Significance
Phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) is the principal mechanism cells use to regulate translation initiation. Specific kinases phosphorylate eIF2α to inhibit protein synthesis under stress conditions; however, eIF2α dephosphorylation is catalyzed by general protein phosphatase 1 (PP1). In mammalian cells, specific trans-acting targeting proteins, growth arrest and DNA damage-inducible protein 34 (GADD34) and constitutive repressor of eIF2α phosphorylation (CReP), bind to PP1 and promote dephosphorylation of eIF2α. We show that GADD34 directly binds to eIF2α, and we identify and demonstrate the function of an eIF2α-binding motif that is shared among GADD34, CReP, and several viral proteins. Thus, these cellular and viral PP1-targeting proteins bind independently to PP1 and to eIF2α to form a trimeric complex and promote the specific dephosphorylation of eIF2α to maintain cellular protein synthesis.
Keywords: DP71L, CReP, canarypox, PP1, PKR
Abstract
Transient protein synthesis inhibition, mediated by phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2α), is an important protective mechanism cells use during stress conditions. Following relief of the stress, the growth arrest and DNA damage-inducible protein GADD34 associates with the broadly acting serine/threonine protein phosphatase 1 (PP1) to dephosphorylate eIF2α. Whereas the PP1-binding motif on GADD34 has been defined, it remains to be determined how GADD34 directs PP1 to specifically dephosphorylate eIF2α. In this report, we map a novel eIF2α-binding motif to the C terminus of GADD34 in a region distinct from where PP1 binds to GADD34. This motif is characterized by the consensus sequence Rx[Gnl]x1–2Wxxx[Arlv]x[Dn][Rg]xRFxx[Rlvk][Ivc], where capital letters are preferred and x is any residue. Point mutations altering the eIF2α-binding motif impair the ability of GADD34 to interact with eIF2α, promote eIF2α dephosphorylation, and suppress PKR toxicity in yeast. Interestingly, this eIF2α-docking motif is conserved among viral orthologs of GADD34, and is necessary for the proteins produced by African swine fever virus, Canarypox virus, and Herpes simplex virus to promote eIF2α dephosphorylation. Taken together, these data indicate that GADD34 and its viral orthologs direct specific dephosphorylation of eIF2α by interacting with both PP1 and eIF2α through independent binding motifs.
The reversible phosphorylation of proteins is one of the most common posttranslational modifications and plays an important role in regulating many cellular processes including protein synthesis, glycogen metabolism, and cell division (1–3). In mammals, four kinases, PKR, PERK, GCN2, and HRI, are known to down-regulate protein synthesis by phosphorylating the α subunit of eukaryotic translation initiation factor 2α (eIF2α) on Ser51 following their activation under specific stress conditions including amino acid deprivation, heat shock, and viral infection (4). The factor eIF2, composed of α, β, and γ subunits, binds GTP and Met-tRNAiMet to form a ternary complex (TC) and then delivers the Met-tRNAiMet to the small ribosomal subunit. After the resulting complex binds an mRNA and scans to select a start codon for protein synthesis, hydrolysis of the GTP bound to eIF2 is completed and an eIF2–GDP binary complex is released from the ribosome. To participate in another round of translation initiation, the GDP on eIF2 must be exchanged for GTP by the guanine–nucleotide exchange factor eIF2B (5). This eIF2 recycling step is an important control point in the translation pathway.
Phosphorylation of eIF2α converts eIF2 from a substrate to a competitive inhibitor of eIF2B. The resultant block of eIF2 recycling and TC formation prevents the subsequent steps in the translation initiation pathway (5). Although eIF2α phosphorylation inhibits general protein synthesis, it also promotes the translation of mRNAs encoding specific stress response factors including GCN4 in yeast and the activating transcription factor 4 (ATF4) in mammals. Like GCN4, the ATF4 protein induces the transcription of downstream genes like CHOP that are critical for the cellular stress response (6). A further downstream target of ATF4/CHOP is the growth arrest and DNA damage-inducible protein GADD34 (PPP1R15A). As indicated by its alternate name, GADD34 is a protein phosphatase 1 (PP1) targeting protein that directs PP1 to dephosphorylate eIF2α (7–11). Consistent with this function, expression of GADD34 is correlated with eIF2α dephosphorylation during later stages of the stress response (7, 10).
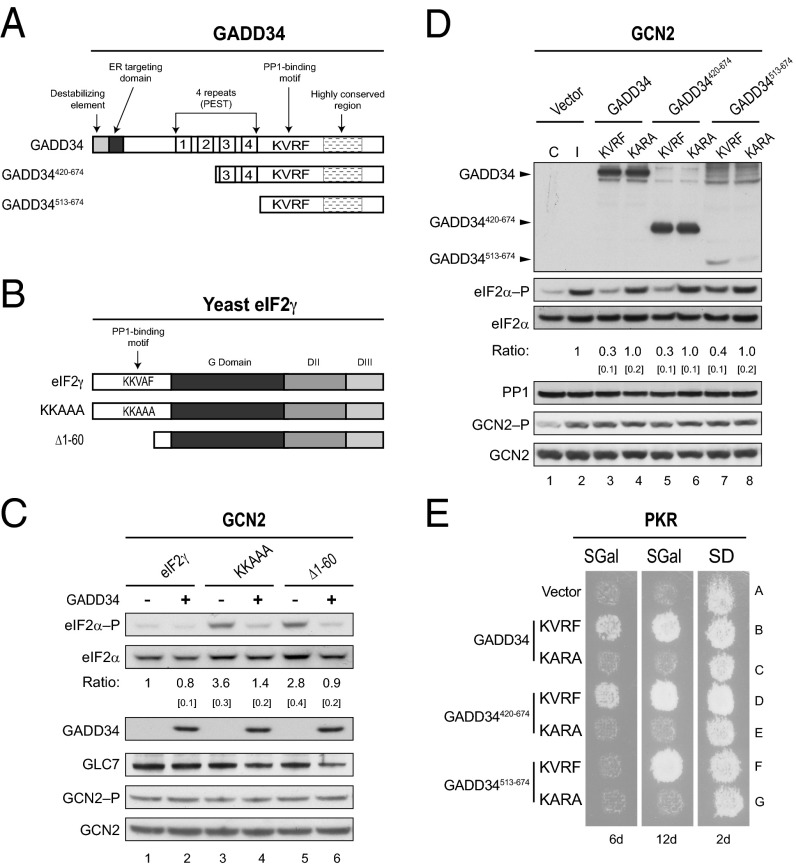
GADD34 is composed of 674 residues organized in several functional regions (Fig. 1A)—a highly basic N terminus, which has been shown to be important to direct the localization of GADD34 to the endoplasmic reticulum (ER) (7), enhance the rate of the GADD34 protein turnover (12), and enable GADD34 induction of apoptosis in mammalian cells (13); a central domain containing four repeated sequence elements rich in proline, acidic, serine, and threonine residues. Sequences enriched in these amino acids are commonly known as PEST motifs and usually have an important role in protein turnover (14). Although these repeated sequences in GADD34 are highly conserved across species, and are important for interaction with the regulator termed inhibitor-1 (8), they do not appear to impact GADD34 turnover (12). Because their function appears distinct from typical PEST motifs, we refer to these sequences simply as repeated sequences. Another characteristic of GADD34 is a well-conserved C-terminal region whose precise role is not yet understood (Fig. 1A). Finally, within this C-terminal region, GADD34 contains a degenerate amino acid sequence motif commonly simplified as RVxF. This motif is essential to recruit PP1 to dephosphorylate eIF2α (7, 8).
Fig. 1.
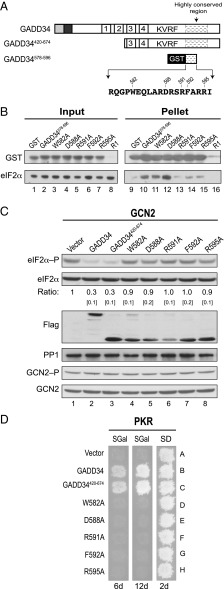
GADD34 promotes eIF2α dephosphorylation in yeast. (A) Schematics of GADD34 and two N-terminally truncated derivatives. Positions of the ER targeting domain, destabilizing element, four repeated sequence elements, PP1-binding motif (KVRF sequence), and the highly conserved region are indicated. (B) Schematic diagram showing S. cerevisiae eIF2γ. The PP1-binding motif (KKVAF sequence) in the N-terminal extension of eIF2γ, the locations of the GTP-binding (G) domain and domains II (DII) and III (DIII), and eIF2γ mutations designed to alter or eliminate the KKVAF motif are indicated. (C) Derivatives of yeast strain YM103 expressing eIF2γ, or its KKAAA or Δ1–60 derivative, and carrying either an empty vector (-) or a plasmid expressing GADD34 (+) under the control of a galactose-inducible promoter were grown in synthetic galactose (SGal) medium to log phase under nonstarvation conditions, and then equivalent amounts of WCEs were subjected to SDS/PAGE, followed by immunoblot analysis with antibodies to detect phosphorylated eIF2α–P. The membrane was then sequentially stripped and probed with antibodies against total yeast eIF2α (SUI2), the Myc-tag on GLC7, and the Flag-tag on GADD34. The relative level of phosphorylated to total eIF2α was determined by quantitative densitometry by using ImageJ software and normalized to the ratio obtained in lane 1, mean and SEs (in brackets) were calculated from at least three independent experiments. GCN2 was immunoprecipitated from yeast and subjected sequentially to immunoblot analysis by using antibodies against phosphorylated Thr882–P or total GCN2. (D) The humanized yeast strain YM100 expressing human eIF2α and human PP1 in place of yeast eIF2α (SUI2) and GLC7 was transformed with empty vector or plasmids expressing the indicated variant of GADD34. Cells were grown in SGal medium and then incubated for 1 h under nonstress control (C) conditions (lane 1) or in the presence of 1 μg/mL SM to induce (I) activation of GCN2 (lanes 2–8); equivalent amounts of WCEs were subjected to sequential immunoblot analysis by using phosphospecific antibodies against phosphorylated Ser51 of eIF2α (eIF2α–P), monoclonal antibodies against the Myc-tag on human eIF2α, monoclonal antibodies against the Flag-tag on GADD34, and monoclonal antibodies against human PP1. GCN2 was immunoprecipitated and analyzed as described for C. The relative levels of phosphorylated to total eIF2α were determined as described above. (E) Transformants of yeast strain YM77 (+PKR) bearing an empty vector or a plasmid that expresses the indicated version of GADD34 were grown to confluence on synthetic dextrose (SD) plates and then replica-plated to SD plates or SGal plates to induce PKR and GADD34 expression. To limit the appearance of revertants, plates were incubated at 18 °C for 6 or 12 d.
In addition to GADD34, mammalian cells encode a constitutively expressed protein named CReP (PPP1R15B) that also interacts with PP1 and is responsible for maintaining the basal levels of eIF2α phosphorylation in unstressed cells (15). Likewise, the Herpes simplex virus (HSV) γ34.5 protein (16) and the African swine fever virus (ASFV) DP71L protein (17), which both share sequence similarity with GADD34 and CReP, recruit PP1 to dephosphorylate eIF2α in infected cells. Whereas the interaction of PP1 with GADD34, CReP, γ34.5, and DP71L has been shown to depend on the RVxF motif (7, 8, 15–17), it remains to be determined how these regulatory subunits direct PP1 to specifically dephosphorylate eIF2α.
