Significance
Prostaglandin E2 (PGE2) plays an important role in maintaining water and sodium homeostasis via its four membrane-associated receptors including EP1, EP2, EP3, and EP4. This study uncovers a unique role for the EP4 receptor in controlling urine volume independent of antidiuretic hormone (arginine vasopressin). EP4 activation increases collecting duct aquaporin 2 (AQP2) expression in a cAMP/cAMP-response element binding protein (CREB)-dependent manner and promotes its membrane sorting via the cAMP/protein kinase A and extracellular signal-regulated kinase pathways. The ability of EP4 to increase AQP2 membrane targeting and cellular abundance makes it a potential therapeutic target for the treatment of clinical disorders including acquired and congenital diabetes insipidus.
Keywords: arachidonic acid, cyclooxygenase, antidiuretic hormone, gene targeting, water homeostasis
Abstract
The antidiuretic hormone arginine vasopressin is a systemic effector in urinary concentration. However, increasing evidence suggests that other locally produced factors may also play an important role in the regulation of water reabsorption in renal collecting ducts. Recently, prostaglandin E2 (PGE2) receptor EP4 has emerged as a potential therapeutic target for the treatment of nephrogenic diabetes insipidus, but the underlying mechanism is unknown. To evaluate the role of EP4 in regulating water homeostasis, mice with renal tubule-specific knockout of EP4 (Ksp-EP4−/−) and collecting duct-specific knockout of EP4 (AQP2-EP4−/−) were generated using the Cre-loxP recombination system. Urine concentrating defect was observed in both Ksp-EP4−/− and AQP2-EP4−/− mice. Decreased aquaporin 2 (AQP2) abundance and apical membrane targeting in renal collecting ducts were evident in Ksp-EP4−/− mice. In vitro studies demonstrated that AQP2 mRNA and protein levels were significantly up-regulated in mouse primary inner medullary collecting duct (IMCD) cells after pharmacological activation or adenovirus-mediated overexpression of EP4 in a cAMP/cAMP-response element binding protein-dependent manner. In addition, EP4 activation or overexpression also increased AQP2 membrane accumulation in a mouse IMCD cell line (IMCD3) stably transfected with the AQP2 gene, mainly through the cAMP/protein kinase A and extracellular signal-regulated kinase pathways. In summary, the EP4 receptor in renal collecting ducts plays an important role in regulating urinary concentration under physiological conditions. The ability of EP4 to promote AQP2 membrane targeting and increase AQP2 abundance makes it a potential therapeutic target for the treatment of clinical disorders including acquired and congenital diabetes insipidus.
Urinary concentration is a key process for maintaining body water homeostasis, which is primarily regulated by the antidiuretic hormone arginine vasopressin (AVP). AVP is produced in the hypothalamus and stored in and released from the posterior pituitary, either in response to increased plasma osmolality or decreased blood volume. It binds to its type 2 receptor (V2R) on the basolateral membrane of the principal cells of renal collecting ducts (CDs), triggering the redistribution of aquaporin 2 (AQP2) from intracellular vesicles into the apical membrane. The prolonged activation of V2R can also increase AQP2 expression in CDs, which is essential for urinary concentration (1). AVP thus increases water permeability of the CDs, resulting in enhanced water reabsorption from the tubule lumens and concentrated urine output (1, 2). In some cases, however, urinary concentration is altered independent of AVP, a phenomenon called vasopressin escape, suggesting additional mechanisms may participate in the process of water reabsorption in renal collecting ducts (3–5).
Among many identified factors affecting urine output, prostaglandin E2 (PGE2) has been shown to play an important role in urinary concentration. PGE2 is one of the major cyclooxygenated metabolites of arachidonic acid produced in the kidney (6). It exerts various biological functions via its four distinct G protein-coupled receptors, designated EP1–4 (7). Substantial evidence suggests a prominent, yet complex, role for PGE2 in the regulation of water homeostasis. For instance, PGE2 and sulprostone, an EP1/3 agonist, have been found to blunt AVP-induced AQP2 trafficking and urinary concentration (8–11). Results from the EP3 gene knockout mice also suggested a diuretic effect of PGE2, likely via the EP3 receptor (12). Olesen et al. have shown that both G-coupled PGE2 receptor EP2 and EP4 agonists increased AQP2 membrane accumulation and that the EP2 agonist butaprost relieved nephrogenic diabetes insipidus (NDI) symptoms in a rat model of NDI (13). Moreover, a selective EP4 agonist has recently been reported to be able to alleviate symptoms in a mouse model of NDI (14). These results indicate a role for EP2 and EP4 receptors in promoting urinary concentration and suggest the potential use of EP2 and EP4 agonists in the treatment of NDI. However, to date, the underlying mechanism is poorly defined and the precise role of each EP receptor in the regulation of urinary concentration under physiological conditions remains unclear.
In the present study, we found a significant increase in renal medullary EP4, but not EP1, EP2, or EP3, expression in wild-type (WT) mice after water deprivation. EP4 renal tubule-specific knockout (Ksp-EP4−/−) mice and collecting duct-specific knockout (AQP2-EP4−/−) mice exhibited an impaired urinary concentrating ability, along with decreased AQP2 expression and membrane targeting. The possible mechanisms by which EP4 regulates AQP2 membrane translocation involve both cAMP/protein kinase A (PKA) and extracellular signal-regulated kinase (ERK) pathways, whereas EP4-elicited AQP2 abundance is dependent on the cAMP/cAMP-response element binding protein (CREB) pathway. Our findings highlight an important role for the EP4 receptor in regulating water reabsorption under physiological conditions and support the use of EP4 agonist as a potential therapeutic treatment for NDI.
