Significance
On activation, blood platelets package components from their cytoplasm into microparticles (MPs), tiny vesicles released by cytoplasmic membrane budding and shedding. Given that MPs can impact other cellular lineages on internalization, we aimed to decipher the mechanisms promoting MP internalization by cellular recipients. We modeled MP internalization by neutrophils and identified a predominant lipid, 12(S)-hydroxyeicosatetranoic acid, as a mediator critical for the promotion of MP internalization. MPs were found inside neutrophils from individuals with rheumatoid arthritis, and their presence in neutrophils in the joints of mice treated with arthritogenic serum is dependent on the expression of enzymes implicated in the generation of 12(S)-hydroxyeicosatetranoic acid. These findings reveal a unique molecular mechanism implicated in MP internalization relevant to inflammatory processes.
Keywords: platelets, microparticles, neutrophils, 12-lipoxygenase, phospholipase A2
Abstract
Platelets are anucleated blood elements highly potent at generating extracellular vesicles (EVs) called microparticles (MPs). Whereas EVs are accepted as an important means of intercellular communication, the mechanisms underlying platelet MP internalization in recipient cells are poorly understood. Our lipidomic analyses identified 12(S)-hydroxyeicosatetranoic acid [12(S)-HETE] as the predominant eicosanoid generated by MPs. Mechanistically, 12(S)-HETE is produced through the concerted activity of secreted phospholipase A2 IIA (sPLA2-IIA), present in inflammatory fluids, and platelet-type 12-lipoxygenase (12-LO), expressed by platelet MPs. Platelet MPs convey an elaborate set of transcription factors and nucleic acids, and contain mitochondria. We observed that MPs and their cargo are internalized by activated neutrophils in the endomembrane system via 12(S)-HETE. Platelet MPs are found inside neutrophils isolated from the joints of arthritic patients, and are found in neutrophils only in the presence of sPLA2-IIA and 12-LO in an in vivo model of autoimmune inflammatory arthritis. Using a combination of genetically modified mice, we show that the coordinated action of sPLA2-IIA and 12-LO promotes inflammatory arthritis. These findings identify 12(S)-HETE as a trigger of platelet MP internalization by neutrophils, a mechanism highly relevant to inflammatory processes. Because sPLA2-IIA is induced during inflammation, and 12-LO expression is restricted mainly to platelets, these observations demonstrate that platelet MPs promote their internalization in recipient cells through highly regulated mechanisms.
Small extracellular vesicles (EVs) are implicated in physio(patho)logical contexts, such as immunity, reproduction, and cancer (1–4). They also include apoptotic bodies, the vesicles produced by apoptotic cells. Exosomes are EVs generated by exocytosis of multivesicular bodies ranging in size between 50 nm and 150 nm, whereas microparticles (MPs), also known as microvesicles, are vesicles of ∼100–1,000 nm diameter shed from the plasma membrane by cellular budding and fission (2). EVs bear cellular components originating from the donor cells, and accumulating evidence suggests that they might transfer their material to recipient cells. The regulatory events implicated in the transfer of the EV cargo remain mostly undefined, however.
Platelets circulate in blood and patrol the vasculature to promote hemostasis. Although any cell lineage might shed MPs, platelets are particularly proficient at this function. Consistent with this, the blood is rich in MPs expressing platelet (and megakaryocyte) surface markers, and levels of platelet MPs increase in diseases in which platelets are activated (5). Albeit anucleated, platelets represent a major blood reservoir of such components as nuclear factors (6, 7), messenger RNA (mRNA) (8, 9), microRNA (miRNA) (10), and mitochondria (11), which may be packaged inside MPs and transferred to nucleated recipients. A key event in the occurrence of such transfer is the binding of platelet MPs to cells. This may implicate selectins (12) and the recognition of phosphatidylserine, a phospholipid frequently exposed on MPs (13), by lactadherin (14) and developmental endothelial locus-1 (15). Indeed, miRNA-containing platelet MPs are internalized by endothelial cells, thereby altering the stability of mRNA in the recipient (16). Platelets are also active participants in immunity (17–20); platelet MPs are found in inflammatory conditions (1, 17, 21) and are ideally positioned to interact with immune cells.
Neutrophils patrol the vasculature and tissues at the ready to respond to an infectious agent or tissue insult (22). Although neutrophils are considered terminally differentiated granulocytes, they can undergo important phenotypical and functional changes once present in inflammatory exudates (23). For instance, in rheumatoid arthritis (RA), the most common form of autoimmune joint inflammation, neutrophils are represented preponderantly in the diseased joint fluid and display a prolonged lifespan and reduced migratory activity, suggesting the accumulation of factor(s) in RA that promote neutrophil plasticity (23–25).