Besides controlling translation initiation, PP1 regulates a great variety of cellular processes through its interaction with many different regulatory subunits (18). Most of these subunits contain the conserved RVxF motif, which has been proposed either to allosterically affect the activity and/or substrate specificity of PP1 or to more simply target PP1 to its substrates (19–23). Notably, the latter idea is favored because binding of the RVxF motif has not been found to induce a conformational change in PP1 (22–24). To determine how GADD34 promotes the specific dephosphorylation of eIF2α by PP1, we established a yeast cell-based assay to monitor the dephosphorylation of human eIF2α mediated by the human GADD34–PP1 complex. Here, we present in vitro and in vivo evidence that GADD34 directly interacts with eIF2α. Moreover, we map an eIF2α-binding site to the C terminus of GADD34 in a region distinct from where PP1 binds to GADD34. This eIF2α-binding motif is highly conserved in several viral orthologs of GADD34 and is essential to promote dephosphorylation of eIF2α. Finally, we provide evidence that the N-terminal region of eIF2α is required for proper recognition by GADD34. Our studies indicate that GADD34 functions as a scaffold to direct the specific dephosphorylation of eIF2α by interacting with both PP1 and eIF2α.
Results
GADD34 Promotes Dephosphorylation of eIF2α in Yeast Cells.
In mammalian cells, the specific dephosphorylation of eIF2α is mediated by the GADD34–PP1 and CReP–PP1 complexes (7, 8, 15). However, no homologs of GADD34 or CReP are found in yeast. We recently found that an N-terminal extension on yeast eIF2γ contains a PP1-binding motif (KKVAF) (Fig. 1B), which facilitates the recruitment of GLC7, the yeast ortholog of PP1, to dephosphorylate yeast eIF2α (SUI2) (25). To determine whether GADD34 can functionally substitute for the N-terminal extension on yeast eIF2γ and direct GLC7 to dephosphorylate eIF2α (SUI2) in yeast, whole-cell extracts (WCEs) from yeast strains expressing different versions of eIF2γ and GADD34 were subjected to immunoblot analysis with antibodies specific for the phospho-Ser51 form of eIF2α. Consistent with our previous findings (25), deletion of residues 1–60, eliminating most of the N-terminal extension, or mutations designed to eliminate the KKVAF motif in yeast eIF2γ (Fig. 1B) impaired eIF2α (SUI2) dephosphorylation in cells grown under nonstarvation conditions where the eIF2α kinase GCN2 has low activity (Fig. 1C, lanes 3 and 5 versus 1). Of interest, we found that expression of GADD34 was able to reduce the high levels of phosphorylated eIF2α (SUI2) observed in cells expressing the KKAAA and Δ1–60 mutant forms of eIF2γ (Fig. 1C, lanes 4 and 6). The decrease in eIF2α phosphorylation observed in cells expressing GADD34 could result from decreased GCN2 kinase activity, the sole eIF2α kinase in yeast, or increased eIF2α dephosphorylation mediated by a GADD34–GLC7 complex. To distinguish between these possibilities, we assayed the phosphorylation level of Thr882 in the GCN2 activation loop as an indicator of GCN2 activation (26). GCN2 was immunoprecipitated from WCEs prepared from yeast either lacking or expressing GADD34, and then subjected to immunoblot analysis by using phospho-specific anti-Thr882 antibodies. Interestingly, expression of GADD34 did not appear to alter the amount of GCN2 or its autophosphorylation on Thr882 (Fig. 1C), thus the reduction in eIF2α phosphorylation is not due to reduced GCN2 kinase activity in cells expressing GADD34. Notably, expression of GADD34 also did not alter GLC7 levels (Fig. 1C). These data indicate that human GADD34 is functional in yeast and is able to recruit GLC7 to dephosphorylate yeast eIF2α (SUI2).
While previous studies suggested that the four repeated sequence elements near the middle of GADD34 collaborate with the conserved C-terminal region of GADD34 to assemble a functional eIF2α phosphatase (12), it remained to be determined how GADD34 directs PP1 to specifically promote dephosphorylation of eIF2α. To identify the minimal functional domain of GADD34 that is essential to promote dephosphorylation of eIF2α, and to analyze the functional interactions of GADD34 with its partner proteins from humans, we designed deletions to eliminate most of the N-terminal region of GADD34 (Fig. 1A). The full-length and truncated forms of GADD34 were then expressed in a humanized yeast strain in which human eIF2α, under the control of the SUI2 promoter, and human PP1, under the control of the yeast PGK1 promoter, were expressed in place of yeast eIF2α (SUI2) and GLC7. As expected, in yeast transformed with an empty vector, eIF2α phosphorylation was low in the absence of stress (Fig. 1D, lane 1) and increased when cells were grown in medium containing the drug sulfometuron methyl (SM) that causes Ile and Val starvation and activation of GCN2 (Fig. 1D, lane 2). Notably, the activation of GCN2 was marked by enhanced autophosphorylation on Thr882 (Fig. 1D, GCN2–P panel, lanes 1 and 2). Yeast expressing full-length GADD34 with an intact PP1-binding motif (KVRF) and grown in the presence of SM showed reduced levels of eIF2α phosphorylation compared with the control strain lacking GADD34 (Fig. 1D, lane 3 versus 2). Like GADD34, the GADD34420-674 and GADD34513-674 proteins containing the KVRF motif were able to promote eIF2α dephosphorylation (Fig. 1D, lanes 5 and 7). When normalized for the total amount of eIF2α in the extracts, GADD34513-674 was noticeably less efficient in lowering eIF2α phosphorylation levels (Fig. 1D, lane 7); however, this reduced activity could be due to the poorer expression of GADD34513-674, compared with full-length GADD34 or GADD34420-674 (Fig. 1D, Upper). Importantly, the KARA mutation that impairs PP1 binding to GADD34 (7, 8) (Fig. 2B, lane 14) reduced the ability of GADD34, GADD34420-674, and GADD34513-674 to promote dephosphorylation of eIF2α (Fig. 1D, lanes 4, 6, and 8). As observed previously, expression of GADD34 had no impact on the GCN2 autophosphorylation on Thr882 or on PP1 levels (Fig. 1D). These results support the idea that GADD34 recruits PP1 to dephosphorylate eIF2α without affecting GCN2 kinase activity. Previously, it was shown that overexpression of human PKR is lethal in yeast because of the high levels of eIF2α phosphorylation and inhibition of translation (27, 28). To test whether expression of GADD34 would suppress the toxicity associated with high-level expression of PKR in yeast, vectors encoding different variants of GADD34 were introduced into a yeast strain containing one copy of the human PKR gene. Expression of both GADD34 and PKR in this strain is under the control of the galactose-inducible GAL1 promoter. Accordingly, GADD34 and PKR expression is repressed when cells are grown on synthetic dextrose (SD) medium and induced when cells are grown on galactose (SGal) medium. As shown in Fig. 1E, yeast cells expressing PKR exhibited a severe growth defect on galactose medium (row A), and coexpression of full-length GADD34 (row B) or the truncated protein GADD34420-674 (row D) with intact KVRF motifs restored cell growth. In contrast, cells expressing GADD34513-674 failed to grow by 6 d, but showed growth after 12 d of incubation (Fig. 1E, row F). The impaired growth of the cells expressing the GADD34513-674 mutant could reflect poorer expression of this GADD34 mutant (Fig. 1D and Fig. S1) or could indicate that this protein is less efficient at promoting dephosphorylation of eIF2α. The latter idea is consistent with the notion that the four repeated sequence elements, which are missing in GADD34513-674, contribute to the function of GADD34, but are not essential to promote eIF2α dephosphorylation. In agreement with the idea that GADD34 suppresses PKR toxicity in yeast by recruiting PP1 to dephosphorylate eIF2α, the KARA mutation impaired the ability of full-length GADD34, GADD34420-674, and GADD34513-674 to restore cell growth (Fig. 1E, rows C, E, and G, respectively). In addition, expression of GADD34 or its truncated derivatives did not alter PKR autophosphorylation on Thr446 (Fig. S1), indicating that GADD34 did not impair PKR activation. Despite the significant suppression of PKR toxicity in the yeast replica-printing assays (Fig. 1E), Western blot analysis of WCEs from the same strains grown in liquid cultures failed to detect a change in eIF2α phosphorylation on Ser51 (Fig. S1). This lack of correlation between eIF2α phosphorylation and yeast growth may reflect differences in the levels or kinetics of PKR and GADD34 expression in yeast grown in liquid cultures versus on plates or may reflect the heightened sensitivity of the replica-plating assay to detect PKR suppression following a modest reduction in eIF2α phosphorylation that is not detectable in the Western blot analyses.
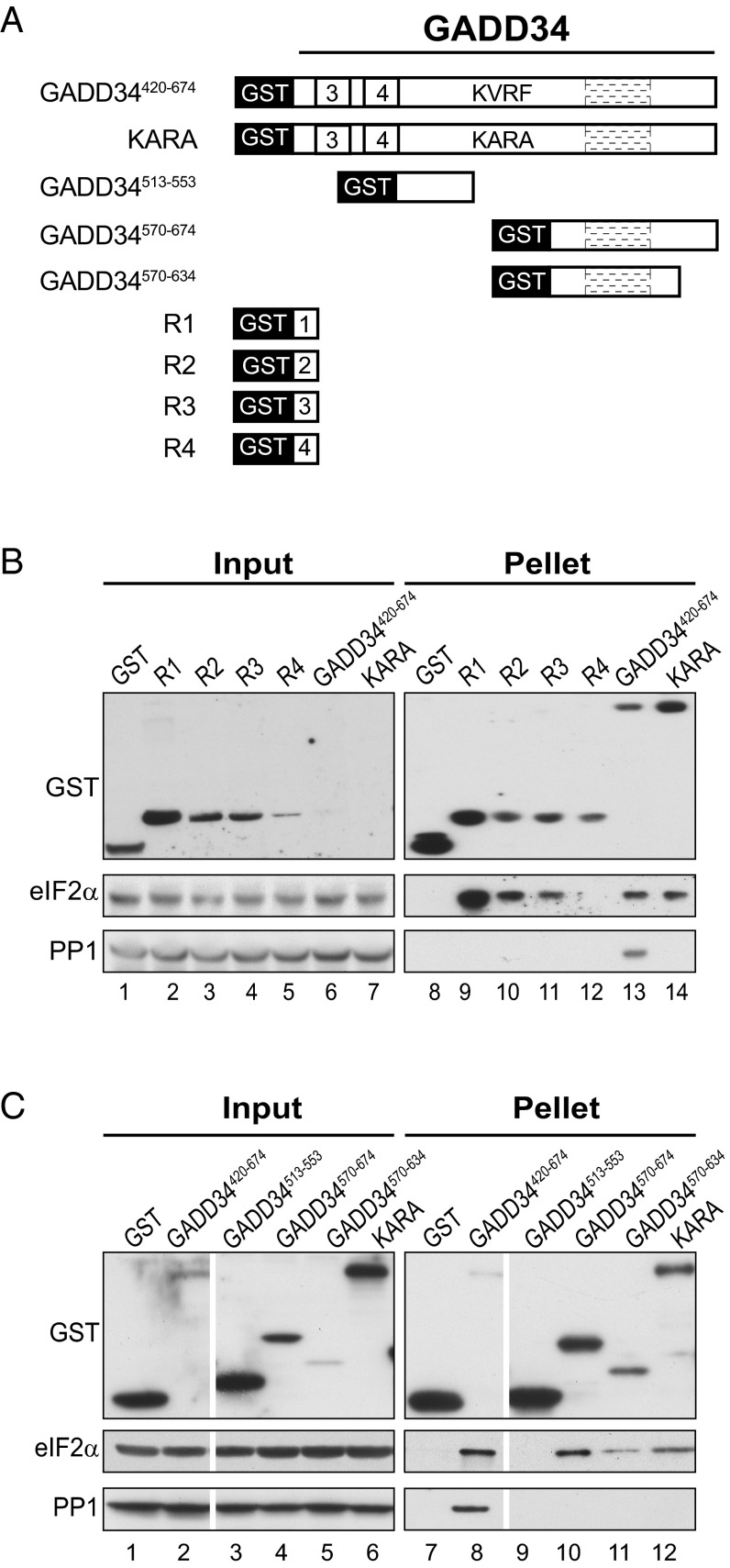
Fig. 2.