Results
Renal PGE2 Synthesis and Medullary EP4 Expression Were Increased in Wild-Type Mice After Water Deprivation.
WT C57BL/6 mice were subjected to water deprivation (WD) for 24 h. Compared with controls, both renal PGE2 content and urinary PGE2 output were significantly increased, as depicted in Fig. 1 A and B. In addition, among the synthases that generate PGE2, the renal expression of cyclooxygenase (COX)-2 and microsomal prostaglandin E synthase (mPGES)-1 was markedly up-regulated in WD mice. No difference was observed in renal COX-1, mPGES-2, or cytosolic PGES (cPGES) levels between the two groups (SI Appendix, Fig. S1 A and B). These results suggest an enhanced PGE2 synthesis in the kidney after WD, possibly via the activation of COX-2 and mPGES-1. Because the renal medulla is the main site where urine concentration occurs, we examined the expression of the four EP receptors in renal medulla. Renal medullary EP4 protein expression was significantly increased after WD, whereas no obvious change in EP1, EP2, or EP3 expression was observed (Fig. 1C). This result suggests the involvement of EP4, rather than the other three receptors, in the process of urine concentration after WD. In support, no abnormalities in the urine volume and urine osmolality were observed in mice deficient for EP1, EP2, or EP3, respectively (15–17) (SI Appendix, Fig. S2 A–F). However, the urine volume (1,313 ± 114.4 in EP4+/+ vs. 1,618 ± 98.48 μL/24 h in EP4+/− mice) was significantly higher, whereas urine osmolality was significantly lower in EP4+/− mice than in control mice (SI Appendix, Fig. S2 G and H).
Fig. 1.
Enhanced renal PGE2 synthesis and EP4 expression in wild-type (WT) mice after water deprivation. (A) Renal and (B) urine PGE2 levels were examined in WT C57BL/6 mice (male, 3 mo old) subjected to 24-h water deprivation (WD) (n = 14). The mice with free access to water were used as controls (n = 13). **P < 0.01 vs. control. (C) Western blot analysis of four EP receptors expression in kidney medulla before and after 24-h WD (n = 3). The results are presented as mean ± SEM.
To verify whether EP4 was regulated in renal CDs during the process of urinary concentration, mouse primary inner medullary collecting duct (IMCD) cells were cultured as described previously (18). Hyperosmotic challenge (700–900 mOsm/kg H2O) induced EP4 protein expression in IMCD cells (SI Appendix, Fig. S3 A and B). Taken together, renal PGE2 synthesis and renal medullary EP4 expression were significantly increased after water restriction, suggesting that PGE2 might promote urinary concentration via the EP4 receptor under normal physiological conditions.
EP4 Disruption in Renal Tubules Impaired Urinary Concentrating Ability.
To assess the role of the EP4 receptor in urinary concentration in vivo, mice with specific deletion of the EP4 gene in renal tubules (Ksp-EP4−/− mice) were generated by the Cre-loxP recombination system (19–21) (SI Appendix, Figs. S4, S5 A–E, and Table S1). At 10 mo of age, urine volume was increased by 78% and urine osmolality was reduced by 28% in Ksp-EP4−/− mice vs. control mice (Fig. 2 A and B). However, no significant difference in daily urine electrolyte excretion, including urea, Na+, K+, Cl−, Ca2+, Mg2+, and phosphate, was observed between Ksp-EP4−/− and the control mice (SI Appendix, Table S2). These findings suggest that EP4 deficiency in renal tubules mainly affects water, but not solute, excretion. Although water deprivation for 24 h resulted in concentrated urine output in both genotypes, Ksp-EP4−/− mice exhibited significantly higher urine volume and lower urine osmolality than control mice (SI Appendix, Fig. S6 A and B). Similarly, a chronic water loading (WL) study revealed a significant increase in hypotonic urine output and a marked reduction in urine osmolality in Ksp-EP4−/− mice compared with Ksp-Cre− mice (SI Appendix, Fig. S6 C and D). Collectively, these results indicate a critical role for EP4 in regulating urinary concentration.
Fig. 2.
Impaired urinary concentrating ability in Ksp-EP4−/− mice. (A) Twenty-four-hour urine volume and (B) osmolality (n = 9 for Ksp-Cre− mice, n = 16 for Ksp-EP4−/− mice) under basal conditions. **P < 0.01 vs. Ksp-Cre− mice. (C) Ksp-Cre− and Ksp-EP4−/− mice were injected i.p. with 0.4 μg/kg of dDAVP. Urine was collected after 2 h and urine osmolality was determined (n = 15). **P < 0.01 vs. Ksp-Cre− mice at 0 h; ##P < 0.01 vs. Ksp-EP4−/− mice at 0 h. (D) Serum AVP concentrations in the Ksp-Cre− and Ksp-EP4−/− mice. The results are presented as mean ± SEM.