Using autoimmune arthritis as a model of inflammation in which both MPs and neutrophils contribute (21, 26), we reveal that MP cargo transfer from anucleated platelets to nucleated recipient neutrophils is dependent on the concerted activities of sPLA2-IIA present in the extracellular milieu and of 12-lipoxygenase (12-LO) present in platelet MPs. Our observations demonstrate that platelet MPs are not passively internalized by neutrophils, but rather that MPs promote their own internalization via a lipid mediator of inflammation. Considering that platelets (i) represent a substantial source of nuclear factors, noncoding RNAs, and functional organelles; (ii) are highly efficient at producing MPs; and (iii) are unique with respect to 12(S)-HETE expression, platelets might be specialized at transferring their material to other cells to modify them.
Results
MP Internalization by Neutrophils.
Assuming that the transfer of materials from platelets to neutrophils is biologically significant, we hypothesized that it necessarily would occur via finely controlled mechanisms. To identify key mediators involved in internalization, we further surmised that these mediators would be concomitantly expressed with platelet MPs. The secreted phospholipase A2 (sPLA2) enzymes hydrolyze membrane phospholipids in the sn-2 position, generating free fatty acids and lysophospholipids (27). Although 10 different groups of sPLA2 enzymes have been identified in humans, sPLA2 group IIA (sPLA2-IIA) is (nonexclusively) expressed by platelets and is induced in inflammation (27, 28). In RA, sPLA2-IIA is overexpressed in joint lubricating synovial fluid (SF) and amplifies the disease (28). Whereas sPLA2-IIA has limited activity on the cellular plasma membrane (27), it uses MPs as a substrate (27, 29, 30). Like sPLA2-IIA, MPs accumulate in SF during RA, where they are frequently associated with neutrophils (11, 21, 28, 31, 32).
In a preliminary set of experiments, we generated platelet MPs to verify whether they are internalized by neutrophils and whether sPLA2-IIA impacts this process. We used collagen to activate human platelets that had been labeled with 5-chloromethylfluorescein diacetate (CMFDA), a probe that passes freely through the platelet cell membrane and is converted to a fluorescent cell-impermeable product by cytosolic esterases (33). Under these conditions, the fluorescent probe was encapsulated within platelets, and 96.8 ± 0.25% of the MPs shed from these platelets fluoresced (SI Appendix, Fig. S1A). MPs isolated by centrifugation contained ∼0.1% remnant platelets and were of heterogeneous size (115 nm–538 nm; average, 346.3 ± 23.4 nm) (SI Appendix, SI Materials and Methods and Fig. S1 B, C, D, and F), consistent with the reported dimensions of platelet MPs (13).
We then verified different categories of markers expected in EVs in MP preparations. Whereas mitochondria are typically absent in exosomes, they can be packaged inside MPs (11, 34). Furthermore, the presence of a protein associated with the endosomal sorting complex required for transport (ESCRT), tumor susceptibility gene 101 protein (TSG101), is recognized in exosomes but underrepresented in MPs (34). Thus, the platelet EV preparations used in this study were enriched in MPs, because a subset contained mitochondrial markers, whereas ESCRT proteins were undetectable (SI Appendix, Fig. S1 E and G).
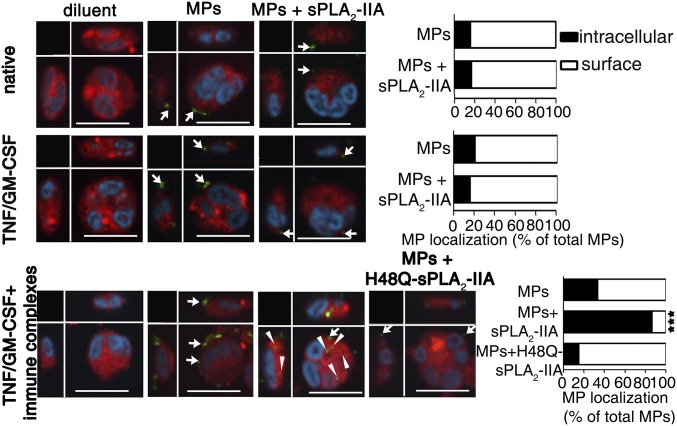
To determine the contribution of inflammatory stimuli and sPLA2-IIA to MP internalization by neutrophils, we treated the latter with autoimmune-relevant inflammatory stimuli [TNF/GM-CSF and immune complexes, an agonist of the receptors for the Fc portion of IgG (FcγR)] in the presence or absence of sPLA2-IIA. We observed that MPs rapidly bound neutrophils independently of sPLA2-IIA and of any costimulation (SI Appendix, Fig. S2 A–E). In contrast, the combination of stimuli (TNF/GM-CSF and immune complexes) and sPLA2-IIA was necessary for efficient internalization of MPs (Fig. 1 and SI Appendix, Fig. S3A), which were identified in the neutrophil’s cytoplasm in the vicinity of the endoplasmic reticulum, Golgi apparatus, and lysosome, but never by the recipient mitochondria (SI Appendix, Fig. S4 A–D). Accordingly, an average of 20 ± 4 and 38 ± 3 MPs were internalized in neutrophils in the presence of sPLA2-IIA within 30 and 60 min, respectively. The internalization process occurred through dynamin-, clathrin-, and caveolin-dependent endocytosis (SI Appendix, Fig. S5), and was not unique to collagen-induced MPs. MPs generated by activating platelets with thrombin, a serine protease, and collagen-related peptide, a specific glycoprotein VI agonist, also were internalized by neutrophils, and this was dependent on the presence of sPLA2-IIA (SI Appendix, Fig. S3B). These results provide an ideal model for identifying the molecular process implicated in MP internalization following adhesion.