Multiple eIF2α binding sites in GADD34. (A) Schematic diagram illustrating serial deletions of GADD34 that were fused in-frame with GST. (B and C) GST or the indicated GST-GADD34 fusion protein was overexpressed in the yeast strain YM100 that expresses human eIF2α and human PP1. WCEs were mixed with glutathione-Sepharose beads, and after washing, bound proteins were eluted with SDS-loading buffer, separated by SDS/PAGE, and detected by immunoblotting with antibodies against GST, PP1, or the Myc-epitope on eIF2α; 5% (vol/vol) of input and 20% (vol/vol) of pellet fractions were analyzed; white lines indicate splicing of lanes from the same original blot to generate the final figure. A longer exposure of the input blot from B is presented in Fig. S2.
Fig. S1.
GADD34 does not reduce PKR activation. Transformants of yeast strain YM77 (+PKR) carrying an empty vector or expressing the indicated version of GADD34 were grown in SD medium and then incubated for 1 h in SGal medium to induce expression of PKR and GADD34. Lanes 2–8 correspond to the strains described in Fig. 1E, and lanes 9–13 correspond to the strains described in Fig. 4D. Lane 1 is from a yeast strain carrying a galactose-inducible PKR-K296R plasmid. Equivalent amounts of WCEs were subjected to SDS/PAGE followed by immunoblot analysis to detect eIF2α–P, eIF2α-Myc, the Flag tag on GADD34, and PP1. In parallel, PKR was immunoprecipitated from the WCEs and subjected sequentially to immunoblot analysis with antibodies against phosphorylated Thr446 or total PKR.
Multiple Elements in GADD34 Bind eIF2α.
Previously, it was shown that GADD34-GFP fusion proteins lacking one or more of the repeated sequence elements exhibited reduced PP1 binding and impaired ability to promote eIF2α phosphatase activity (12), suggesting that the repeat region of GADD34 contributes to eIF2α phosphatase activity; however, the role of this region in binding eIF2α has not been reported. To test whether GADD34 interacts with eIF2α, a set of GST-GADD34 fusion proteins (Fig. 2A) was expressed in the humanized yeast strain containing human eIF2α and human PP1. WCEs from the strains were incubated with glutathione beads, and the products of the pull-down reactions were subjected to immunoblot analysis. As shown in Fig. 2B, a GST-GADD34420-674 fusion protein readily interacted with both PP1 and eIF2α (Fig. 2B, lane 13 and Fig. S2). Consistent with the idea that the KVRF motif in GADD34 mediates the interaction with PP1 (7), the KARA mutation in the GST-GADD34420-674 fusion impaired binding of PP1 (Fig. 2B, compare lanes 13 and 14). Interestingly, the KARA mutation in GST-GADD34420-674 did not affect its interaction with eIF2α (Fig. 2B, compare lanes 13 and 14), indicating that GADD34 interacts with eIF2α in a PP1-independent manner. Because the data in Fig. 1 D and E suggested that the four repeated sequence elements might contribute to the function of GADD34 in vivo, we tested the hypothesis that these repeats interact with eIF2α. Following expression in yeast, GST fusion proteins containing repeats R1, R2, or R3, but not R4, bound to eIF2α (Fig. 2B, lanes 9–12). Because these repeated sequence elements are not essential to promote eIF2α dephosphorylation (Fig. 1 D and E), we predict that there must be an additional, functionally important interaction between GADD34 and eIF2α, and that these repeated sequence elements contribute to the function of GADD34 by increasing the local concentration of eIF2α. To test the idea that another region(s) of GADD34 binds eIF2α, we produced GST fusion proteins containing the regions of GADD34 flanking the KVRF motif. As shown in Fig. 2C, GST-GADD34513-553 failed to interact with either PP1 or eIF2α (lane 9); however, the GST-GADD34570-674 fusion, which includes the C-terminal highly conserved region of GADD34, interacted with eIF2α but not with PP1 (lane 10). Following removal of 40 residues from the C terminus, the GST-GADD34570-634 fusion retained the ability to bind eIF2α (Fig. 2C, lane 11). Taken together, these results suggest that GADD34 either directly or indirectly (via other proteins in the yeast WCE) interacts with eIF2α and that this interaction is independent of PP1.
Fig. S2.
Longer exposure of GST blot from Input image of Fig. 2B. GST or the indicated GST-GADD34 fusion protein was overexpressed in the yeast strain YM100 that expresses human eIF2α and human PP1. WCEs [5% (wt/vol) of the input samples used for GST pull-down assays] were subjected to SDS/PAGE and immunoblot analysis by using antibodies against GST, the Myc-epitope on eIF2α, or PP1.
An eIF2α-Binding Motif Is Conserved Among Viral Orthologs of GADD34.
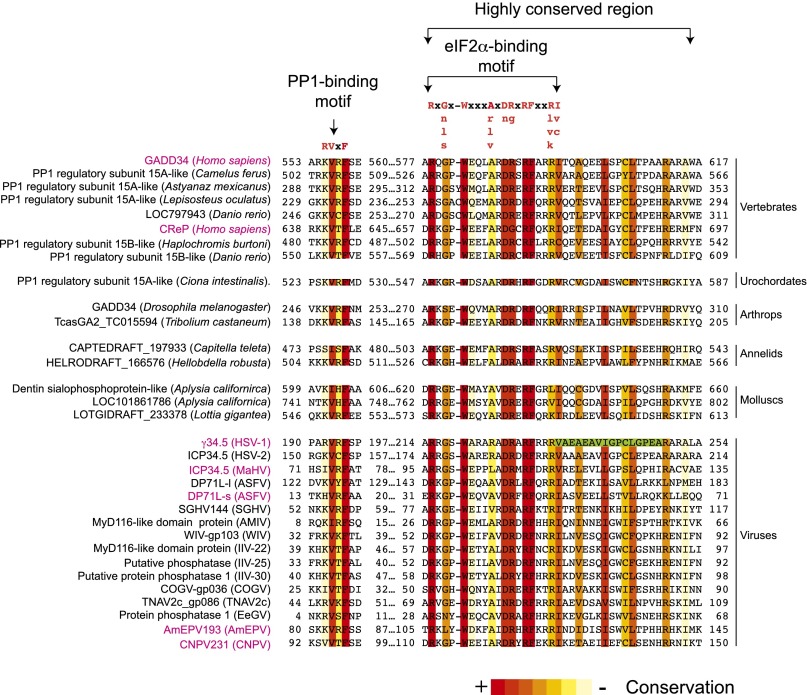
If the primary function of the C-terminal region of GADD34 is to bind eIF2α, then we reasoned it should be possible to find an eIF2α-binding site in this region that facilitates the formation of the eIF2α–GADD34–PP1 complex. Consistent with this idea, deletion of residues 233–248 of the HSV γ34.5 protein (Fig. 3, green box), which shows similarity to GADD34, was shown to impair eIF2α binding (29). To determine whether there is an eIF2α-binding motif in the C-terminal region of GADD34, the amino acid sequences of CReP, γ34.5, and DP71L were compared with the GADD34 sequence (Fig. 3). Surprisingly, only a few residues of the γ34.5 233–248 peptide were highly conserved in GADD34 (Fig. 3, green box). In contrast, residues 578–596 of GADD34, located immediately N-terminal to the γ34.5 peptide, are highly conserved in CReP, γ34.5, and ASFV DP71L (Fig. 3). To test whether these residues are conserved in other proteins, the GADD34578-596 peptide sequence was used as a query for a BLAST search against the nonredundant GenBank database, and matching motifs were identified in proteins from a variety of organisms including most, if not all, vertebrates, urochordates, annelids, arthropods, and molluscs. For simplicity, only a few representative sequences are presented in Fig. 3. Interestingly, besides HSV and ASFV, this motif was also found in proteins from other viruses including Canarypox virus (CNPV), Macropoid herpes virus (MaHV), human herpes virus 2 (HSV-2), and the invertebrate viruses: Amsacta moorei entomopoxvirus “L” (AmEPV), Wiseana iridescent virus (WIV), Anopheles minimus irodovirus (AMIV), Trichoplusia ni ascovirus 2c (TNAV2c), Choristoneura occidentalis granulovirus (COGV), Erinnys ello granulo virus (EeGV), Glossina pallidipes salivary gland hypertrophy virus (GpSGHV), Invertebrate iridescent virus 22 (IIV-22), Invertebrate iridescent virus 25 (IIV-25), and Invertebrate iridescent virus 30 (IIV-30). Based on the conservation of the sequence, we defined a 19-residue motif with the consensus sequence Rx[Gnls]x1–2Wxxx[Arlv]x[Dn][Rg]xRFxx[Rlvk][Iv], where capital letters are preferred and x is any residue. Interestingly, all these proteins also have a conserved PP1 binding motif (Fig. 3).
Fig. 3.
Identification of a conserved eIF2α-binding motif in GADD34. Multiple sequence alignment of the C-terminal portion of human GADD34 and 31 related proteins was built by using the T-coffee web server (48, 49). Numbers correspond to residue positions in the full-length proteins. Residues are colored according to conservation. The consensus PP1-binding RVxF motif and the eIF2α-binding motif are indicated; uppercase letters indicate the most preferred residue in the eIF2α-binding motif. The green box highlights residues that have been shown to be important for the interaction between γ34.5 and eIF2α (29).
Point Mutations Altering the eIF2α-Binding Motif of GADD34 Impair eIF2α Dephosphorylation.
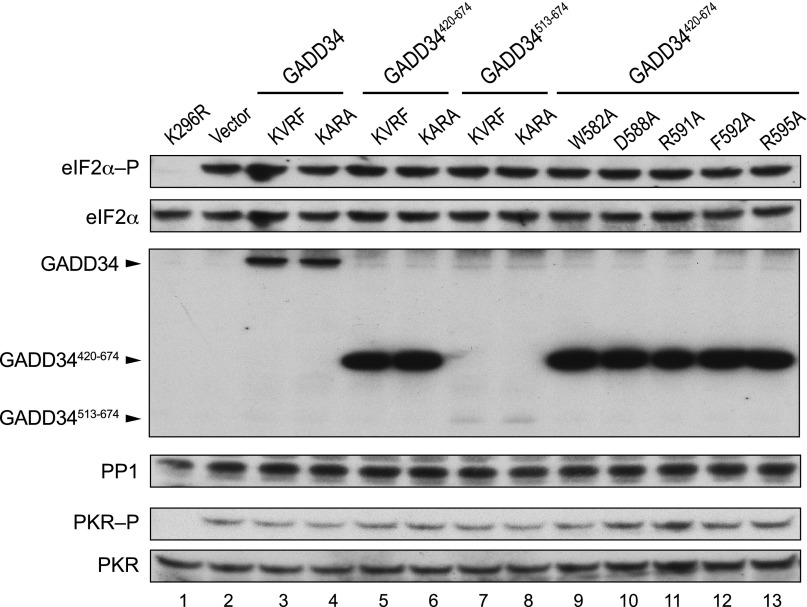
In an effort to map the eIF2α-binding motif in GADD34, we tested the ability of a GST-GADD34578-596 fusion protein (Fig. 4A) to bind eIF2α. The GST fusion and eIF2α were independently expressed in Escherichia coli, the bacterial cells were then mixed, lysed together, and then the WCEs were mixed with glutathione-Sepharose beads. Immunoblot analysis of the pull-down products revealed that GST-GADD34578-596 readily and directly interacted with eIF2α (Fig. 4B, lane 10). In addition, because PP1 and eIF2α kinases are not present in the reaction, these results show that GADD34 interacts with eIF2α in a PP1-independent manner, and that this interaction does not require phosphorylation of eIF2α (Fig. 4B, lane 10). To test the importance of conserved residues in the eIF2α-binding motif of GADD34, alanine residues were substituted for residues Trp582, Asp588, Arg591, Phe592, and Arg595 of the GST-GADD34578-596 fusion protein. As shown in Fig. 4B, alanine substitutions at Arg591, Phe592, and Arg595 reduced the interaction between GST-GADD34578-596 and eIF2α by 70%, 60%, and 50%, respectively (lanes 13–15), highlighting the importance of the conserved motif for binding eIF2α. In contrast to the important role of these residues, the alanine substitution at Trp582 did not affect the interaction with eIF2α (Fig. 4B, lane 11), whereas the alanine substitution at Asp588 increased the binding between eIF2α and the GST-GADD34578-596 fusion by up to 200% (Fig. 4B, lane 12). We also attempted to determine whether the conserved repeat element R1 directly interacted with eIF2α; however, the instability of the GST-R1 fusion protein in bacteria (Fig. 4B, lanes 8 and 16) prevented the test. These results provide strong evidence that GADD34 directly interacts with eIF2α through an eIF2α-binding motif located near the C terminus of GADD34.