To assess whether the AVP signaling pathway remained normal in the animals, Ksp-EP4−/− and Ksp-Cre− mice were injected with desmopressin (dDAVP). Urine osmolality was monitored 2 h after the injection. A similar upward tendency in urine osmolality was observed in both genotypes, indicating an intact AVP signaling in Ksp-EP4−/− mice (Fig. 2C). Furthermore, serum AVP levels in Ksp-EP4−/− was comparable to Ksp-Cre− mice, excluding the possibility that the difference in urine output between the two genotypes was caused by the alteration in circulating AVP levels (Fig. 2D).
To further confirm the findings in Ksp-EP4−/− mice, renal collecting duct-specific EP4 gene knockout mice (AQP2-EP4−/− mice) were generated (22). Similar to the findings in Ksp-EP4−/− mice, polyuria and normal dDAVP responsiveness were observed in AQP2-EP4−/− mice at the age of 4 mo (SI Appendix, Fig. S7 A–H).
AQP2 Protein Abundance and Membrane Targeting Were Reduced in Ksp-EP4−/− Mice.
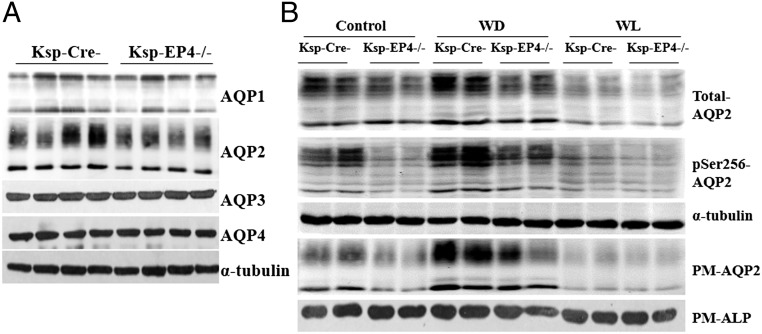
Among four major AQPs (AQP1–4) expressed in the kidney, only AQP2 expression was significantly reduced in the Ksp-EP4−/− mice compared with Ksp-Cre− mice under normal water intake condition (Fig. 3A). After water restriction, total AQP2 expression in the kidneys was increased in both groups. However, AQP2 levels were still significantly lower in Ksp-EP4−/− mice than in Ksp-Cre− mice (Fig. 3B). In contrast, chronic water loading significantly reduced AQP2 expression in both genotypes, with much lower levels in Ksp-EP4−/− mice (Fig. 3B). Because AQP2 phosphorylation at Ser256 is critical for its membrane targeting (23), expression of pSer256-AQP2 was measured and a significant reduction was observed in the Ksp-EP4−/− mice compared with controls under both hydrated and dehydrated conditions (Fig. 3B). As Ser256-AQP2 is a target for PKA-induced phosphorylation (23), the phosphorylated PKA substrates were measured and a significant suppression was evident in Ksp-EP4−/− mice (SI Appendix, Fig. S5F). This result suggested that the decreased pSer256-AQP2 levels were probably due to decreased PKA activity after EP4 gene deletion. Consistently, AQP2 protein expression on plasma membrane (PM-AQP2) was markedly reduced in the Ksp-EP4−/− mice compared with the Ksp-Cre− mice (Fig. 3B). In addition, immunohistochemical (SI Appendix, Fig. S8A) and immunofluorescent (SI Appendix, Fig. S8B) analyses also showed decreased AQP2 abundance and membrane targeting in Ksp-EP4−/− mice under both hydrated and dehydrated conditions.
Fig. 3.
Reduced total, phosphorylated, and membrane-targeted AQP2 levels in Ksp-EP4−/− mice. (A) Western blot analysis of AQP1, AQP2, AQP3, and AQP4 expression in the kidneys of Ksp-Cre− and Ksp-EP4−/− mice under basal conditions. (B) Western blot analysis of total AQP2, pSer256-AQP2, and PM-AQP2 expression in Ksp-Cre− and Ksp-EP4−/− mice under basal condition and following 24-h water deprivation (WD) or 5-d water loading (WL). ALP, alkaline phosphatase; PM, plasma membrane.
EP4 Agonist CAY10580 Increased AQP2 Membrane Targeting in Vitro.