Fig. 1.
Internalization of platelet MPs in neutrophils. (Left) Representative confocal microscopy analyses of neutrophil cytoplasm (red) and nuclei (cyan) on incubation with MPs (green) for 2 h at 37 °C in the absence or presence of recombinant human sPLA2-IIA or its catalytically inactive mutant H48Q-sPLA2-IIA. Neutrophils were left native (without stimulation), primed (TNF/GM-CSF), or activated (TNF/GM-CSF and immune complexes) before MP incubation. MPs are seen at the surface (white arrows) and internalized (arrowheads). (Scale bars: 10 µm.) (Right) Graph bars indicating the relative localization (surface vs. intracellular) of the MPs, depending on the neutrophil and MP treatments. Data were obtained from 100 neutrophils per condition repeated at least three times with cells from different donors (n > 3). ***P < 0.0001 compared with MP condition, Mann–Whitney test.
Although the sPLA2-IIA enzyme generates potent lipid mediators, it also acts as a receptor ligand (27, 35–37). Thus, sPLA2-IIA might promote internalization either through the production of lipid mediators or through receptor binding and signaling. To assess the contribution of sPLA2-IIA catalytic activity to MP internalization, we made use of an inactive enzyme mutant, H48Q-sPLA2-IIA (27). An important observation is that sPLA2-IIA catalytic activity was critical for the promotion of MP internalization (Fig. 1), ruling out the role of sPLA2-IIA receptor binding and pointing to the role of lipid mediator(s) in this process.
Platelet Microparticle Lipidomics.
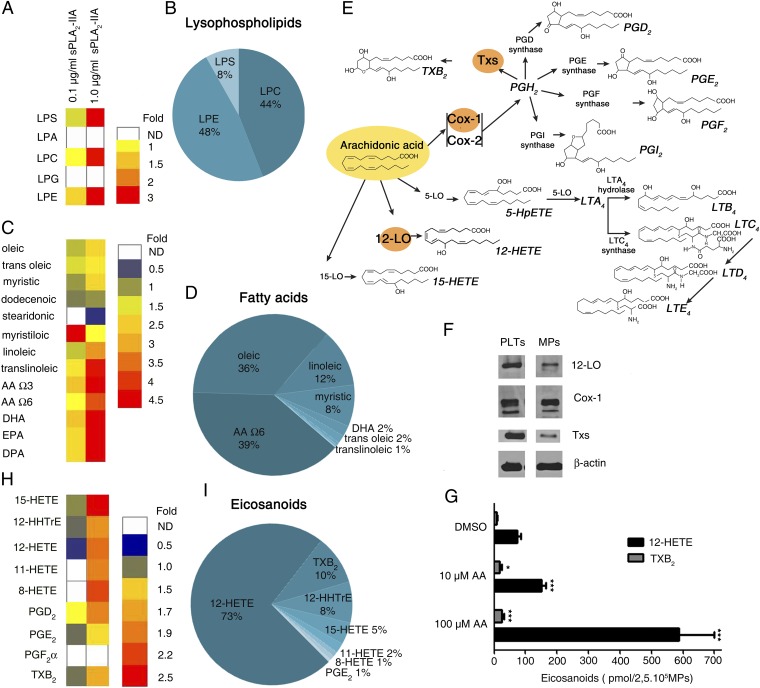
We next sought to identify the lipid trigger implicated in MP internalization. sPLA2-IIA generates lysophospholipids from MPs (27, 29); however, the complete set of lipid mediators expressed by MPs is unknown. Using tandem mass spectrometry to survey MP-derived lipid mediators, we confirmed that MPs are used as substrates by sPLA2-IIA, generating diverse lysophospholipids and fatty acids, including arachidonic acid (AA; 20:4) (Fig. 2 A–D and SI Appendix, Fig. S6 A and B). AA can be metabolized into eicosanoids, highly versatile mediators of multiple physiological and pathological processes (Fig. 2E) (38, 39). Indeed, examination of platelet MPs revealed, as in platelets (40), the presence of enzymes [i.e., cyclooxygenase 1 (Cox-1), thromboxane synthase (TXs), and 12-LO] that metabolize AA into the eicosanoids thromboxane A2 (TXA2) and 12(S)-HETE (Fig. 2F). These pathways are active in MPs as exogenous AA was metabolized into thromboxane B2 (TXB2; a stable metabolite of TXA2) and 12(S)-HETE (Fig. 2G). Consistent with this was the identification of TXB2 and 12(S)-HETE [12(S)-HETE >>TXB2] by lipidomics as the predominant eicosanoids produced by sPLA2-IIA from MPs (Fig. 2 H and I and SI Appendix, Fig. S6C).
