atmosphere into the deep ocean. In model runs where PO4 at low latitudes is highly depleted, atmospheric CO2 is reduced by as much as 100 ppm.
Fig. 1.
Salient features of global four-box model with fixed (A) vs. variable (B) stoichiometry. Thermohaline circulation (black arrows) creates an overall ocean circulation from low to high latitudes to deep water and back to low latitudes, which partially accounts for the movement of carbon (red) and phosphorus (blue). However, carbon exchanges with the atmosphere and both C and P sink into deep water at both high and low latitudes. Total C and P inventories are considered fixed at this time scale. The effect of allowing for variable stoichiometry is to increase the C:P ratio (decrease the P:C) of particles at low latitudes, which at steady state redistributes carbon from the atmosphere into the deep ocean.
Hopefully, this line of reasoning will increase the attention paid to marine particulate nutrient chemistry. What was once thought to be fixed now seems to be variable enough in ways that potentially mediate planetary distribution of carbon. There is much more detail that could be added and it is hard to know at this point if that detail will add to the importance of variable stoichiometry or subtract from it. A more realistic representation of vertical particle flux of C vs. P is an obvious candidate for future research.
There have been suggestions for some time that the composition of marine particulate matter may have a globally relevant impact on carbon and climate (13). However, we are only now seeing the weaving together of several critical threads: adaptive autotroph flexibility, ocean circulation, and global element distribution. This new work by Galbraith and Martiny (2) suggests that variable marine stoichiometry is not a sideshow; it may well be significant at the global scale and even be central to our understanding of glacial–interglacial cycles.
Footnotes
The author declares no conflict of interest.
See companion article on page 8199.
References
- 1.Sterner RW, Elser JJ. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton Univ Press; Princeton, NJ: 2002. [Google Scholar]
- 2.Galbraith ED, Martiny AC. A simple nutrient-dependence mechanism for predicting the stoichiometry of marine ecosystems. Proc Natl Acad Sci USA. 2015;112:8199–8204. doi: 10.1073/pnas.1423917112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redfield AC. The biological control of chemical factors in the environment. Am Sci. 1958;46(3):205–221. [PubMed] [Google Scholar]
- 4.Redfield AC. On the proportions of organic derivatives in sea water and their relation to the composition of plankton. In: Daniel RJ, editor. James Johnstone Memorial Volume. Liverpool Univ Press; Liverpool, UK: 1934. pp. 176–192. [Google Scholar]
- 5.Williams PJlB An appreciation of Alfred C. Redfield and his scientific work. Limnology and Oceanography Bulletin. 2006;15(4):1–28. [Google Scholar]
- 6.Goldman JC, McCarthy JJ, Peavey DG. Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature. 1979;279:210–215. [Google Scholar]
- 7.Tett P, Heaney SI, Droop MR. The Redfield ratio and phytoplankton growth rate. J Mar Biol Assoc U K. 1985;65(2):487–504. [Google Scholar]
- 8.Elser JJ, Hassett RP. A stoichiometric analysis of the zooplankton-phytoplankton interaction in marine and freshwater ecosystems. Nature. 1994;370:211–213. [Google Scholar]
- 9.Sterner RW, et al. Scale-dependent carbon:nitrogen:phosphorus seston stoichiometry in marine and freshwaters. Limnol Oceanogr. 2008;53(3):1169–1180. [Google Scholar]
- 10.Martiny AC, et al. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat Geosci. 2013;6:279–283. [Google Scholar]
- 11.Sterner RW, Elser JJ, Fee EJ, Guildford SJ, Chrzanowski TH. The light:nutrient ratio in lakes: The balance of energy and materials affects ecosystem structure and process. Am Nat. 1997;150(6):663–684. doi: 10.1086/286088. [DOI] [PubMed] [Google Scholar]
- 12.Vitousek P. Nutrient cycling and nutrient use efficiency. Am Nat. 1982;119(4):553–572. [Google Scholar]
- 13.Broecker WS. Ocean chemistry during glacial time. Geochemica et Cosmochimica ACTA. 1982;46(10):1689–1706. [Google Scholar]