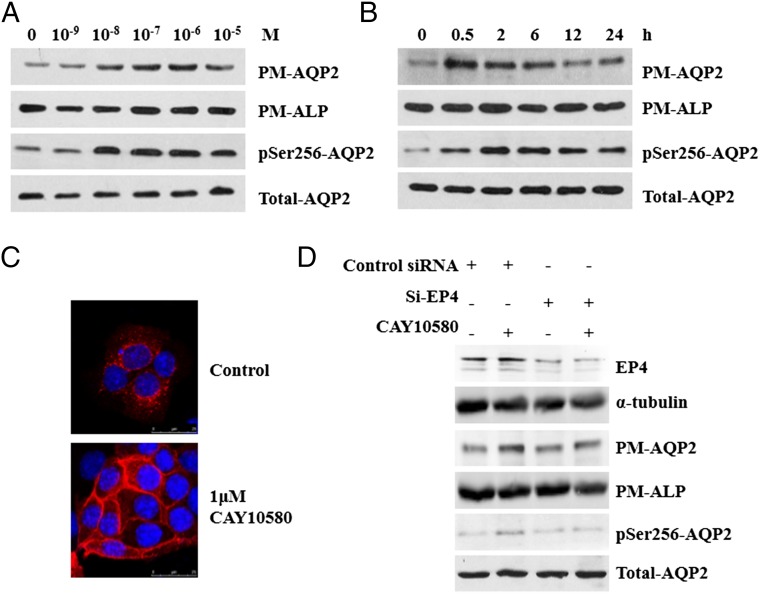
A mouse IMCD3 cell line stably transfected with AQP2 construct (AQP2-IMCD3) was generated to investigate the effect of EP4 on AQP2 membrane targeting. Treatment with an EP4 agonist CAY10580 (10−9 to 10−5 M) for 30 min resulted in a significant increase in PM-AQP2 and pSer256-AQP2 in a concentration-dependent manner (Fig. 4A). CAY10580 at 10−6 M was then used in the following experiments: To examine the time course of AQP2 membrane targeting, AQP2-IMCD3 cells were treated with CAY10580 for 0.5 h up to 24 h (Fig. 4B). PM-AQP2 and pSer256-AQP2 levels began to increase at 0.5 h and remained at high levels for at least 6 h. Immunofluorescence analysis showed that AQP2 was diffusely distributed in the cytoplasm in the resting state, but translocated to the plasma membrane after stimulation with CAY10580 for 30 min, indicating an increased membrane targeting of AQP2 (Fig. 4C). Observation of IMCD3 cells transfected with an EGFP-AQP2 plasmid (SI Appendix, Fig. S9A) by total internal reflection fluorescence (TIRF) microscopy confirmed the increased membrane accumulation of AQP2 in living cells at the presence of CAY10580 (SI Appendix, Fig. S9B). The increase in EGFP-AQP2 targeting on plasma membrane reached a significant level as early as 45 s after EP4 activation (SI Appendix, Fig. S9C). Collectively, these results revealed that EP4 activation promoted AQP2 membrane targeting as quickly as less than 1 min and sustained for at least 6 h.
Fig. 4.
Increased AQP2 membrane targeting and phosphorylation stimulated by the EP4 agonist CAY10580. (A) AQP2 expression on plasma membrane (PM-AQP2) and AQP2 phosphorylation at Ser256 (pSer256-AQP2) were elevated in a dose-dependent manner after treatment with CAY10580 for 30 min in AQP2-IMCD3 cells. Alkaline phosphatase (ALP) and total AQP2 served as loading control of plasma membrane protein and total protein, respectively. (B) Time course of PM-AQP2 and pSer256-AQP2 expression in AQP2-IMCD3 cells stimulated with 1 μM of CAY10580. (C) Immunofluorescence of AQP2 in AQP2-IMCD3 cells after stimulation with 1 μM of CAY10580 for 30 min. (D) Western blot analysis of EP4, PM-AQP2, and pSer256-AQP2 in AQP2-IMCD3 cells stimulated with 1 μM CAY10580 for 30 min after transfection with control or EP4 siRNA (Si-EP4) for 36 h.
EP4 siRNA (si-EP4) was used to exclude the possibility of off-target effect of CAY10580 on AQP2 membrane targeting. As shown in Fig. 4D, CAY10580 treatment no longer increased PM-AQP2 and pSer256-AQP2 levels after siRNA knockdown of EP4 gene in AQP2-IMCD3 cells. This result demonstrated that the effect of CAY10580 on promoting AQP2 membrane targeting was due to its specific activation of the EP4 receptor. This conclusion was also further supported by another EP4 agonist PGE1-OH (SI Appendix, Fig. S9 D–H) and EP4 adenovirus (Ad-EP4) (SI Appendix, Fig. S11 A–D), which also increased AQP2 membrane targeting in AQP2-IMCD3 cells.
EP4 Activation Increased AQP2 Membrane Targeting Through the cAMP/PKA and ERK Pathways.
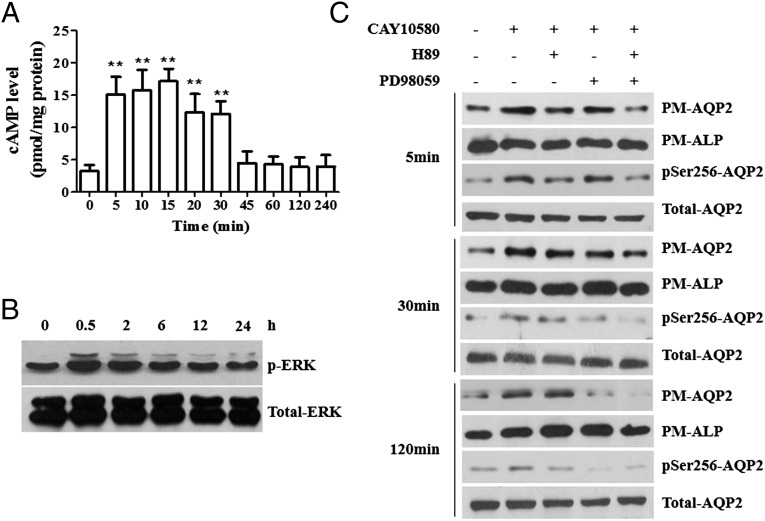
The cAMP/PKA pathway plays an important role in regulating AQP2 phosphorylation at Ser256 and translocation to plasma membrane (23). Intracellular cAMP levels were measured after treatment of IMCDs with CAY10580 and a transient increase within 30 min was found (Fig. 5A). Because mitogen-activated protein kinase (MAPK) family has also been known to increase AQP2 membrane targeting (24) and EP4 could activate ERKs (25), the expression of total ERK and phosphorylated-ERK (p-ERK) in cells was determined. An increased ERK activation from 0.5 h to 6 h after CAY10580 treatment was evident (Fig. 5B). However, different from EP4 agonists, EP4 overexpression caused a persistent increase in cAMP levels and ERK activation in a dose-dependent manner (SI Appendix, Fig. S11 E, G, and H). Consistently, phosphorylated PKA substrates were also increased in a similar pattern (SI Appendix, Fig. S11F). These findings further support that both cAMP/PKA and ERK pathways participate in EP4-elicited AQP2 phosphorylation and apical membrane insertion.