Fig. 2.
sPLA2-IIA promotes the release of multiple lipid mediators from MPs. Mass spectrometry measurements of the indicated lysophospholipids (A and B), fatty acids (C and D), and eicosanoids (H and I) released and metabolized after sPLA2-IIA activity on MPs. (A, C, and H) Heat maps showing fold change of each molecule produced in response to a 6-h incubation of MPs with the indicated concentration of human recombinant sPLA2-IIA. The concentrations of lysophospholipids (A), fatty acids (C), and eicosanoids (H) measured in MPs incubated with diluent (no sPLA2-IIA) were used to determine the relative fold changes. LPS, lysophosphatidylserine; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; LPG, lysophosphatidylglycerol; LPE, lysophosphatidylethanolamine; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; DP, docosapentaenoic acid; 15-HETE, 15-hydroxyeicosatetraenoic acid, 11-HETE, 11-hydroxy eicosatetraenoic acid; 8-HETE, 8-hydroxyeicosatetraenoic acid; PGE2, prostaglandin E2. (B, D, and I) Pie chart representations of lysophospholipids, fatty acids, and eicosanoids present in MPs treated with sPLA2-IIA for 6 h. The data presented are based on the mass composition (molar expression) of each lysophospholipid, fatty acid, and eicosanoid detected by mass spectrometry (n = 2 different blood donors). (E) Graphic representation of the metabolism of AA into eicosanoids. (F) Immunoblot of 12-LO, TXs, COX-1, and β-actin in platelets (PLTs) and MPs. Data are representative of three independent experiments performed using platelets and MPs from three blood donors. (G) Indicated amounts of exogenous AA were added to platelet MPs, and 12-HETE and TXB2 were measured by HPLC (n = 3). *P < 0.05; ***P < 0.0001 compared with respective DMSO control, t test.
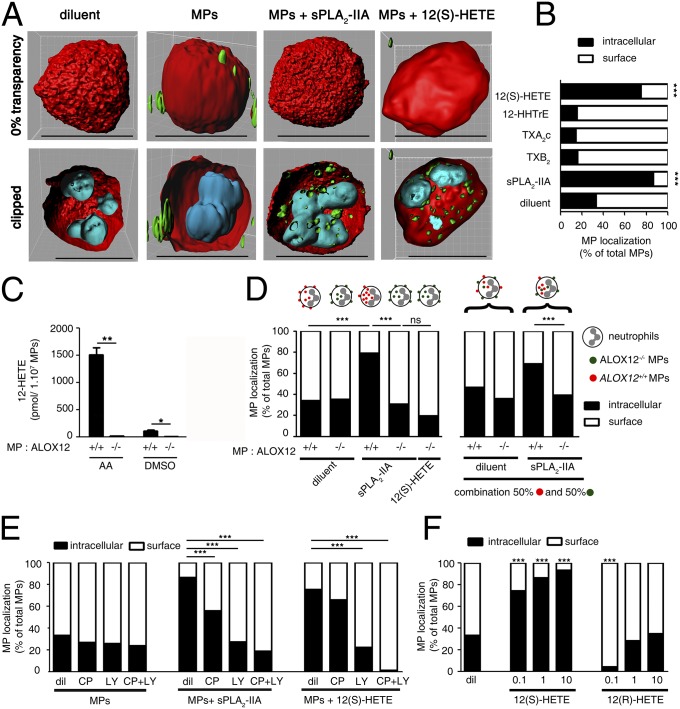
Consequently, we added exogenous lipids to activated neutrophils and examined their role in internalization. We observed that, similar to sPLA2-IIA, 12(S)-HETE was sufficient to promote internalization by inducing neutrophils to internalize MPs (Fig. 3 A and B and Movies S1 and S2). Conversely, lysophosphatidylcholine (SI Appendix, Fig. S7A), carbocyclic TXA2 (TXA2c; a stable analog of TXA2), TXB2, and 12-hydroxyheptadecatrienoic acid (12-HHTrE) produced concurrently with TXA2 by TXs (41) had no impact on MP internalization (Fig. 3B). Moreover, a COX inhibitor demonstrated no effect on MP internalization, confirming that the COX products are dispensable (SI Appendix, Fig. S7B).
Fig. 3.