Fig. 4.
The C-terminal region of GADD34 contains an eIF2α-binding motif that is essential to promote dephosphorylation of eIF2α. (A) Schematic of GADD34. The eIF2α-binding motif (residues 578–596 of GADD34) is indicated; numbers indicate positions of mutated residues. (B) E. coli cells expressing GST or the indicated GST-GADD34578-596 fusion protein were mixed with E. coli cells expressing human eIF2α. WCEs were prepared and mixed with glutathione-Sepharose beads, and after washing, bound proteins were eluted with SDS-loading buffer and subjected to immunoblot analysis by using monoclonal antibodies against the His-tag on eIF2α and polyclonal antibodies against the GST tag on GADD34. (C) Derivatives of the yeast strain YM100 expressing full-length GADD34, GADD34420-674, or the indicated GADD34420-674 mutant were grown in SGal medium, and then shifted to SGal medium supplemented with 1 μg/mL SM for 1 h to trigger activation of GCN2. Equivalent amounts of WCEs were subjected to immunoblot analysis to detect phosphorylated eIF2α–P, total eIF2α-Myc, human PP1, and Flag-GADD34. GCN2 was immunoprecipitated from yeast and subjected sequentially to immunoblot analysis by using antibodies against Thr882–P or total GCN2. eIF2α phosphorylation ratios were calculated from three independent experiments as described for Fig. 1C. (D) Transformants of yeast strain YM77 (+PKR) expressing the indicated form of GADD34 as described in C were grown to confluence on SD plates, and then replica-plated to SD or SGal plates and incubated for 2, 6, or 12 d at 18 °C.
We next asked whether the eIF2α-binding motif in GADD34 was important to promote eIF2α dephosphorylation. To this end, the alanine mutations described above were introduced into GADD34420-674 (Fig. 4A). The mutants were expressed in the humanized yeast strain containing human eIF2α and PP1 and tested for the ability to reduce eIF2α phosphorylation under amino acid starvation conditions where the eIF2α kinase GCN2 is activated. Importantly, expression of the various forms of GADD34 did not affect PP1 levels or GCN2 autophosphorylation (Fig. 4C). Whereas expression of full-length GADD34 or GADD34420-674 resulted in lower eIF2α phosphorylation levels (Fig. 4C, lanes 2 and 3), all of the alanine mutations blocked the ability of GADD34420-674 to promote dephosphorylation of eIF2α in vivo (Fig. 4C). Thus, the C-terminal eIF2α-binding motif in GADD34 is critical to promote eIF2α dephosphorylation. In further support of this idea, the alanine mutations blocked the ability of GADD34420-674 to suppress the growth inhibition due to expression of PKR in yeast (Fig. 4D, compare rows D–H versus C). As described previously, the ability of GADD34 to suppress PKR toxicity in yeast was not due to inhibition of PKR autophosphorylation or to an increase in PP1 levels (Fig. S1). The fact that all of the Ala mutant forms of the GADD34420-674 construct retained two of the four repeated sequence elements but still failed to promote eIF2α dephosphorylation provides further support for the idea that the four repeats, while able to bind eIF2α, are not essential for GADD34 to promote eIF2α dephosphorylation. Notably, aside from the D588A mutation, the Ala mutations did not affect GADD34 protein levels in yeast (Fig. 4C, Bottom), indicating that the mutations were likely affecting the proper binding of eIF2α to the C-terminal region of GADD34 rather than abundance of GADD34 in the cell. Because the W582A and D588A mutations in GADD34 did not impair eIF2α binding (Fig. 4B, lanes 11 and 12) but blocked the ability of GADD34 to promote eIF2α dephosphorylation (Fig. 4C) and suppression of PKR toxicity (Fig. 4D), we suggest that W582 and D588 are required to properly orient eIF2α in the eIF2α–GADD34–PP1 complex such that the Ser51 residue can access the PP1 active site.
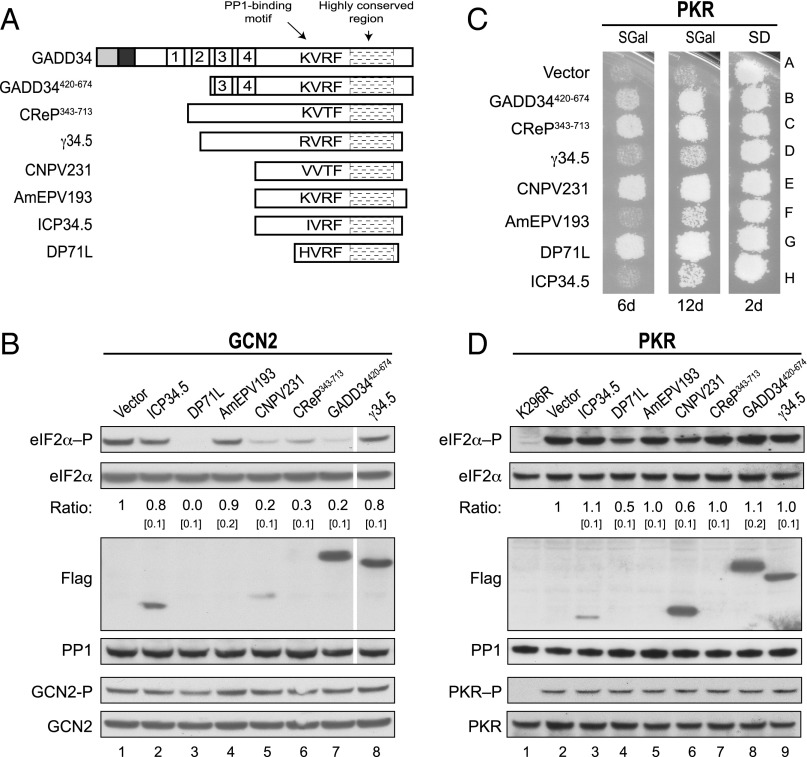
Viral Targeting Subunits of PP1 Promote eIF2α Dephosphorylation.
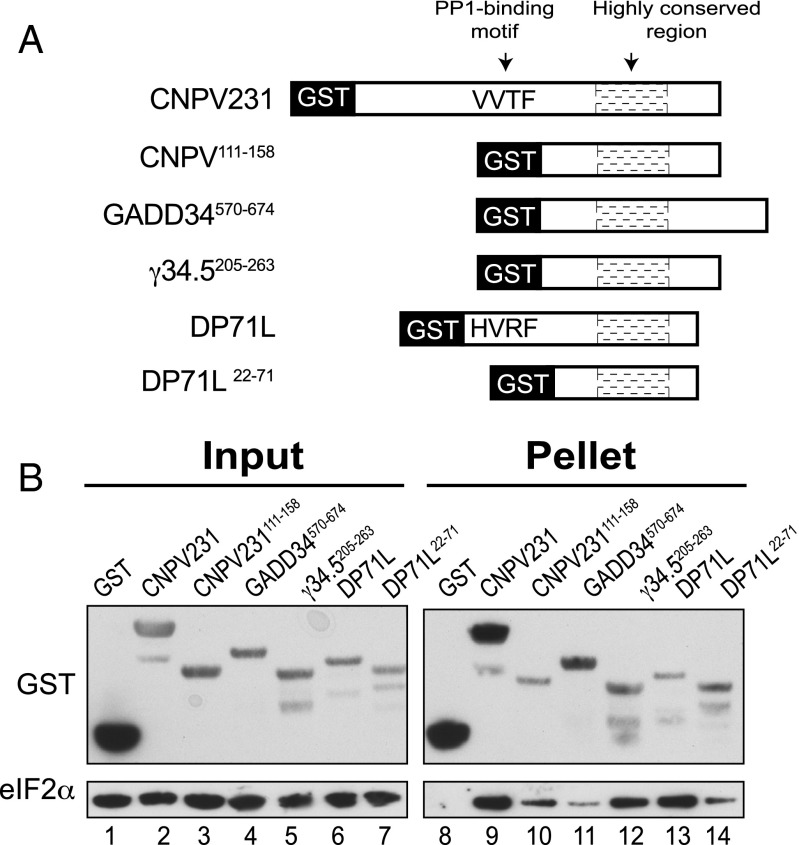
Our database search revealed that the eIF2α-binding motif in GADD34 is highly conserved in proteins produced by different viruses (Fig. 3), and we hypothesized that in addition to HSV (γ34.5) and ASFV (DP71L), other viruses produce PP1-targeting proteins that are able to interact with eIF2α and, thereby, promote its dephosphorylation. To test this idea, we first constructed bacterial expression vectors to produce GST fusion proteins containing the full-length forms of the CNPV protein CNPV231 or the ASFV protein DP71L, both of which include a predicted PP1 binding motif (Fig. 5A). In addition, vectors were constructed to express GST proteins lacking the PP1-binding motif and containing only the highly conserved region of GADD34, CNPV231, DP71L, or γ34.5 (Fig. 5A). Bacteria expressing the various GST fusions were mixed with bacteria expressing human eIF2α, the cells were broken together, and the ability of the GST fusions to pull down eIF2α was examined by immunoblot analysis. As shown in Fig. 5B, the CNPV231 protein, and DP71L, bound eIF2α (Fig. 5B, lanes 9 and 13), and this interaction did not require the PP1-binding site, because the truncated proteins CNPV231111-158 and DP71L22-71 were also able to bind eIF2α (Fig. 5B, lanes 10 and 14). Similarly, truncated GADD34570-674 and γ34.5205-263 were able to bind eIF2α (Fig. 5B, lanes 11 and 12). To test whether the viral proteins containing both a PP1-binding motif and an eIF2α-binding site can substitute for GADD34 and function to subvert the antiviral response mediated by eIF2α phosphorylation, we expressed full-length forms MaHV ICP34.5 protein, ASFV DP71L protein, AmEPV AmEPV193 protein, CNPV CNPV231 protein, HSV γ34.5 protein, or the control proteins CReP or GADD34 (Fig. 6A) in our yeast strain expressing human eIF2α and human PP1. Following activation of GCN2, high levels of eIF2α phosphorylation were detected in the control strain containing an empty vector (Fig. 6B, lane 1), and eIF2α phosphorylation was reduced to different levels in cells expressing GADD34, CReP, or the indicated viral protein (Fig. 6B). Markedly, as shown in Fig. 6B (Flag panel), even when DP71L was expressed at undetectable levels (lane 3) and CNPV231 was expressed at very low levels (lane 5), compared with GADD34420-674 (lane 7), the viral proteins efficiently promoted eIF2α dephosphorylation (Top). However, expression of the AmEPV AmEPV193, MaHV ICP34.5, and HSV γ34.5 proteins only modestly lowered eIF2α phosphorylation levels (Fig. 6B). Importantly, expression of these viral proteins did not alter GCN2 autophosphorylation on Thr882 (Fig. 6B). Consistent with the ability of the viral proteins, GADD34, and CReP to lower the level of eIF2α phosphorylation under starvation conditions where GCN2 is activated, expression of DP71L, CNPV231, CReP, and GADD34 reduced PKR toxicity in yeast as observed by growth after 6 d of incubation at 18 °C (Fig. 6 C, Left). After 12 d of incubation, yeast expressing AmEPV193, γ34.5, or ICP34.5 exhibited weak growth, although greater than that observed in the vector control strain (Fig. 6C, Middle), indicating that all of the viral proteins have the ability to reverse growth inhibition due to eIF2α kinase activity. As expected, high levels of eIF2α phosphorylation were detected in cells expressing wild-type PKR (Fig. 6D, lane 2), but not in cells expressing catalytically dead PKR-K296R (Fig. 6D, lane 1). Consistent with the abilities of DP71L and CNPV231 to suppress PKR toxicity in the replica-plating assay (Fig. 6C), lower levels of eIF2α, but not PKR, phosphorylation were detected in WCEs prepared from these strains when they were grown in liquid cultures (Fig. 6D, lanes 4 and 6). However, DP71L and CNPV231 did not reduce eIF2α phosphorylation mediated by PKR as efficiently as they reduced phosphorylation of eIF2α mediated by GCN2 (compare Fig. 6B versus 6D). As described previously, the level of eIF2α phosphorylation in WCEs prepared from yeast grown in liquid cultures does not always correlate with cell growth in replica-printing assays. Accordingly, despite the ability of GADD34, CReP, and the viral proteins AmEPV193, γ34.5, and ICP34.5 to suppress PKR toxicity (Fig. 6C, Middle), there was no detectable reduction in eIF2α phosphorylation (Fig. 6D). We suggest that the replica-printing assay, which requires only a few cell doublings to score positive growth, is a more sensitive reporter on reversal of PKR functions than the Western blot analyses of eIF2α phosphorylation in extracts prepared from cells grown in liquid cultures. Taken together, these results indicate that the primary function of C-terminal conserved region in GADD34 and its viral orthologs is to bind eIF2α and enable its dephosphorylation by PP1.