Fig. 5.
EP4 activation increased AQP2 membrane insertion and phosphorylation through the cAMP/PKA and ERK pathways. (A) cAMP levels in AQP2-IMCD3 cells stimulated with 1 μM of CAY10580 for the indicated time periods. **P < 0.01 vs. basal levels, n = 7. (B) Western blot analysis of p-ERK and ERK expression in IMCD3 cells stimulated with 1 μM of CAY10580 for the indicated time periods. (C) Western blot analysis of PM-AQP2 and pSer256-AQP2 in AQP2-IMCD3 cells stimulated with 1 μM of CAY10580 for 5, 30, and 120 min after pretreatment with 10 μM of H89 or 20 μM PD98059 for 30 min. The results are presented as mean ± SEM.
To elucidate the precise role for PKA and ERK in EP4-elicited AQP2 membrane targeting, AQP2-IMCD3 cells were pretreated with the PKA inhibitor H89 and/or ERK inhibitor PD98059 for 30 min, followed by treatment with CAY10580 for 5, 30, and 120 min, respectively. After treatment with CAY10580 for 5 min, H89, but not PD98059, significantly decreased pSer256-AQP2 and PM-AQP2 levels (Fig. 5C). When treated for 30 min, both H89 and PD98059 reduced CAY10580-induced pSer256-AQP2 and PM-AQP2 expression. In contrast, the inhibitory effect of H89 on AQP2 membrane trafficking was markedly diminished after treatment for 120 min, whereas PD98059 significantly decreased CAY10580-induced pSer256-AQP2 and PM-AQP2 levels (Fig. 5C). Interestingly, the combined use of H89 and PD98059 almost completely abolished the effects of CAY10580 on AQP2 membrane targeting at all time points. These results indicate that PKA is likely to be the initial kinase triggering AQP2 phosphorylation and translocation, whereas ERK appears to be the kinase responsible for maintaining the effects of EP4.
EP4 Activation or Overexpression Up-Regulated AQP2 Protein Abundance in Vitro.
To further characterize the role of EP4 in regulating AQP2 expression, primary IMCD cells were cultured. Both mRNA and total protein expression of AQP2 were significantly increased after the treatment of CAY10580 in a time-dependent manner (Fig. 6 A and B). Similarly, adenovirus-mediated EP4 overexpression caused a robust increase in AQP2 expression at both transcriptional and translational levels (SI Appendix, Fig. S12 A and B). Because AQP2 transcription is directly regulated by cAMP through cAMP-responsive element (CRE) (26), the expression of p-CREB and total CREB (t-CREB) were measured by Western blot. A significant increase in the ratio of p-CREB/t-CREB was observed after both EP4 pharmacological activation and overexpression (Fig. 6B and SI Appendix, Fig. S12B). To verify whether EP4 activation or overexpression affects AQP2 transcription, an AQP2 gene promoter-driven luciferase reporter was transfected into IMCD cells in the presence or absence of CAY10580 or Ad-EP4. Both CAY10580 and Ad-EP4 significantly increased AQP2 promoter activity (Fig. 6C and SI Appendix, Fig. S12C). As expected, the mutation of the CRE region abolished EP4-induced AQP2 promoter transcription activity (Fig. 6C and SI Appendix, Fig. S12C), suggesting that EP4 activation or overexpression increased AQP2 protein abundance mainly through the CREB pathway.
Fig. 6.
Effect of EP4 activation on AQP2 protein and mRNA levels in primary mouse IMCD cells. (A) AQP2 mRNA levels in primary IMCD cells stimulated with 1 μM of CAY10580 for the indicated time periods. *P < 0.05 vs. basal level, n = 4. (B) Western blot analysis of AQP2 and CREB in primary IMCD cells stimulated with 1 μM of CAY10580 for the indicated time periods. (C) EP4 activation by CAY10580 (1 μM) significantly increased the AQP2 luciferase reporter activity. Mutation of CRE completely abolished the stimulatory effect. **P < 0.01 vs. control, n = 6. The results are presented as mean ± SEM.