The 12-LO product 12(S)-HETE is the trigger of MP internalization. (A) Representative confocal microscopy 3D reconstruction of activated neutrophils (cytoplasm in red and nuclei in cyan), interacting with platelet MPs (green) in the presence of human recombinant sPLA2-IIA or 12(S)-HETE (1 µM). (B, D–F) Bar graphs indicating the relative localization (surface vs. intracellular) of the MPs incubated with activated neutrophils for 2 h at 37 °C under the indicated conditions. Data were obtained by confocal microscopy from 100 neutrophils per condition (n = 3 for each panel). ***P < 0.0001 comparing diluents or indicated conditions, Mann–Whitney test. (C) MPs were generated using platelets from WT mice (red ALOX12+/+ MPs) and mice lacking 12-LO expression (green ALOX12−/− MPs). Detection of 12-HETE was determined by HPLC after a 30-min incubation at 37 °C of AA (100 µM) or diluent DMSO with ALOX12+/+ MPs or ALOX12−/− MPs (n = 3). *P < 0.05; **P < 0.001 on indicated conditions, Mann–Whitney test. (D) Activated neutrophils were incubated for 2 h at 37 °C in the presence of combinations of ALOX12+/+ MPs and ALOX12−/− MPs (50:50) or single groups of MPs (ALOX12+/+ MPs or ALOX12−/− MPs) and treated with or without recombinant sPLA2-IIA or with or without 12(S)-HETE (1 µM). (E) Activated neutrophils were treated for 10 min at 37 °C with the antagonists of BLT1 [CP105696 (CP), 10 nM] and BLT2 [LY255283 (LY), 100 nM] receptor alone and in combination before the addition of platelet MPs and sPLA2-IIA. (F) Activated neutrophils incubated with MPs were treated with exogenous 12(S)-HETE (0.1–10 µM) or 12(R)-HETE (0.1–10 µM).
Next, to confirm that 12(S)-HETE is the lipid trigger implicated in sPLA2-IIA–induced MP internalization, we produced fluorescent MPs from platelets isolated from platelet-type 12-LO–deficient (ALOX12−/−) mice and from their wild type (WT) control littermates (ALOX12+/+). Remarkably, ALOX12−/− MPs, which cannot metabolize AA into 12(S)-HETE (Fig. 3C), were not internalized by neutrophils, even in the presence of sPLA2-IIA (Fig. 3D), demonstrating the critical involvement of platelet MP 12-LO in this process. Neutrophils express the 12(S)-HETE high-affinity receptor BLT2 and the leukotriene B4 (LTB4) high-affinity receptor BLT1. Using an antagonist of BLT2 (LY255283), along with an antagonist of BLT1 (CP105696) for comparison, we confirmed the involvement of 12(S)-HETE and its receptor BLT2 in the internalization process (Fig. 3E). The contribution of BLT1 was significant, but less prominent, in agreement with the lower affinity of 12(S)-HETE for this receptor, or suggestive of a modest role for LTB4 in this process (42).
Platelet-type 12-LO expressed by human platelets generates the S enantiomer of 12-hydroxyeicosatetraenoic acid (12-HETE), designated 12(S)-HETE, whereas the R enantiomer, 12(R)-HETE, is produced by the 12-LO expressed by leukocytes and skin fibroblasts, or through the noncatalytic derivation of AA by cytochrome P450 (43). To verify whether this mechanism of internalization might apply to other cell lineage MPs (deficient in platelet-type 12-LO), we compared the relative impacts of 12(S)-HETE and 12(R)-HETE on internalization. We found that 12(S)-HETE, but not 12(R)-HETE, is specifically involved in MP internalization (Fig. 3F). ALOX12−/− MPs were not internalized even in the presence of exogenous 12(S)-HETE, providing further support for the contribution of platelet-type 12-LO in this process (Fig. 3D). In addition, using red fluorescent ALOX12−/− platelets and green fluorescent ALOX12+/+ platelets to generate red ALOX12−/− MPs and green ALOX12+/+ MPs, respectively, we found that 12(S)-HETE produced from MPs is incapable of promoting internalization of neighboring MPs lacking 12-LO (Fig. 3D), suggesting that the internalization process revealed here might be unique to 12(S)-HETE–expressing MPs (i.e., platelet MPs).
MP Internalization in Disease.