Fig. 5.
Viral GADD34-related proteins directly bind eIF2α. (A) Schematic diagram of GST fusion proteins containing the indicated full-length or truncated versions of GADD34 or related viral proteins. The RVxF motifs in CNPV231 (sequence VVTF) and DP71L (sequence HVRF) are indicated. (B) E. coli cells expressing the indicated GST fusion protein were mixed with E. coli cells expressing human eIF2α. WCEs were prepared and mixed with glutathione-Sepharose beads, and after washing, bound proteins were eluted with SDS-loading. Five percent (vol/vol) of input and 20% (vol/vol) of pellet fractions were subjected to immunoblot analysis by using monoclonal antibodies against the His-tag on eIF2α and polyclonal antibodies against GST.
Fig. 6.
Viral GADD34-related proteins promote eIF2α dephosphorylation. (A) Schematic diagram showing the relation among GADD34, GADD34420-674, CReP343-713, and full-length γ34.5, CNPV231, AmEPV193, ICP34.5, and DP71L; their respective PP1-binding motifs and highly conserved regions are indicated. (B) Derivatives of yeast strain YM100 containing an empty vector or expressing Flag-tagged versions of GADD34 or the indicated viral protein were grown in SGal medium and then incubated for 1 h in the presence of 1 μg/mL SM. Equivalent amounts of WCEs were subjected to SDS/PAGE followed by immunoblot analysis to detect eIF2α–P, eIF2α-Myc, and the indicated Flag-tagged protein. The relative level of phosphorylated to total eIF2α was determined by quantitative densitometry as described for Fig. 1C. White lines indicate splicing of lanes from the same original blot to generate the final figure. GCN2 was immunoprecipitated from yeast cells and subjected sequentially to immunoblot analysis with antibodies against Thr882–P or total GCN2. (C) Transformants of yeast strain YM77 (+PKR) carrying an empty vector or expressing GADD34, CReP, or the indicated viral protein were grown to confluence on SD plates, and then replica-plated to SD or SGal plates and incubated for 2, 6, or 12 d at 18 °C. (D) Yeast strains described in C were grown in SD medium and then incubated for 1 h in SGal medium to induce expression of PKR and the indicated viral protein. Equivalent amounts of WCEs were subjected to SDS/PAGE followed by immunoblot analysis to detect eIF2α–P, eIF2α-Myc, PP1, and the indicated Flag-tagged protein. The relative level of phosphorylated to total eIF2α was determined as described for Fig. 1C. In parallel, PKR was immunoprecipitated from the WCEs and subjected sequentially to immunoblot analysis with antibodies against phosphorylated Thr446-P or total PKR.
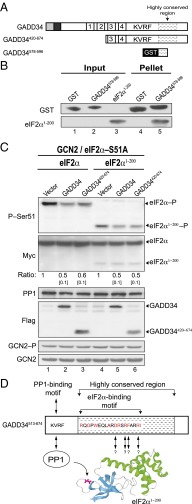
The N-Terminal Domain of eIF2α Is Sufficient for GADD34 Recognition.
Human eIF2α is composed of three domains: an N-terminal β-barrel (Fig. 7D, cyan), followed by a helical domain (Fig. 7D, green), and a C-terminal α-β domain (30). The Ser51 phosphorylation site is located in domain 1 of eIF2α; however, mutational analyses of eIF2α revealed that substrate recognition by the eIF2α kinases, as well as binding of eIF2α to eIF2B, requires residues that are remote from the phosphorylation site (31, 32). Moreover, biochemical and structural studies revealed that the two N-terminal domains of eIF2α assemble into a stable ∼200-residue protein and that further truncations lead to instability (30–35). We anticipated that this N-terminal region of eIF2α might also be important for GADD34 recognition, although we could not rule out contributions from the eIF2α C-terminal domain. To define the GADD34 recognition domain in eIF2α, GST or a GST-GADD34578-596 fusion protein containing the eIF2α-binding motif (Fig. 7A) was expressed in E. coli and tested for its ability to bind recombinant, purified C-terminally truncated eIF2α1-200 in vitro. In contrast to the GST control, the GST-GADD34578-596 fusion was able to pull down eIF2α1-200 (Fig. 7B, lane 5), indicating that GADD34 interacts with the N-terminal domain of eIF2α. As stated above, we were unable to test larger C-terminal truncations in eIF2α because of protein instability (31, 32). To determine whether GADD34 or GADD34420-674 was able to promote eIF2α1-200 dephosphorylation in vivo, vectors encoding GADD34, GADD43420-674, full-length human eIF2α, or human eIF2α1-200 were introduced into a yeast strain expressing human PP1 in place of GLC7 and also expressing a nonphosphorylatable (S51A) mutant of human eIF2α to maintain cell viability. As shown in Fig. 7C, activation of GCN2 by growth in amino acid starvation conditions resulted in high-level phosphorylation of full-length human eIF2α (lane 1) and eIF2α1-200 (lane 4). Expression of full-length GADD34 or truncated GADD34420-674 resulted in an ∼50% reduction in the level of phosphorylation for both full-length and truncated eIF2α1-200 (Fig. 7C, lanes 2, 3, 5, and 6). Accordingly, we conclude that the C-terminal region of GADD34 functionally interacts with PP1 and the N-terminal region of eIF2α to assemble a trimeric PP1–GADD34–eIF2α complex and promote eIF2α dephosphorylation (Fig. 7D).
Fig. 7.
GADD34 recognizes the N-terminal 200 residues of human eIF2α to promote Ser51 dephosphorylation. (A) Schematic representation of GADD34 derivatives. (B) GST or a GST-GADD34578-596 fusion protein were immobilized on glutathione-Sepharose beads, incubated for 1 h with purified human eIF2α1-200, and after washing, bound proteins were eluted with SDS-loading buffer. Five percent (vol/vol) of input and 20% (vol/vol) of pellet fractions were subjected to immunoblot analysis by using monoclonal antibodies against the His-tag on eIF2α1-200 and polyclonal antibodies against GST. (C) Plasmids encoding WT human eIF2α, eIF2α1-200, and the indicated form of GADD34 were introduced into the strain YM107, in which human PP1 and the phosphorylation site mutant eIF2α-S51A are expressed in place of yeast GLC7 and eIF2α (SUI2). WCEs were prepared from cells grown in SGal medium and treated with 1 μg/mL SM to induce activation of GCN2. Proteins were separated by SDS/PAGE and analyzed by immunoblotting to detect phospho-Ser51, the Myc-tag on eIF2α, human PP1, or the Flag-tag on GADD34. The relative level of phosphorylated eIF2α was determined as described for Fig. 1C. GCN2 was immunoprecipitated from yeast cells grown in the presence of SM and subjected sequentially to immunoblot analysis by using antibodies against Thr882–P or total GCN2. (D) Model depicting the PP1–GADD34–eIF2α complex. The PP1-binding motif in GADD34 (KVRF) is indicated. Residues in red are conserved among multiple orthologs of GADD34; arrows indicate residues that are critical for GADD34 binding and Ser51 dephosphorylation. The ribbons image presents the N-terminal β-barrel (cyan) and the helical (green) domains of eIF2α1-200; Ser51 in eIF2α is shown in magenta.
Discussion
Phosphorylation of Ser51 on eIF2α integrates many cellular signals and results in rapid attenuation of protein synthesis, producing both positive and negative consequences for cells. For example, during the early phase of the unfolded protein response (UPR) (reviewed in ref. 36), transient phosphorylation of eIF2α is protective as it represses translation initiation to reduce the protein load in the ER. Moreover, phosphorylation of eIF2α induces ATF4 production leading to increased expression of ER chaperones and enabling cells to deal with the accumulation of unfolded proteins (36). However, sustained eIF2α phosphorylation triggers apoptosis (37). Therefore, dephosphorylation of eIF2α is required to restore protein synthesis, terminate the stress signal, and maintain cell viability.
To dephosphorylate eIF2α, mammalian cells express the proteins GADD34 and CReP. Whereas CReP is expressed constitutively to provide basal levels of eIF2α phosphatase activity (38), GADD34 expression is induced by eIF2α phosphorylation to provide a negative feedback loop and limit eIF2α phosphorylation (7, 10, 11). Both CReP and GADD34 contain the PP1-binding motif RVxF that is required for the formation of the functional GADD34–PP1 and CReP–PP1 complexes that dephosphorylate eIF2α. However, how these complexes direct the specific dephosphorylation of eIF2α was unknown. PP1-targeting subunits have been proposed to activate the phosphatase activity of PP1 or to target the phosphatase to its substrate (21). Because binding of an RVxF peptide does not appear to alter the conformation of PP1 (22–24), it seems likely that binding of a regulatory subunit containing an RVxF motif to PP1 is not sufficient to activate PP1 to dephosphorylate a specific substrate. However, in support of the scaffolding role of the PP1-targeting subunits, we previously showed that yeast eIF2γ contains a PP1-binding motif that enables recruitment of the phosphatase PP1/GLC7 to its substrate eIF2α via the eIF2 complex (25). A similar idea has been proposed for the HSV γ34.5 protein, which is able to interact with both PP1 and eIF2α (29).