Discussion
The kidney is the central organ in the maintenance of water homeostasis. Approximately 85% of the filtered water is constitutively reabsorbed in the proximal tubules and the descending thin limbs of Henle. The remaining 15% is reabsorbed in the connecting tubules and collecting ducts, which are highly regulated by both systemic hormone AVP and local autocrine and paracrine agents including bradykinin, ATP, endothelin, nitric oxide, and PGE2 (27, 28). In the present study, we generated both renal tubule- and collecting duct-specific EP4 gene knockout mice and showed that both mouse lines produced large amount of diluted urine along with decreased collecting duct AQP2 expression and apical membrane targeting under both hydrated and dehydrated conditions. Mechanistic study found that EP4-regulated AQP2 membrane translocation involves both cAMP/PKA and ERK pathways, whereas EP4-elicited AQP2 abundance is dependent on the cAMP/CREB pathway. Our findings demonstrate an important role for the EP4 receptor in regulating water reabsorption and support the use of EP4 agonist as a potential therapeutic treatment for NDI.
The principal cells of the collecting ducts are central to water transport as reflected by its containing AQP2, AQP3, and AQP4 water channels. Among these three channels, AQP2 is located in the luminal plasma membrane and is highly regulated in a complex manner (27). This regulation involves short-term modulation through alterations in AQP2 trafficking between intracellular vesicles and apical membrane, and long-term regulation via changes in AQP2 transcription and translation. Apical AQP2 mediates the entry of water into principal cells and constitutes the rate-limiting step for water transport in the collecting ducts. AQP2 is primarily controlled by AVP released from the pituitary in response to plasma osmolality and volume. However, many locally produced agents including PGE2 may also regulate AQP2 abundance or trafficking. Increasing evidence demonstrates that the collecting ducts produce relatively large amounts of PGE2, which exert natriuretic and diuretic effects through its four G protein-coupled receptors, i.e., EP1–4 (29). Here we show that among the four PGE2 receptors, EP4, rather than the other three receptors, plays an important role in the regulation of water reabsorption under physiological conditions.
In the kidney, EP4 receptor is expressed in the glomerulus, vasa recta, and the collecting ducts (30, 31). Studies on the role of EP4 in vivo have been hampered by the early postnatal death of EP4 gene global knockout mice due to lethal patent ductus arteriosus (32). In the present study, we found that both entire renal tubule (Ksp-EP4−/−)- and collecting duct (AQP2-EP4−/−)-EP4 gene targeting mice exhibited a significant increase in hypoosmotic urine output compared with their wild-type controls, although AQP2-EP4−/− mice displayed the polyuric phenotype much earlier than Ksp-EP4−/− mice. The defect in urinary concentrating ability in the knockout mice appears to be the result of renal EP4 deficiency, because similar response of urine volume was observed after dDAVP injection and no difference was found in hypothalamic AVP mRNA levels and plasma AVP concentrations between EP4 gene-deficient mice and wild-type mice.
Decreased AQP2 abundance and apical membrane targeting in renal collecting ducts were observed in both Ksp-EP4−/− and AQP2-EP4−/− mice, which may be responsible for the polyuric phenotype in these mice. The findings also suggest that EP4 may affect AQP2 protein expression and apical translocation in the collecting ducts. In support, in vitro studies demonstrated that EP4 activation or overexpression increased AQP2 membrane accumulation and phosphorylation at the ser256 site in a dose- and time-dependent manner in a mouse IMCD cell line (IMCD3) stably transfected with the AQP2 gene. Increased membrane insertion of AQP2 occurred within seconds and lasted for at least 6 h. EP4-induced AQP2 membrane targeting appears to be attributed to the sequential activation of the cAMP/PKA and ERK pathways. An early PKA activation may mediate the short-term induction of AQP2 membrane targeting, whereas ERK activation may contribute to the sustained AQP2 membrane trafficking induced by EP4 activation. Increased PKA activity seems to be the result of a rapid, but transient increase in cAMP levels after EP4 activation. However, ERK activation after EP4 agonist treatment might involve β-arrestins, which is slower in onset and more sustained in duration (33). It is anticipated that activation of EP4 receptor leads to the recruitment of β-arrestins that trigger internalization and desensitization of the EP4 receptor, decrease cAMP levels, and then activate ERK. Collectively, our findings suggest the induction of AQP2 membrane targeting by EP4 activation via both cAMP/PKA and ERK pathways.
AQP2 mRNA and protein levels were also significantly up-regulated in mouse primary cultured IMCD cells after pharmacological activation with selective EP4 agonists or adenovirus-mediated overexpression of EP4 in a cAMP/CREB-dependent manner. It is well documented that AVP regulates AQP2 abundance via the PKA/CREB pathway. Kortenoeven et al. have proposed that long-term regulation of AQP2 by AVP in mpkCCD cells involves the activation of Epac, which is independent of PKA and CREB (34). In the present study, we found a significant increase in p-CREB in cultured mouse primary IMCD cells stimulated by EP4 agonists or adenovirus-mediated overexpression. Moreover, data from the luciferase study indicated that EP4-induced activation of AQP2 promoter was totally abolished when the CRE site was mutated. Together, these results suggest that EP4 stimulates the phosphorylation of CREB and the subsequent binding of CREB to the CRE region on the AQP2 promoter, which leads to an increase in AQP2 transcription and translation.