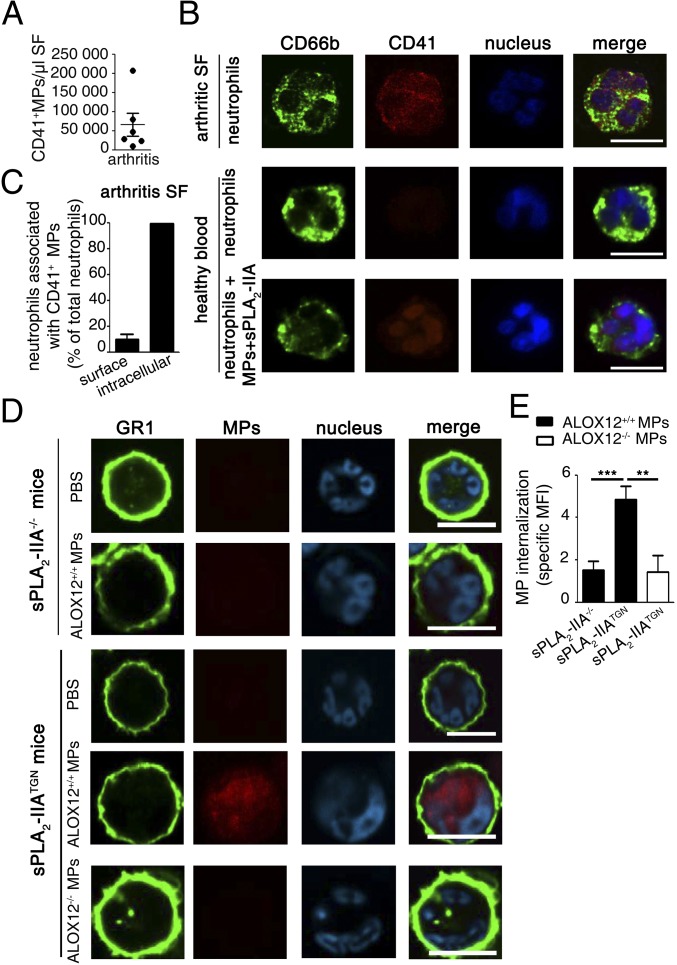
Neutrophils, platelet MPs (11, 21, 31, 32), sPLA2-IIA (28), and inflammatory stimuli all coexist in RA SF. We used SF from patients with RA that contained an abundance of platelet MPs (Fig. 4A) to determine whether platelet MPs can be found within neutrophils. Using anti-CD41 and anti-CD66b monoclonal antibodies to label platelet MPs and neutrophils, respectively, we could discern significant platelet MP signals both at the surface and inside neutrophils (mostly near or at the nucleus) in RA SF (Fig. 4 B and C). We observed a similar pattern of CD41+MP expression inside peripheral neutrophils that had been incubated with platelet MPs, but only in the presence of sPLA2-IIA (Fig. 4B).
Fig. 4.
The concerted action of sPLA2-IIA and 12-LO in MP internalization. (A) Platelet MPs in SF of patients with RA were quantified by high-sensitivity flow cytometry using an antibody directed against the CD41 epitope (n = 6 RA patients). (B) Representative confocal microscopy picture of purified CD66b+ neutrophils (detected using an FITC-conjugated anti-CD66b antibody) isolated from the SF of patients with RA (n = 6). For comparison, circulating neutrophils isolated from healthy blood donors (n = 6) were activated and incubated with MPs and sPLA2-IIA. Platelet MPs were detected using a PE-conjugated anti-CD41 antibody (red), and nuclei were labeled with Hoechst (blue). (Scale bars: 10 µm.) (C) Bar graph indicating the relative localization (surface vs. intracellular) of the CD41+ MPs in neutrophils isolated from arthritic patients. The quantification was performed on 100 neutrophils per donor using confocal microscopy (n = 6). (D and E) Equivalent arthritis scores (clinical index of 10 at day 5; details in Materials and Methods) were induced in sPLA2-IIA–deficient (sPLA2-IIA−/−) and –sufficient (sPLA2-IIATGN) mice to assess MP internalization by neutrophils in arthritic ankles. Fluorescently labeled MPs (ALOX12+/+ or ALOX12−/−) were injected i.v. into the mouse tail vein, and the neutrophils retrieved in the arthritic joints were collected for confocal microscopy analyses. (D) Representative confocal microscopy images of neutrophils identified using FITC-conjugated anti-GR1 and the presence of neutrophil distinctive polymorphonuclei (cyan). The presence of MPs was determined as red fluorescence. (E) MFI of the red fluorescence signals present intracellularly was measured on 100 neutrophils per mice. Bar graph presents specific MFI (i.e., MFI of MP injected mice minus PBS-injected mice) (n = 6) **P < 0.005; ***P < 0.0001, Mann–Whitney test.
We next used the K/BxN serum transfer model of arthritis, a disease model in which both sPLA2-IIA and platelet MPs are implicated (21, 28), to validate the MP internalization process in inflammation. Because C57Bl6/J mice naturally lack sPLA2-IIA (28), we used transgenic mice expressing human sPLA2-IIA (sPLA2-IIATGN, in a C57Bl6/J background) (44) and included WT C57Bl6/J mice as controls. We observed that on injection into the tail vein, fluorescent ALOX12+/+ MPs quickly localized inside neutrophils in the arthritic ankles of sPLA2-IIA–expressing mice (Fig. 4 D and E). An important observation is the absence of MP localization inside neutrophils in mice lacking sPLA2-IIA, confirming the essential role of sPLA2-IIA in the internalization process in vivo. The internalization was through MP-derived 12(S)-HETE; ALOX12−/− MPs failed to localize in neutrophils, even in mice expressing sPLA2-IIA (Fig. 4 D and E). Thus, the concerted actions of sPLA2-IIA– and MP-derived 12-LO trigger MP internalization during inflammation.
Transfer of an Elaborate Microparticle Cargo Inside Neutrophils.
