Abstract
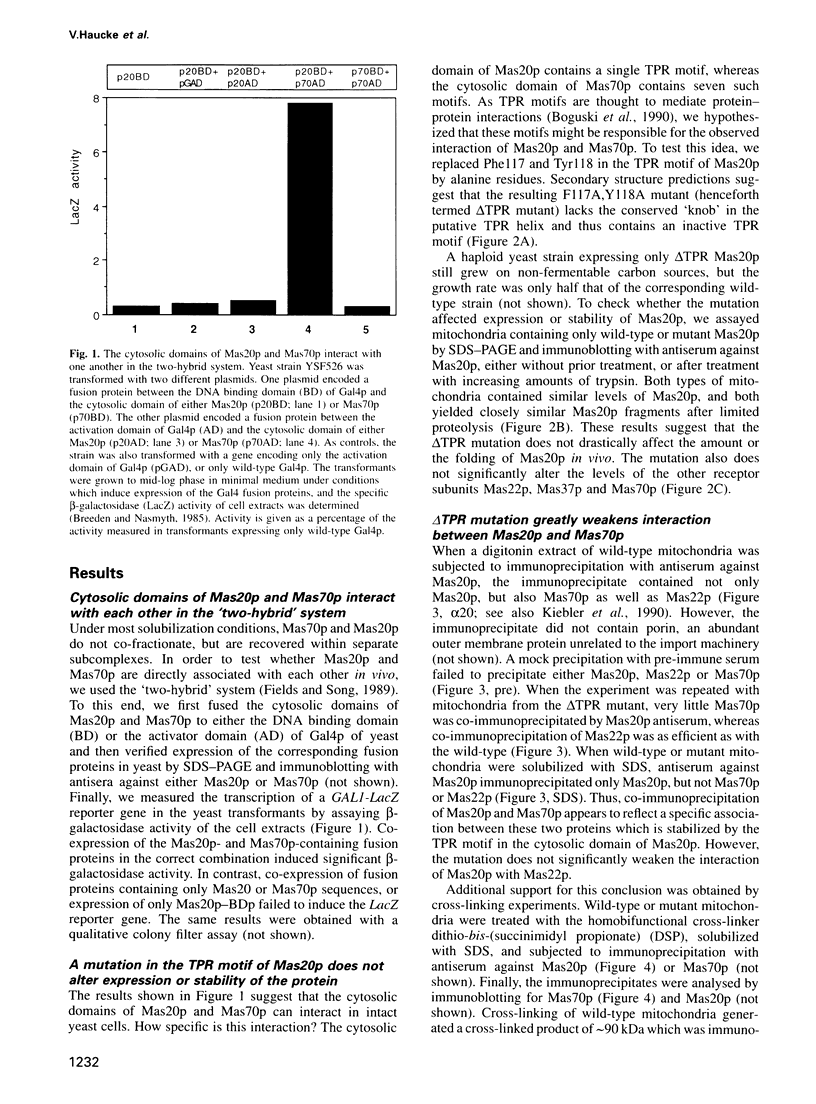
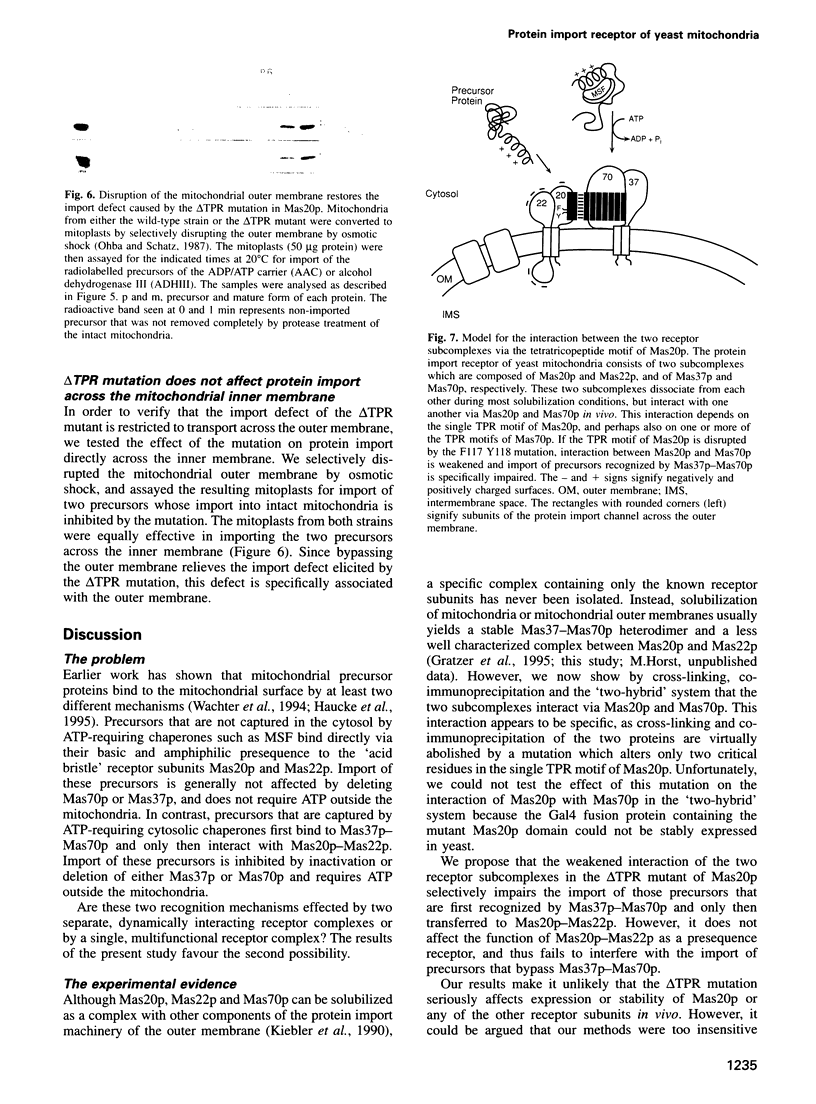
Protein import into yeast mitochondria is mediated by four integral outer membrane proteins which function as import receptors. These proteins (termed Mas20p, Mas22p, Mas37p and Mas70p) appear to exist as two subcomplexes: a Mas37p-Mas70p heterodimer and a less well characterized Mas20p-Mas22p complex. The subcomplexes interact functionally during protein import, but it has remained uncertain whether they are in direct contact with each other in vivo. Here we show that Mas20p and Mas70p can be cross-linked in intact mitochondria, or co-immunoprecipitated from digitonin-solubilized mitochondria. Furthermore, the cytosolic domains of these two proteins interact in the 'two-hybrid' system. Association of Mas20p and Mas70p is virtually abolished by a mutation in the single tetratricopeptide motif in Mas20p. This mutation specifically inhibits import of precursors that are first recognized by Mas37p-Mas70p and only then transferred to Mas20p-Mas22p. We conclude that the two receptor subcomplexes of the mitochondrial protein import receptor interact in vivo via their Mas20p and Mas70p subunits and that this interaction is functionally important.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boguski M. S., Sikorski R. S., Hieter P., Goebl M. Expanding family. Nature. 1990 Jul 12;346(6280):114–114. doi: 10.1038/346114a0. [DOI] [PubMed] [Google Scholar]
- Bolliger L., Junne T., Schatz G., Lithgow T. Acidic receptor domains on both sides of the outer membrane mediate translocation of precursor proteins into yeast mitochondria. EMBO J. 1995 Dec 15;14(24):6318–6326. doi: 10.1002/j.1460-2075.1995.tb00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeden L., Nasmyth K. Regulation of the yeast HO gene. Cold Spring Harb Symp Quant Biol. 1985;50:643–650. doi: 10.1101/sqb.1985.050.01.078. [DOI] [PubMed] [Google Scholar]
- Brunelli J. P., Pall M. L. A series of yeast shuttle vectors for expression of cDNAs and other DNA sequences. Yeast. 1993 Dec;9(12):1299–1308. doi: 10.1002/yea.320091203. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Goebl M., Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991 May;16(5):173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- Gratzer S., Lithgow T., Bauer R. E., Lamping E., Paltauf F., Kohlwein S. D., Haucke V., Junne T., Schatz G., Horst M. Mas37p, a novel receptor subunit for protein import into mitochondria. J Cell Biol. 1995 Apr;129(1):25–34. doi: 10.1083/jcb.129.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya N., Alam R., Sakasegawa Y., Sakaguchi M., Mihara K., Omura T. A mitochondrial import factor purified from rat liver cytosol is an ATP-dependent conformational modulator for precursor proteins. EMBO J. 1993 Apr;12(4):1579–1586. doi: 10.1002/j.1460-2075.1993.tb05802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya N., Mihara K., Suda K., Horst M., Schatz G., Lithgow T. Reconstitution of the initial steps of mitochondrial protein import. Nature. 1995 Aug 24;376(6542):705–709. doi: 10.1038/376705a0. [DOI] [PubMed] [Google Scholar]