Although AVP plays the central role in maintaining water homeostasis (35), the present study demonstrates that the prostaglandin E2/EP4 receptor signaling pathway represents an independent and indispensable mechanism regulating water transport in renal collecting ducts. Collecting duct deficiency for EP4 receptor impairs urine concentrating ability via reducing protein expression and apical membrane targeting of AQP2, leading to an NDI phenotype. Recently, a selective EP4 agonist, ONO-AE1-329, has been shown to be effective in attenuating polyuria and polydipsia in a mouse model of X-linked NDI model that lacks V2R after birth (14), further supporting the critical role of EP4 in water reabsorption. It should be noted that although PGE2 has been previously shown to be a natriuretic factor (36), urinary excretion of sodium, potassium, and chloride remained unchanged in renal tubule-specific EP4 gene knockout mice, suggesting a natriuretic effect of PGE2 is likely mediated by the other three EP receptors located in renal collecting ducts.
In conclusion, EP4 receptor is an independent and indispensable local regulator of urinary concentration in renal collecting ducts under physiological conditions (SI Appendix, Fig. S13). EP4 activation increases AQP2 expression and membrane translocation in a cAMP/CREB- and a PKA/ERK-dependent manner, respectively. The ability of EP4 to promote AQP2 membrane targeting and increase AQP2 abundance makes it a potential therapeutic target for the treatment of clinical disorders including acquired and congenital diabetes insipidus.
Materials and Methods
Experimental Animals.
All experiments were reviewed and approved by the Animal Care and Use Review Committee of Peking University Health Science Center. Renal tubule- and collecting duct-specific EP4 gene deficiency mice were generated using the Cre/loxP system. The Ksp-Cre transgenic mice on 129 background was kindly provided by Baoxue Yang (Peking University) (19, 20). EP4flox/flox mice on C57BL/6 background were generated as previously reported (21). The mouse line expressing Cre recombinase specifically in renal collecting ducts under the control of AQP2 promoter (AQP2-Cre) on C57BL/6 background was purchased from The Jackson Laboratory. For details, see SI Appendix, SI Materials and Methods.
Cell Culture.
Primary mouse IMCD cells were cultured as described previously (18).
Extraction of Cell Membrane Protein.
The cell membrane protein of IMCD3 and primary IMCD cells was isolated with the Pierce Cell Surface Protein Isolation kit (Thermo) according to the manufacturer’s instructions.
Reagents, antibodies, siRNAs, adenoviruses, and experimental procedures are described in detail in SI Appendix, SI Materials and Methods.
Statistical Analysis.
Data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism5 software. Comparisons between two groups were analyzed by Student’s t test. For multiple comparisons, one-way analysis of variance (ANOVA) was performed followed by Bonferroni’s test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) (Grants 2012CB517504 and 2013CB945202), the National Natural Science Foundation of China (Grants 81030003, 81270275, 81200511, and 81390351), and the Swedish Research Council.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509565112/-/DCSupplemental.
References
- 1.Rojek A, Füchtbauer EM, Kwon TH, Frøkiaer J, Nielsen S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci USA. 2006;103(15):6037–6042. doi: 10.1073/pnas.0511324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci USA. 1993;90(24):11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ecelbarger CA, et al. Escape from vasopressin-induced antidiuresis: Role of vasopressin resistance of the collecting duct. Am J Physiol. 1998;274(6 Pt 2):F1161–F1166. doi: 10.1152/ajprenal.1998.274.6.F1161. [DOI] [PubMed] [Google Scholar]
- 4.Ecelbarger CA, et al. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest. 1997;99(8):1852–1863. doi: 10.1172/JCI119352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbalis JG, Murase T, Ecelbarger CA, Nielsen S, Knepper MA. Studies of renal aquaporin-2 expression during renal escape from vasopressin-induced antidiuresis. Adv Exp Med Biol. 1998;449:395–406. doi: 10.1007/978-1-4615-4871-3_51. [DOI] [PubMed] [Google Scholar]
- 6.Qi Z, Cai H, Morrow JD, Breyer MD. Differentiation of cyclooxygenase 1- and 2-derived prostanoids in mouse kidney and aorta. Hypertension. 2006;48(2):323–328. doi: 10.1161/01.HYP.0000231934.67549.b7. [DOI] [PubMed] [Google Scholar]
- 7.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: Structures, properties, and functions. Physiol Rev. 1999;79(4):1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 8.Jackson BA. Prostaglandin E2 synthesis in the inner medullary collecting duct of the rat: Implications for vasopressin-dependent cyclic AMP formation. J Cell Physiol. 1986;129(1):60–64. doi: 10.1002/jcp.1041290109. [DOI] [PubMed] [Google Scholar]
- 9.Maeda Y, Terada Y, Nonoguchi H, Knepper MA. Hormone and autacoid regulation of cAMP production in rat IMCD subsegments. Am J Physiol. 1992;263(2 Pt 2):F319–F327. doi: 10.1152/ajprenal.1992.263.2.F319. [DOI] [PubMed] [Google Scholar]