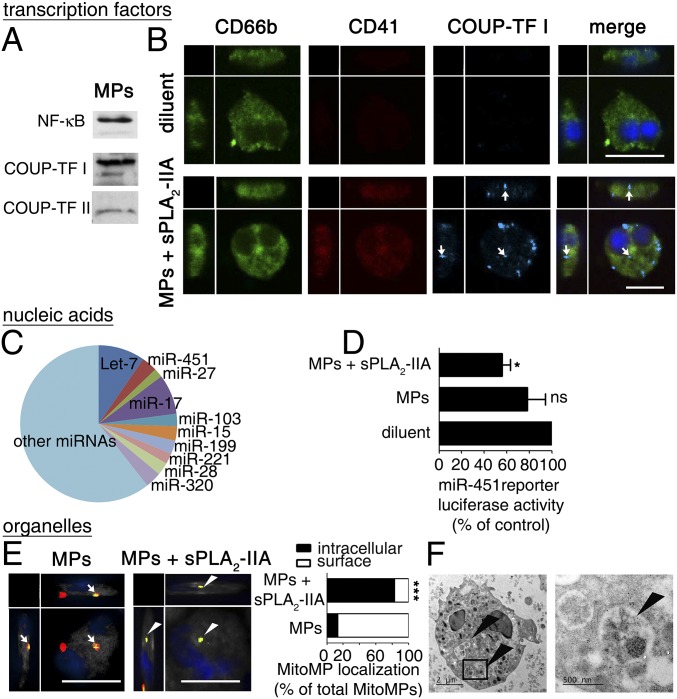
Having confirmed efficient internalization of fluorescently labeled platelet MPs, we proceeded to verify the actual transfer of three distinct platelet components (i.e., a cytosolic protein, a nucleic acid, and an organelle) to neutrophils. Because megakaryocytes perform several rounds of endomitosis before platelet production, the platelet content can be particularly enriched in cellular components. Although lacking a nucleus (and thus transcription), the transcription factors NF-κB (7) and peroxisome proliferator-activated receptor (PPAR) have been reported in the platelet cytosol (6). The complete set of transcription factors expressed by platelets is unknown, however, and whether they are encapsulated in MPs has not been investigated. We determined that 80 different transcription factors, belonging to seven distinct structural families (45) (SI Appendix, Dataset S1) are found in platelets (SI Appendix, Fig. S8A) and are packaged in MPs (SI Appendix, Fig. S8B), including NF-κB and chicken ovalbumin upstream promoter-transcription factor (COUP-TFI) (Fig. 5A). The latter, which is involved in neural maturation (46), was highly expressed in platelet MPs and was undetectable by immunofluorescence in neutrophils (Fig. 5B and SI Appendix, Dataset S1). We used this information to monitor the transfer of transcription factors to the recipient. We observed that through sPLA2-IIA activity, COUP-TFI was efficiently transferred to neutrophils, where it remained in the cytosol (Fig. 5B). Furthermore, the COUP-TFI protein detected in the platelet MPs retained its DNA-binding activity, as revealed by DNA–protein interaction assays (SI Appendix, Fig. S8C), thereby indicating that it might impact recipient cell transcription on translocation to the nucleus.
Fig. 5.
Platelet MPs transfer an elaborate cargo in neutrophils. (A) Representative immunoblots of COUP-TF I, COUP-TF II, and NF-κB in platelet MPs (n = 3). (B) Representative confocal microscopy images of activated neutrophils after incubations with or without MPs and sPLA2-IIA. COUP-TF I (cyan) is denoted by white arrows in CD66b+ (green) neutrophils with blue nuclei. Platelet MPs were detected using an anti-CD41 antibody (red). (Scale bars: 10 µm.) (C) Pie chart representation of the top-10 families of miRNA found in MPs based on intensity expression in miRNA arrays (n = 3). (D) Luciferase reporter assay of miR-451 activity measured in the PLB-985 differentiated neutrophil-like cell line incubated for 48 h at 37 °C in the presence of MPs untreated or treated with human recombinant sPLA2-IIA (n = 3). *P < 0.05 compared with diluent, t test. (E) Representative confocal microscopy images of activated neutrophils incubated with MPs in the presence or absence of sPLA2-IIA. MPs were labeled with CMFDA (green) and MitoTracker (red). Green and red MPs (mitochondria-containing MP or mitoMPs) are denoted by white arrows on the neutrophil surfaces or by white arrowheads inside neutrophils. (Scale bars: 10 µm.) Graph bars indicate the relative localization (surface vs. intracellular) of the MitoMPs, depending on the neutrophil and MitoMP treatments. Data were obtained from 100 neutrophils per condition repeated at least three times with cells from different donors (n = 3). ***P < 0.0001 compared with the MP condition, Mann–Whitney test. (F) Representative TEM image of activated neutrophils incubated with MPs in the presence of sPLA2-IIA. Arrowheads denote mitoMPs internalized in neutrophils.