Because γ34.5 shares significant homology with GADD34, we hypothesized that GADD34 also functions as a scaffold to physically link PP1 and eIF2α. Consistent with this idea, we found that GADD34 directly interacts with eIF2α (Figs. 2 and 4B). First, three of four of the repeated sequence elements in the middle of GADD34 were able to pull down eIF2α from yeast WCEs, indicating that these repeats directly or indirectly bind to eIF2α. Nevertheless, eliminating these repeats only partially impaired the ability of GADD34 to promote eIF2α dephosphorylation in yeast cells (Fig. 1 D and E). These findings are consistent with the notion that the repeats contribute to the function of GADD34 by increasing the local concentration of eIF2α, but that they are dispensable for triggering eIF2α dephosphorylation. Given that GADD34 is polyubiquitinated and rapidly degraded by the 26S proteasome (12), the ability of GADD34 to increase the local concentration of eIF2α could enhance the ability of GADD34 to efficiently promote dephosphorylation of eIF2α in a short time frame. Second, we identified a key eIF2α-binding motif at the C terminus of GADD34 in a region distinct from where PP1 binds to GADD34. Based on sequence conservation among a variety of cellular and viral proteins, we characterized the consensus sequence Rx[Gnl]x1–2Wxxx[Arlv]x[Dn][Rg]xRFxx[Rlvk][Iv], where capital letters are preferred and x is any residue (Fig. 3). This eIF2α-binding motif allows direct interaction between GADD34 and eIF2α and is essential for GADD34 to promote the specific dephosphorylation of eIF2α (Fig. 4). In agreement with this idea, point mutations altering this eIF2α-binding site in GADD34 impaired the ability of GADD34 to interact with eIF2α in a pull-down assay and blocked the ability of GADD34 to promote eIF2α dephosphorylation and suppress PKR toxicity in yeast (Fig. 4). Interestingly, we also identified mutations in the eIF2α-binding motif of GADD34 (e.g., D588A) that either did not alter or, in fact, increased the binding of GST-GADD34578-596 to eIF2α, yet still reduced the ability of GADD34 to promote dephosphorylation of eIF2α. This second class of mutations in the eIF2α-binding motif of GADD34 suggests that the scaffolding role of GADD34 to promote eIF2α dephosphorylation is more complex than a simple tether linking PP1 to eIF2α.
Because eIF2α phosphorylation impairs both cellular and viral protein synthesis, many viruses have evolved mechanisms to inactivate eIF2α kinases (39). As an alternative, HSV and ASFV circumvent the deleterious effects of eIF2α phosphorylation expressing of γ34.5 and DP71L, respectively, which stimulate dephosphorylation of eIF2α. The C-terminal region of these viral proteins shares homology with GADD34, and here we showed that the eIF2α-docking site in GADD34 is well conserved in γ34.5, DP71L, and proteins produced by other viruses including, CNPV, AmEPV, and MaHV (Fig. 3). Based on the strong conservation of the PP1- and eIF2α-binding motifs, we proposed that these viral proteins function as scaffolds to recruit PP1 and promote specific dephosphorylation of eIF2α. Consistent with this hypothesis, we demonstrated that DP71L, as well as CNPV231, directly interact with eIF2α (Fig. 5B). Furthermore, we found that in addition to γ34.5 and DP71L, the viral proteins CNPV231, AmEPV193, and ICP34.5 (from MaHV) are able to promote dephosphorylation of eIF2α and reduce the associated PKR toxicity in yeast (Fig. 6 B and C). Thus, rather than directly inhibiting the activity of PKR (Fig. 6D), our work demonstrates that a variety of viruses have adopted the tactics of HSV and ASFV and express targeting subunits that direct PP1 to dephosphorylate eIF2α.
In conclusion, our results show that GADD34 and its viral orthologs function as scaffold proteins that promote specific dephosphorylation of eIF2α by interacting with both PP1 and eIF2α (Fig. 7D). Whereas GADD34 is a complex protein and targets PP1 to other substrates in addition to eIF2α, our work reveals that a conserved C-terminal motif in GADD34 functionally interacts with the N-terminal region of eIF2α (Fig. 7D). Given that inhibition of PP1 results in the inactivation of a large number of holophosphatase complexes with a broad range of substrate specificities, one implication of our findings is that a drug able to block the specific interaction of eIF2α with GADD34-related viral proteins could be a useful approach for the development of antiviral therapies. Notably, salubrinal has been shown to inhibit GADD34–PP1 and CReP–PP1 complexes (40), and guanabenz specifically inhibits formation of the GADD34–PP1 complex (41), suggesting that PP1 regulatory subunits are a druggable target. Finally, although we have identified orthologs of GADD34 containing both the eIF2α-binding site and the PP1-binding motif in different animals and viruses, clear orthologs of GADD34 are absent in plants and fungi. We propose that the direct targeting of PP1 to eIF2 via the N terminus of eIF2γ that we discovered in Saccharomyces cerevisiae (25) has been replaced during evolution by an unknown mechanism in plants and other fungi, and by the metazoan scaffolding proteins GADD34 and CReP, which, in turn, have been mimicked by viruses to thwart the mammalian antiviral response.
Materials and Methods
Plasmids and Strains.
Plasmid and strain construction are described in SI Materials and Methods. Plasmids are listed in Table S1; strains are listed in Table S2.
Table S1.
Plasmids used in this study
| Name | Description | Promoter | Backbone | Source |
| p1358 | lc, URA3, GLC7 | GLC7 | Yep352 | 42 |
| p1421 | hc, URA3, PKR-K296R | GAL1 | pEMBLYex4 | 27 |
| pC1657 | lc, LEU2, SUI2 | SUI2 | PRS315 | 31 |
| pC2872 | lc, LEU2, His8-GCD11 (eIF2γ) | GCD11 | YCplac111 | 43 |
| pC4031 | lc, LEU2, His8-GCD11-KKAAA | GCD11 | YCplac111 | 25 |
| pC4032 | lc, LEU2, His8-GCD11-Δ1–60 | GCD11 | YCplac111 | 25 |
| pC4043 | hc, URA3, Flag-GADD34 | GAL1 | pEMBLYex4 | This study |
| pC4209 | lc, LEU2, SUI2 | SUI2 | PRS315 | This study |
| pC4214 | lc, LEU2, h-eIF2α-Myc | SUI2 | PRS315 | This study |
| pC4229 | lc, TRP1, h-Flag-PP1 | PGK1 | pRS314 | This study |
| pC4520 | lc, TRP1, h-PP1 | PGK1 | pRS314 | This study |
| pC4539 | hc, URA3, Flag-GADD34- V556a/F558a (KARA) | GAL1 | pEMBLYex4 | This study |
| pC4554 | hc, URA3, Flag-GADD34420-674 | GAL1 | pEMBLYex4 | This study |
| pC4558 | hc, URA3, Flag-GADD34420-674- V556a/F558a (KARA) | GAL1 | pEMBLYex4 | This study |
| pC4565 | hc, URA3, Flag-GADD34513-674 | GAL1 | pEMBLYex4 | This study |
| pC4567 | hc, URA3, GST-GADD34420-674 | GAL1 | pEGKT | This study |
| pC4573 | hc, URA3, GST-GADD34513-553 | GAL1 | pEGKT | This study |
| pC4574 | hc, URA3, GST-CReP343-713 | GAL1 | pEGKT | This study |
| pC4586 | hc, URA3, Flag-γ34.5 | GAL1 | pEMBLYex4 | This study |
| pC4589 | hc, URA3, Flag-GADD34513-674- V556a/F558a (KARA) | GAL1 | pEMBLYex4 | This study |
| pC4594 | hc, URA3, GST-GADD34570-674 | GAL1 | pEGKT | This study |
| pC4595 | hc, URA3, GST-GADD34570-634 | GAL1 | pEGKT | This study |
| pC4597 | hc, URA3, Flag-GADD34420-674 -R595A | GAL1 | pEMBLYex4 | This study |
| pC4598 | hc, URA3, Flag-GADD34420-674 -F592A | GAL1 | pEMBLYex4 | This study |
| pC4599 | hc, URA3, Flag-GADD34420-674 -W582A | GAL1 | pEMBLYex4 | This study |
| pC4600 | hc, URA3, Flag-GADD34420-674 -R591A | GAL1 | pEMBLYex4 | This study |
| pC4607 | hc, URA3, Flag-GADD34420-674 -D588A | GAL1 | pEMBLYex4 | This study |
| pC4608 | hc, URA3, GST-GADD34420-674 -V556a/F558a (KARA) | GAL1 | pEGKT | This study |
| pC4611 | lc, LEU2, h-eIF2α-S51a-Myc | SUI2 | PRS315 | This study |
| pC4614 | lc, LEU2, h-eIF2α1-200 –Myc | SUI2 | PRS315 | This study |
| pC4923 | lc, TRP1, h-eIF2α-Myc, h-PP1 | SUI2, PGK1 | PRS314 | This study |
| pC4924 | hc, URA3, Flag-CNPV231 | GAL1 | pEMBLYex4 | This study |
| pC4925 | hc, URA3, Flag-AmEPV193 | GAL1 | pEMBLYex4 | This study |
| pC4926 | Hc, URA3, Flag-DP71L | GAL1 | pEMBLYex4 | This study |
| pC4927 | Hc, URA3, Flag-ICP34.5 | GAL1 | pEMBLYex4 | This study |
| pC4965 | GST-CNPV231 | TAC | pGEX-6P-1 | This study |
| pC4986 | His6-h-eIF2α1-200 | T7 | pET28a | This study |
| pC4988 | GST-CNPV231111-158 | TAC | pGEX-6P-1 | This study |
| pC4989 | GST-GADD34570-674 | TAC | pGEX-6P-1 | This study |
| pC4990 | GST-γ34.5205-263 | TAC | pGEX-6P-1 | This study |
| pC4991 | GST-DP71L | TAC | pGEX-6P-1 | This study |
| pC4992 | GST-DP71L22-71 | TAC | pGEX-6P-1 | This study |
| pC4993 | hc, URA3, GST-GADD34331-376 | GAL1 | pEGKT | This study |
| pC4994 | hc, URA3, GST-GADD34377-420 | GAL1 | pEGKT | This study |
| pC4995 | hc, URA3, GST-GADD34421-466 | GAL1 | pEGKT | This study |
| pC4996 | hc, URA3, GST-GADD34471-516 | GAL1 | pEGKT | This study |
| MR283 | GST-GADD34578-596 | TAC | pGEX-6P1 | This study |
| MR284 | GST-GADD34578-596 -W582A | TAC | pGEX-6P1 | This study |
| MR285 | GST-GADD34578-596 -D588A | TAC | pGEX-6P1 | This study |
| MR286 | GST-GADD34578-596 -R591A | TAC | pGEX-6P1 | This study |
| MR287 | GST-GADD34578-596 -F592A | TAC | pGEX-6P1 | This study |
| MR288 | GST-GADD34578-596 -R595A | TAC | pGEX-6P1 | This study |
| MR298 | GST-GADD34331-376 | TAC | pGEX-6P1 | This study |
hc, high copy-number plasmid; lc, low copy number plasmid.
Table S2.