- 10.Noland TD, Carter CE, Jacobson HR, Breyer MD. PGE2 regulates cAMP production in cultured rabbit CCD cells: Evidence for dual inhibitory mechanisms. Am J Physiol. 1992;263(6 Pt 1):C1208–C1215. doi: 10.1152/ajpcell.1992.263.6.C1208. [DOI] [PubMed] [Google Scholar]
- 11.Hébert RL, Jacobson HR, Fredin D, Breyer MD. Evidence that separate PGE2 receptors modulate water and sodium transport in rabbit cortical collecting duct. Am J Physiol. 1993;265(5 Pt 2):F643–F650. doi: 10.1152/ajprenal.1993.265.5.F643. [DOI] [PubMed] [Google Scholar]
- 12.Fleming EF, et al. Urinary concentrating function in mice lacking EP3 receptors for prostaglandin E2. Am J Physiol. 1998;275(6 Pt 2):F955–F961. doi: 10.1152/ajprenal.1998.275.6.F955. [DOI] [PubMed] [Google Scholar]
- 13.Olesen ET, Rützler MR, Moeller HB, Praetorius HA, Fenton RA. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci USA. 2011;108(31):12949–12954. doi: 10.1073/pnas.1104691108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JH, et al. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest. 2009;119(10):3115–3126. doi: 10.1172/JCI39680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan Y, et al. Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J Clin Invest. 2007;117(9):2496–2505. doi: 10.1172/JCI29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy CR, et al. Salt-sensitive hypertension and reduced fertility in mice lacking the prostaglandin EP2 receptor. Nat Med. 1999;5(2):217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, et al. Inactivation of the E-prostanoid 3 receptor attenuates the angiotensin II pressor response via decreasing arterial contractility. Arterioscler Thromb Vasc Biol. 2012;32(12):3024–3032. doi: 10.1161/ATVBAHA.112.254052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, et al. Farnesoid X receptor (FXR) gene deficiency impairs urine concentration in mice. Proc Natl Acad Sci USA. 2014;111(6):2277–2282. doi: 10.1073/pnas.1323977111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002;13(7):1837–1846. doi: 10.1097/01.asn.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- 20.Lin F, et al. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA. 2003;100(9):5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider A, et al. Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis. 2004;40(1):7–14. doi: 10.1002/gene.20048. [DOI] [PubMed] [Google Scholar]
- 22.Nelson RD, et al. Expression of an AQP2 Cre recombinase transgene in kidney and male reproductive system of transgenic mice. Am J Physiol. 1998;275(1 Pt 1):C216–C226. doi: 10.1152/ajpcell.1998.275.1.C216. [DOI] [PubMed] [Google Scholar]
- 23.Moeller HB, Olesen ET, Fenton RA. Regulation of the water channel aquaporin-2 by posttranslational modification. Am J Physiol Renal Physiol. 2011;300(5):F1062–F1073. doi: 10.1152/ajprenal.00721.2010. [DOI] [PubMed] [Google Scholar]
- 24.Hasler U, et al. Acute hypertonicity alters aquaporin-2 trafficking and induces a MAPK-dependent accumulation at the plasma membrane of renal epithelial cells. J Biol Chem. 2008;283(39):26643–26661. doi: 10.1074/jbc.M801071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74(2-3):143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol. 1997;8(6):861–867. doi: 10.1681/ASN.V86861. [DOI] [PubMed] [Google Scholar]
- 27.Pearce D, et al. Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol. 2015;10(1):135–146. doi: 10.2215/CJN.05760513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sands JM, Layton HE. Advances in understanding the urine-concentrating mechanism. Annu Rev Physiol. 2014;76:387–409. doi: 10.1146/annurev-physiol-021113-170350. [DOI] [PubMed] [Google Scholar]
- 29.Kömhoff M, et al. Enhanced expression of cyclooxygenase-2 in high grade human transitional cell bladder carcinomas. Am J Pathol. 2000;157(1):29–35. doi: 10.1016/S0002-9440(10)64513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breyer MD, et al. Regulation of renal function by prostaglandin E receptors. Kidney Int Suppl. 1998;67:S88–S94. doi: 10.1046/j.1523-1755.1998.06718.x. [DOI] [PubMed] [Google Scholar]
- 31.Jensen BL, Stubbe J, Hansen PB, Andreasen D, Skøtt O. Localization of prostaglandin E(2) EP2 and EP4 receptors in the rat kidney. Am J Physiol Renal Physiol. 2001;280(6):F1001–F1009. doi: 10.1152/ajprenal.2001.280.6.F1001. [DOI] [PubMed] [Google Scholar]
- 32.Segi E, et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246(1):7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 33.Ma L, Pei G. Beta-arrestin signaling and regulation of transcription. J Cell Sci. 2007;120(Pt 2):213–218. doi: 10.1242/jcs.03338. [DOI] [PubMed] [Google Scholar]
- 34.Kortenoeven ML, et al. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve Epac. Am J Physiol Renal Physiol. 2012;302(11):F1395–F1401. doi: 10.1152/ajprenal.00376.2011. [DOI] [PubMed] [Google Scholar]
- 35.Boone M, Deen PM. Physiology and pathophysiology of the vasopressin-regulated renal water reabsorption. Pflugers Arch. 2008;456(6):1005–1024. doi: 10.1007/s00424-008-0498-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breyer MD, Breyer RM. Prostaglandin E receptors and the kidney. Am J Physiol Renal Physiol. 2000;279(1):F12–F23. doi: 10.1152/ajprenal.2000.279.1.F12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.