- Haucke V., Lithgow T., Rospert S., Hahne K., Schatz G. The yeast mitochondrial protein import receptor Mas20p binds precursor proteins through electrostatic interaction with the positively charged presequence. J Biol Chem. 1995 Mar 10;270(10):5565–5570. doi: 10.1074/jbc.270.10.5565. [DOI] [PubMed] [Google Scholar]
- Hines V., Brandt A., Griffiths G., Horstmann H., Brütsch H., Schatz G. Protein import into yeast mitochondria is accelerated by the outer membrane protein MAS70. EMBO J. 1990 Oct;9(10):3191–3200. doi: 10.1002/j.1460-2075.1990.tb07517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönlinger A., Kübrich M., Moczko M., Gärtner F., Mallet L., Bussereau F., Eckerskorn C., Lottspeich F., Dietmeier K., Jacquet M. The mitochondrial receptor complex: Mom22 is essential for cell viability and directly interacts with preproteins. Mol Cell Biol. 1995 Jun;15(6):3382–3389. doi: 10.1128/mcb.15.6.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Becker K., Pfanner N., Neupert W. Mitochondrial protein import: specific recognition and membrane translocation of preproteins. J Membr Biol. 1993 Sep;135(3):191–207. doi: 10.1007/BF00211091. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Keil P., Schneider H., van der Klei I. J., Pfanner N., Neupert W. The mitochondrial receptor complex: a central role of MOM22 in mediating preprotein transfer from receptors to the general insertion pore. Cell. 1993 Aug 13;74(3):483–492. doi: 10.1016/0092-8674(93)80050-o. [DOI] [PubMed] [Google Scholar]
- Kiebler M., Pfaller R., Söllner T., Griffiths G., Horstmann H., Pfanner N., Neupert W. Identification of a mitochondrial receptor complex required for recognition and membrane insertion of precursor proteins. Nature. 1990 Dec 13;348(6302):610–616. doi: 10.1038/348610a0. [DOI] [PubMed] [Google Scholar]
- Lithgow T., Glick B. S., Schatz G. The protein import receptor of mitochondria. Trends Biochem Sci. 1995 Mar;20(3):98–101. doi: 10.1016/s0968-0004(00)88972-0. [DOI] [PubMed] [Google Scholar]
- Lithgow T., Junne T., Suda K., Gratzer S., Schatz G. The mitochondrial outer membrane protein Mas22p is essential for protein import and viability of yeast. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11973–11977. doi: 10.1073/pnas.91.25.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow T., Schatz G. Import of the cytochrome oxidase subunit Va precursor into yeast mitochondria is mediated by the outer membrane receptor Mas20p. J Biol Chem. 1995 Jun 16;270(24):14267–14269. doi: 10.1074/jbc.270.24.14267. [DOI] [PubMed] [Google Scholar]
- Millar D. G., Shore G. C. Mitochondrial Mas70p signal anchor sequence. Mutations in the transmembrane domain that disrupt dimerization but not targeting or membrane insertion. J Biol Chem. 1994 Apr 22;269(16):12229–12232. [PubMed] [Google Scholar]