Yeast strains used in this study
| Name | Description | Source |
| H1645 | MATa ura3-52, leu2-3, leu2-112, trp1Δ63, sui2Δ, p[SUI2, URA3] | 3 |
| JC821A | MATa leu2-3, leu2-112, ura3-52, can1-100, trp1, his3-11, his3-15, glc7::HIS3, p[GLC7, URA3] | 50 |
| YM12 | MATa ura3-52, leu2-3, leu2-112, trp1Δ63, sui2Δ, glc7Δ::NatMx, pC4214 [Human eIF2α-Myc, LEU2], pC4229 [Human Flag-PP1,TRP1] | This study |
| YM35 | MATa ura3-52, leu2-3, leu2-112, trp1Δ63, sui2Δ, pC4214 [Human eIF2α-Myc, LEU2] | This study |
| YM53 | MATα leu2-3,-112, ura3-52, his3, gcd11Δ::KanMX, GLC7::2myc::NAtMx, gcn2Δ::loxP, p[URA3, GCD11 (eIF2γ)] | 25 |
| YM56 | MATa leu2-3, leu2-112, ura3-52, can1-100, trp1, his3-11, his3-15, glc7::HIS3, pC4229 [Human Flag-PP1, TRP1] | This study |
| YM77 | MATa leu2-3, leu2-112, ura3-52, can1-100, trp1, his3-11, his3-15, glc7::HIS3, gal-PKR::LEU2, pC4229 [Human Flag-PP1, TRP1] | This study |
| YM81 | MATα leu2-3,-112, ura3-52, his3, gcd11Δ::KanMX, GLC7::2myc::NAtMx, pC2872 [His8-GCD11 (eIF2γ), LEU2] | This study |
| YM82 | MATα leu2-3,-112 ura3-52, his3, gcd11Δ::KanMX, GLC7::2myc::NAtMx, pC4031 [His8-GCD11-KKAAA, LEU2] | This study |
| YM83 | MATα leu2-3,-112 ura3-52, his3, gcd11Δ::KanMX, GLC7::2myc::NAtMx, pC4032 [His8-GCD11-Δ1–60, LEU2] | This study |
| YM99 | MATa ura3-52, leu2-3, leu2-112, trp1Δ63, sui2Δ, glc7Δ::NatMx, pC4214 [Human eIF2α-Myc, LEU2], p1358 [GLC7,URA3] | This study |
| YM100 | MATa ura3-52, leu2-3, leu2-112, trp1Δ63, sui2Δ, glc7Δ::NatMx, pC4214 [Human eIF2α-Myc, LEU2], pC4520 [Human PP1,TRP1] | This study |
| YM103 | MATα leu2-3,-112, ura3-52, his3, GLC7::2myc::NAtMx gcd11Δ::KanMX, p[GCD11, URA3] | This study |
| YM107 | MATa ura3-52, leu2-3, leu2-112, trp1Δ63, sui2Δ, glc7Δ::NatMx, pC4923[Human eIF2α-S51a-Myc, human PP1, TRP1] | This study |
Immunoblot Analysis.
Yeast cells were grown in 5 ml of SD medium with minimal supplements at 30 °C to midlogarithmic phase, then cells were harvested and washed with SGal medium [synthetic medium containing 2% (wt/vol) galactose]. Cells were then seeded in 5 mL of SGal medium and incubated overnight to induce expression of the indicated protein. To activate GCN2, cells were again harvested and seeded in 5 mL of fresh SGal medium for 1 h in the presence of 1 μg/mL SM. Finally, cells were harvested by centrifugation, mixed with 2 vol 20% (wt/vol) trichloroacetic acid, and then broken by agitation with glass beads. Proteins were extracted with SDS loading buffer [2% (wt/vol) SDS, 2 mM EDTA, 50 mM Tris⋅HCl (pH 6.8), 10% (vol/vol) glycerol, 0.01% bromophenol blue]; following neutralization with 1 M Tris base, samples were boiled for 5 min and then subjected to SDS-polyacrylamide gel electrophoresis (SDS/PAGE) and immunoblot analysis by using rabbit polyclonal antibodies specific for phospho-Ser51 on eIF2α (BioSource International). Blots were stripped and reprobed with polyclonal anti-yeast eIF2α antiserum (3). Monoclonal anti-Myc (9E10), anti-His (H-5), and anti-FLAG (F-tag-01) antibodies and polyclonal anti-GST (Z5) antibodies were purchased from Santa Cruz Biotechnology. GCN2 and PKR were immunoprecipitated from WCEs prepared as described (26). The immunoprecipitates were subjected to immunoblot analysis by using antibodies against phosphorylated Thr882 on GCN2 (26) or antibodies against phosphorylated Thr446 on PKR (Santa Cruz), respectively. The blots were then stripped and reprobed with polyclonal antibodies against GCN2 or PKR (Santa Cruz), respectively. Immune complexes were detected by using horseradish peroxidase-conjugated anti-rabbit and anti-mouse secondary antibodies (GE Healthcare) and enhanced chemiluminescence.
Yeast GST Pull-Down Assays.
Yeast strain YM100 expressing various GST-GADD34 fusion proteins was grown in 50 mL of SD medium at 30 °C to midlog phase, harvested, and washed with SGal medium. Cells were then seeded in 50 mL of SGal medium and incubated for 6 h to induce expression of the GST fusion protein, harvested, and frozen at −80 °C until further use. Cells were suspended in 500 μL of lysis buffer [20 mM Tris⋅HCl (pH 7.4), 100 mM NaCl, 0.2 mM EDTA, 1 mM DTT, 12.5% (vol/vol) glycerol, 1% Triton X-100, containing one tablet of complete protease inhibitor mixture (Roche) and 2 μM each aprotinin, leupeptin, and pepstatin], and WCEs were prepared by homogenizing the cells by vigorous mixing with glass beads on a vortex. Glutathione-Sepharose 4B beads (Amersham Biosciences) were washed several times with binding buffer [20 mM Tris⋅HCl (pH 7.4), 100 mM NaCl, 0.2 mM EGTA, 1 mM DTT, 0.1% Triton X-100, containing protease inhibitor mixture as described above], suspended in 1 mL binding buffer plus 5% (wt/vol) BSA, incubated with rotation at 4 °C for 1 h, and then washed several times with binding buffer. WCEs were mixed with 50 μL of the treated glutathione-Sepharose beads and incubated with rotation at 4 °C for 2 h. Proteins attached to the beads were washed three times with binding buffer, resuspended in SDS loading buffer, boiled for 5 min, separated by SDS/PAGE, and then analyzed by immunoblotting.
Bacterial GST Pull-Down Assays.
BL21-CodonPlus (DE3)-RIL competent cells (Stratagene) harboring a vector encoding a GST-GADD34 fusion protein or human His6-eIF2α were grown overnight at 37 °C in 5 mL of LB medium containing the required antibiotic. Then, 500 μL of the overnight culture was used to inoculate 5 mL of LB medium supplemented with 0.1 mM IPTG and 50 mg/L kanamycin or 100 mg/mL ampicillin, as required. Following incubation overnight at 18 °C, 5 mL of cells expressing the GST fusion protein were mixed with 5 mL of cells expressing His6-eIF2α. The cells were harvested, washed, resuspended in 1 mL of lysis buffer, and then disrupted by sonication. The WCEs were clarified by centrifugation, mixed with glutathione-Sepharose 4B beads, which had been pretreated as described above, and then incubated with rotation at 4 °C for 2 h. Proteins attached to the beads were washed three times with binding buffer, resuspended in SDS loading buffer, and boiled for 5 min. Finally, the eluted proteins were separated by SDS/PAGE and then analyzed by immunoblotting. Alternatively, GST or the GST-GADD34578-596 fusion protein was immobilized on BSA-pretreated glutathione-Sepharose 4B beads and then incubated with 1 mL of binding buffer and purified His6-eIF2α1-200, described in SI Materials and Methods, for 2 h at 4 °C. Following binding, the beads were washed and the bound proteins were analyzed as described above.
SI Materials and Methods
Plasmids.
Plasmids are listed in Table S1. Standard techniques were used for DNA manipulation. Plasmids p1358 encoding GLC7 (42), p1421 encoding PKR-K296R (27), pC1657 encoding SUI2 (31), and pC2872, pC4031, and pC4032 (25, 43) encoding different versions of eIF2γ were described. A SacI-HindIII PCR product encoding the desired residues of GADD34 was obtained by PCR using as a template a GADD34 expression vector (12) kindly donated by Shirish Shenolikar, Duke-NUS Graduate Medical School, Singapore. These PCR products were inserted under the control of galactose-inducible GAL1 promoter in the URA3 2μ yeast expression vector pEMBLYex4 (44) to create plasmids pC4043, pC4554, and pC4565. The R595A, F592A, W582A, R591A, and D588A mutations were introduced into pC4554 by using a QuikChange site-directed mutagenesis kit (Stratagene) generating the plasmids pC4597, pC4598, pC4599, pc4600, and pC4607, respectively. A PCR fragment encoding the indicated residues of GADD34 was cloned into the yeast GST expression vector pEGKT (45) between the BamHI and HindIII sites to generate the plasmids pC4567, pC4573, pC4594, pC4595, pC4993, pC4994, pC4995, and pC4996, respectively. PCR fragments encoding GADD34 residues 578–596, 331–376, or 570–674 were cloned into the vector pGEX-6P-1 (GE Healthcare) between the BamHI and XhoI sites to generate MR283, MR298, and pc4989, respectively. The W582A, D588A, R591A, F592A, and R595A mutations were introduced into MR283, generating the plasmids MR284, MR285, MR286, MR287, and MR288, respectively. The V55a/F55a (KARA) mutations were introduced into pC4043, pC4554, pC4565, and pC4567, generating the plasmids pC4539, pC4558, pC4589, and pC4608, respectively. Plasmids encoding Flag–γ34.5, Flag–CNPV231, Flag–AmEPV193, Flag–DP71L, and Flag–ICP34.5 were prepared commercially (Invitrogen), and the coding sequences were isolated by digestion with BamHI and HindIII and inserted into the vector pEMBLYex4 (44) to generate the plasmids pC4586, pC4924, pC4925, pC4926, and pC4927, respectively. The GST-CNPV231 expression vector pC4965 was generated by subcloning a BamHI-PvuII fragment from the commercially synthesized CNPV231 plasmid between the BamHI and SmaI sites of pGEX-6P-1. BamHI-XhoI fragments encoding the indicated residues of different viral proteins were inserted into pGEX-6P-1 generating the expression vectors pC4988 (GST-CNPV231111-158), pC4990 (GST-γ34.5205-263), pC4991 (GST-DP71L), and pC4992 (DP71L22-71). To generate plasmid pC4209, the XbaI site in the polylinker of the SUI2 expression vector pC1657 (31) was destroyed. An XbaI-SnaBI fragment encoding human eIF2α-Myc, eIF2α-S51a-Myc, or eIF2α1-200-Myc was amplified by PCR and used to replace the SUI2 coding sequence in pC4209, resulting in pC4214, pC4611, and pC4614, respectively. Plasmids designed to express human PP1 were constructed as follows: (i) an ApaI-BamHI PCR fragment containing the yeast PGK1 promoter was amplified from the vector YEp1PT (46) and inserted into pRS314 (47) generating pC4253; (ii) a BamHI-NotI fragment encoding human PP1 or Flag-PP1 was amplified by using pCDNA3.1-Flag-PP1, kindly provided by Anne-Claude Gingras, Lunenfeld-Tanenbaum Research Institute, Toronto, as DNA template and then inserted into pC4253 to generate the expression vectors pC4520 and pC4229, respectively. An NaeI-SmaI fragment encoding human eIF2α-S51a-Myc under the control of the SUI2 promoter was isolated from pC4611 and inserted into the EcoRV site of pC4520 destroying the TRP1 gene and creating the vector pC4613. A BstZ171-KpnI fragment encoding human PP1 and human eIF2α-S51a-Myc was isolated from pC4613 and inserted into pRS314 (47) to generate pC4923. An NdeI-XhoI fragment encoding human eIF2α1-200 was amplified by PCR from pC4214 and inserted into pET28a (Novagen) to generate pC4986. Finally, a BamHI-HindIII fragment encoding human CReP343-713 was amplified by PCR using a commercially generated template (Invitrogen) and inserted into pEGKT (45), creating the GST-CReP343-713 expression vector pC4574. The sequence of all genes and the presence of the desired mutations were verified by DNA sequencing.
Yeast Strains.
Yeast strains and genotypes are listed in Table S2. Strains H1645 (3) and YM53 (25) were described. YM103 was constructed by transforming YM53 with a PCR product containing the GCN2 gene and selecting for growth on synthetic dextrose (SD) medium supplemented with 1 μg/mL sulfometuron-methyl (SM). Proper integration of the GCN2 coding sequence was confirmed by PCR amplification and also by phosphorylation of eIF2α. The LEU2 plasmids pC2872, pC4031, and pC4032 were introduced into YM103 by plasmid shuffling to generate the strains YM81, YM82, and YM83, respectively. Strain YM35 was generated by shuffling the vector pC4214 into H1645. To create YM12, the strain YM35 was first transformed with pC4229 encoding human Flag-PP1, then the chromosomal GLC7 gene was replaced with a glc7Δ::NatMx cassette. The GLC7 expression vector p1358 was shuffled into YM12 to generate YM99. Strains YM100 and YM107 were created by shuffling the plasmids pC4520 and pC4923, respectively, into YM99. YM56 was obtained by plasmid shuffling after transformation of F356 with pC4229, and YM77 was constructed by integrating the LEU2 vector pC3696 containing a galactose-inducible human PKR gene at the leu2 locus of the strain YM56.
Purification of Human His6-eIF2α1-200.
BL21-CodonPlus (DE3)-RIL competent cells (Stratagene) harboring the vector pC4986 were grown overnight at 37 °C in 5 mL of Luria broth (LB) medium containing 50 mg/L kanamycin. Then, the overnight culture was used to inoculate 500 mL of LB medium supplemented with 0.1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG), and 50 mg/L kanamycin. Following incubation overnight at 18 °C, cells were harvested, washed with buffer A [20 mM Tris⋅HCl (pH 8), 0.2 mM EGTA, 100 mM NaCl, 10% (vol/vol) glycerol, 1 mM DTT, 10 mM Imidazole, 1 mM phenylmethylsulfonyl fluoride, and complete protease inhibitor mixture (Roche)], and then resuspended in 10 mL of buffer A plus 1% Triton X-100. Cells were disrupted by sonication, and whole-cell extracts (WCEs) were clarified by centrifugation. WCEs were mixed with prewashed Ni-NTA resin and incubated with rotation at 4 °C for 2 h. The Ni-NTA resin was washed three times with buffer A, three times with Buffer A supplemented with 50 mM imidazole, and finally His6-eIF2α1-200 was eluted with buffer A containing 250 mM imidazole.
Supplementary Material
Acknowledgments
We thank Anne-Claude Gingras and Shirish Shenolikar for providing reagents, Chune Cao for technical support, and Alan Hinnebusch and members of the T.E.D. and Hinnebusch laboratories for helpful discussions. This work was supported, in part, by the Intramural Research Program of the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (to T.E.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501557112/-/DCSupplemental.
References
- 1.Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez A, Brautigan DL, Lamb NJ. Protein phosphatase type 1 in mammalian cell mitosis: Chromosomal localization and involvement in mitotic exit. J Cell Biol. 1992;116(6):1421–1430. doi: 10.1083/jcb.116.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dever TE, et al. Phosphorylation of initiation factor 2 α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68(3):585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- 4.Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16(1):3–12. doi: 10.1016/j.semcdb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Hinnebusch AG. Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In: Sonenberg N, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor Lab Press; Plainview, NY: 2000. pp. 185–243. [Google Scholar]
- 6.Harding HP, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 7.Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 α to the endoplasmic reticulum and promotes dephosphorylation of the α subunit of eukaryotic translation initiation factor 2. Mol Cell Biol. 2003;23(4):1292–1303. doi: 10.1128/MCB.23.4.1292-1303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21(20):6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kojima E, et al. The function of GADD34 is a recovery from a shutoff of protein synthesis induced by ER stress: Elucidation by GADD34-deficient mice. FASEB J. 2003;17(11):1573–1575. doi: 10.1096/fj.02-1184fje. [DOI] [PubMed] [Google Scholar]
- 10.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J Cell Biol. 2001;153(5):1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novoa I, et al. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22(5):1180–1187. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brush MH, Shenolikar S. Control of cellular GADD34 levels by the 26S proteasome. Mol Cell Biol. 2008;28(23):6989–7000. doi: 10.1128/MCB.00724-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler HT, et al. Leukemic HRX fusion proteins inhibit GADD34-induced apoptosis and associate with the GADD34 and hSNF5/INI1 proteins. Mol Cell Biol. 1999;19(10):7050–7060. doi: 10.1128/mcb.19.10.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rechsteiner M. PEST sequences are signals for rapid intracellular proteolysis. Semin Cell Biol. 1990;1(6):433–440. [PubMed] [Google Scholar]
- 15.Harding HP, et al. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 α (eIF2α) dephosphorylation in mammalian development. Proc Natl Acad Sci USA. 2009;106(6):1832–1837. doi: 10.1073/pnas.0809632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94(3):843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera J, et al. The MyD116 African swine fever virus homologue interacts with the catalytic subunit of protein phosphatase 1 and activates its phosphatase activity. J Virol. 2007;81(6):2923–2929. doi: 10.1128/JVI.02077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen PT. Protein phosphatase 1—targeted in many directions. J Cell Sci. 2002;115(Pt 2):241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 19.Bollen M. Combinatorial control of protein phosphatase-1. Trends Biochem Sci. 2001;26(7):426–431. doi: 10.1016/s0968-0004(01)01836-9. [DOI] [PubMed] [Google Scholar]
- 20.Carmody LC, Baucum AJ, 2nd, Bass MA, Colbran RJ. Selective targeting of the gamma1 isoform of protein phosphatase 1 to F-actin in intact cells requires multiple domains in spinophilin and neurabin. FASEB J. 2008;22(6):1660–1671. doi: 10.1096/fj.07-092841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egloff MP, et al. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 1997;16(8):1876–1887. doi: 10.1093/emboj/16.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurley TD, et al. Structural basis for regulation of protein phosphatase 1 by inhibitor-2. J Biol Chem. 2007;282(39):28874–28883. doi: 10.1074/jbc.M703472200. [DOI] [PubMed] [Google Scholar]
- 23.Ragusa MJ, et al. Spinophilin directs protein phosphatase 1 specificity by blocking substrate binding sites. Nat Struct Mol Biol. 2010;17(4):459–464. doi: 10.1038/nsmb.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen R, et al. G-actin provides substrate-specificity to eukaryotic initiation factor 2α holophosphatases. eLife. 2015;4:e04871. doi: 10.7554/eLife.04871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojas M, Gingras AC, Dever TE. Protein phosphatase PP1/GLC7 interaction domain in yeast eIF2γ bypasses targeting subunit requirement for eIF2α dephosphorylation. Proc Natl Acad Sci USA. 2014;111(14):E1344–E1353. doi: 10.1073/pnas.1400129111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherkasova V, Qiu H, Hinnebusch AG. Snf1 promotes phosphorylation of the α subunit of eukaryotic translation initiation factor 2 by activating Gcn2 and inhibiting phosphatases Glc7 and Sit4. Mol Cell Biol. 2010;30(12):2862–2873. doi: 10.1128/MCB.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dever TE, et al. Mammalian eukaryotic initiation factor 2 α kinases functionally substitute for GCN2 protein kinase in the GCN4 translational control mechanism of yeast. Proc Natl Acad Sci USA. 1993;90(10):4616–4620. doi: 10.1073/pnas.90.10.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chong KL, et al. Human p68 kinase exhibits growth suppression in yeast and homology to the translational regulator GCN2. EMBO J. 1992;11(4):1553–1562. doi: 10.1002/j.1460-2075.1992.tb05200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, et al. ICP34.5 protein of herpes simplex virus facilitates the initiation of protein translation by bridging eukaryotic initiation factor 2α (eIF2α) and protein phosphatase 1. J Biol Chem. 2011;286(28):24785–24792. doi: 10.1074/jbc.M111.232439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito T, Marintchev A, Wagner G. Solution structure of human initiation factor eIF2α reveals homology to the elongation factor eEF1B. Structure. 2004;12(9):1693–1704. doi: 10.1016/j.str.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Dey M, et al. PKR and GCN2 kinases and guanine nucleotide exchange factor eukaryotic translation initiation factor 2B (eIF2B) recognize overlapping surfaces on eIF2α. Mol Cell Biol. 2005;25(8):3063–3075. doi: 10.1128/MCB.25.8.3063-3075.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krishnamoorthy T, Pavitt GD, Zhang F, Dever TE, Hinnebusch AG. Tight binding of the phosphorylated α subunit of initiation factor 2 (eIF2α) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol Cell Biol. 2001;21(15):5018–5030. doi: 10.1128/MCB.21.15.5018-5030.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nonato MC, Widom J, Clardy J. Crystal structure of the N-terminal segment of human eukaryotic translation initiation factor 2α. J Biol Chem. 2002;277(19):17057–17061. doi: 10.1074/jbc.M111804200. [DOI] [PubMed] [Google Scholar]
- 34.Dhaliwal S, Hoffman DW. The crystal structure of the N-terminal region of the α subunit of translation initiation factor 2 (eIF2α) from Saccharomyces cerevisiae provides a view of the loop containing serine 51, the target of the eIF2α-specific kinases. J Mol Biol. 2003;334(2):187–195. doi: 10.1016/j.jmb.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 35.Dar AC, Dever TE, Sicheri F. Higher-order substrate recognition of eIF2α by the RNA-dependent protein kinase PKR. Cell. 2005;122(6):887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Pavitt GD, Ron D. New insights into translational regulation in the endoplasmic reticulum unfolded protein response. Cold Spring Harb Perspect Biol. 2012;4(6):a012278. doi: 10.1101/cshperspect.a012278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teng Y, et al. Inhibition of eIF2α dephosphorylation enhances TRAIL-induced apoptosis in hepatoma cells. Cell Death Dis. 2014;5:e1060. doi: 10.1038/cddis.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jousse C, et al. Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J Cell Biol. 2003;163(4):767–775. doi: 10.1083/jcb.200308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.García MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: Virus and cell control. Biochimie. 2007;89(6-7):799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Boyce M, et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307(5711):935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 41.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332(6025):91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 42.Wek RC, Cannon JF, Dever TE, Hinnebusch AG. Truncated protein phosphatase GLC7 restores translational activation of GCN4 expression in yeast mutants defective for the eIF-2 α kinase GCN2. Mol Cell Biol. 1992;12(12):5700–5710. doi: 10.1128/mcb.12.12.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alone PV, Cao C, Dever TE. Translation initiation factor 2γ mutant alters start codon selection independent of Met-tRNA binding. Mol Cell Biol. 2008;28(22):6877–6888. doi: 10.1128/MCB.01147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cesareni G, Murray JAH. Plasmid vectors carrying the replication origin of filamentous single-stranded phages. In: Setlow JK, Hollaender A, editors. Genetic Engineering: Principals and Methods. Vol 9. Plenum; New York: 1987. pp. 135–154. [Google Scholar]
- 45.Mitchell DA, Marshall TK, Deschenes RJ. Vectors for the inducible overexpression of glutathione S-transferase fusion proteins in yeast. Yeast. 1993;9(7):715–722. doi: 10.1002/yea.320090705. [DOI] [PubMed] [Google Scholar]
- 46.Hitzeman RA, et al. Secretion of human interferons by yeast. Science. 1983;219(4585):620–625. doi: 10.1126/science.6186023. [DOI] [PubMed] [Google Scholar]
- 47.Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Tommaso P, et al. T-Coffee: A web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 2011;39(Web Server issue):W13–W17. doi: 10.1093/nar/gkr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kemena C, Notredame C. Upcoming challenges for multiple sequence alignment methods in the high-throughput era. Bioinformatics. 2009;25(19):2455–2465. doi: 10.1093/bioinformatics/btp452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramaswamy NT, Li L, Khalil M, Cannon JF. Regulation of yeast glycogen metabolism and sporulation by Glc7p protein phosphatase. Genetics. 1998;149(1):57–72. doi: 10.1093/genetics/149.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]