- Millar D. G., Shore G. C. The signal anchor sequence of mitochondrial Mas70p contains an oligomerization domain. J Biol Chem. 1993 Sep 5;268(25):18403–18406. [PubMed] [Google Scholar]
- Nakai M., Endo T. Identification of yeast MAS17 encoding the functional counterpart of the mitochondrial receptor complex protein MOM22 of Neurospora crassa. FEBS Lett. 1995 Jan 3;357(2):202–206. doi: 10.1016/0014-5793(94)01362-5. [DOI] [PubMed] [Google Scholar]
- Ohba M., Schatz G. Disruption of the outer membrane restores protein import to trypsin-treated yeast mitochondria. EMBO J. 1987 Jul;6(7):2117–2122. doi: 10.1002/j.1460-2075.1987.tb02478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfanner N., Müller H. K., Harmey M. A., Neupert W. Mitochondrial protein import: involvement of the mature part of a cleavable precursor protein in the binding to receptor sites. EMBO J. 1987 Nov;6(11):3449–3454. doi: 10.1002/j.1460-2075.1987.tb02668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage L., Junne T., Hahne K., Lithgow T., Schatz G. Functional cooperation of mitochondrial protein import receptors in yeast. EMBO J. 1993 Nov;12(11):4115–4123. doi: 10.1002/j.1460-2075.1993.tb06095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Hase T., van Loon A. P., Grivell L. A., Suda K., Schatz G. Import of proteins into mitochondria: a 70 kilodalton outer membrane protein with a large carboxy-terminal deletion is still transported to the outer membrane. EMBO J. 1983;2(12):2161–2168. doi: 10.1002/j.1460-2075.1983.tb01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer P. E., Manning-Krieg U. C., Jenö P., Schatz G., Horst M. Identification of a 45-kDa protein at the protein import site of the yeast mitochondrial inner membrane. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11930–11934. doi: 10.1073/pnas.89.24.11930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger H. F., Söllner T., Kiebler M., Dietmeier K. A., Pfaller R., Trülzsch K. S., Tropschug M., Neupert W., Pfanner N. Import of ADP/ATP carrier into mitochondria: two receptors act in parallel. J Cell Biol. 1990 Dec;111(6 Pt 1):2353–2363. doi: 10.1083/jcb.111.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söllner T., Griffiths G., Pfaller R., Pfanner N., Neupert W. MOM19, an import receptor for mitochondrial precursor proteins. Cell. 1989 Dec 22;59(6):1061–1070. doi: 10.1016/0092-8674(89)90762-9. [DOI] [PubMed] [Google Scholar]
- Söllner T., Pfaller R., Griffiths G., Pfanner N., Neupert W. A mitochondrial import receptor for the ADP/ATP carrier. Cell. 1990 Jul 13;62(1):107–115. doi: 10.1016/0092-8674(90)90244-9. [DOI] [PubMed] [Google Scholar]
- Wachter C., Schatz G., Glick B. S. Protein import into mitochondria: the requirement for external ATP is precursor-specific whereas intramitochondrial ATP is universally needed for translocation into the matrix. Mol Biol Cell. 1994 Apr;5(4):465–474. doi: 10.1091/mbc.5.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]