Platelets contain mRNAs (8, 47) and noncoding RNAs (ncRNAs) (10), encapsulated inside MPs on budding and shedding (16). We have established the repertoire of nucleic acids from platelet MPs, showing that MPs express a variety of mRNAs coding for proteins implicated in multiple biological processes (SI Appendix, Fig. S8D and Dataset S2). Of interest, ncRNAs, such as transfer RNAs, ribosomal RNAs, and miRNAs, also were identified in MPs. We performed a more precise assessment of MP miRNAs using miRNA arrays, which identified the presence of immature and mature miRNAs (SI Appendix, Dataset S3). An miRNA produced independently of Dicer activity, miR-451 (48), has been implicated in immunity and inflammation (49) and appeared to be one of the most abundantly expressed miRNAs in MPs (Fig. 5C and SI Appendix, Fig. S8E). Using a neutrophil-like cell line transfected with a specific DNA sequence regulated by miR-451, we demonstrated the occurrence of 12(S)-HETE–dependent MP internalization (SI Appendix, Fig. S9) and transfer of miR-451 to these recipient cells (Fig. 5D). Furthermore, we found that MP-derived miR-451 cleaved its cytoplasmic target sequence, establishing that platelet MPs can transfer functional nucleic acids to recipient cells through sPLA2-IIA (Fig. 5D).
Platelets contain an average of four mitochondria, which can be packaged inside MPs, thereby forming mitochondria-containing MPs (11). Because only a subset of MPs contains mitochondria (11), we used confocal microscopic analyses and a combination of cytosolic and mitochondrial fluorescent dyes to distinguish mitochondria-deficient MPs and mitochondria-containing MPs. We found that the internalization of mitochondria-containing MPs by neutrophils depends on the presence of sPLA2-IIA (Fig. 5E). The efficient transfer of mitochondria through MPs was further validated by electron microscopy (Fig. 5F). Thus, the activity of sPLA2-IIA on MPs mediates the transfer of a broad repertoire of platelet components to neutrophils, including cytosolic proteins (e.g., transcription factors), nucleic acids (e.g., miRNA) and organelles (e.g., mitochondria).
Concerted Action of sPLA2-IIA and 12-LO in Vivo.
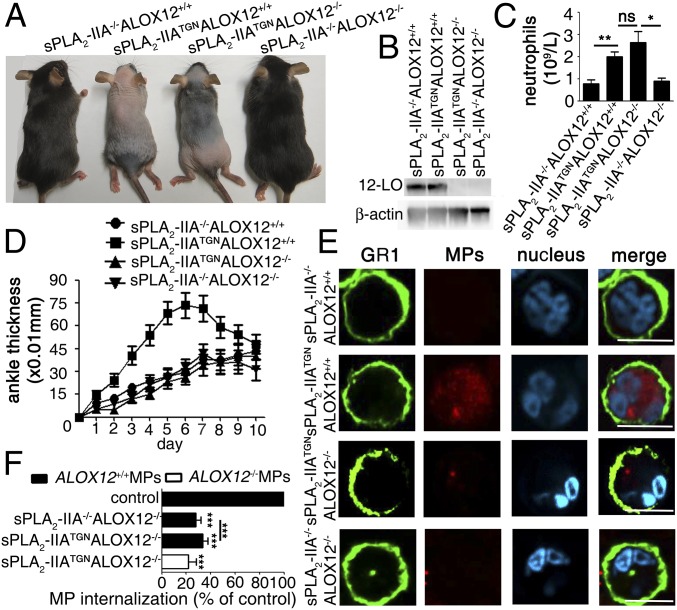
The internalization of MPs by activated neutrophils requires 12(S)-HETE; however, whether this process is proinflammatory or anti-inflammatory is unknown. Under the hypothesis that the internalization of MPs by neutrophils might be biologically relevant, we verified the impact of the concerted activities of sPLA2-IIA and 12-LO in vivo. To this end, we crossed ALOX12−/− mice with sPLA2-IIATGN mice, which reportedly exhibit skin abnormalities reminiscent of psoriasis but with no neutrophil infiltration (44), to generate sPLA2-IIATGNALOX12−/− mice. We observed that ablation of the ALOX12 gene in sPLA2-IIATGN mice had no effect on the skin phenotype, although 12-LO expression was eliminated in blood platelets (Fig. 6 A and B). Furthermore, we also confirmed that neutrophilia, which has been reported in sPLA2-IIATGN mice (50), occurred independently of 12-LO, and that the other blood cell lineages were unaffected in the transgenic mice (Fig. 6C and SI Appendix, Fig. S10). Taken together, these data suggest that the concerted actions of sPLA2-IIA and 12-LO do not support neutrophil-independent psoriasis-like disease or neutropoiesis.
Fig. 6.
Concerted sPLA2-IIA and 12-LO activity promotes inflammation. (A) Phenotypic observations of the indicated groups of mice. sPLA2-IIATGNALOX12+/+ and sPLA2-IIATGNALOX12−/− mice exhibit alopecia and hyperkeratosis with features reminiscent of psoriasis (without leukocyte infiltration), which are absent in control animals. (B) Immunoblot of 12-LO and β-actin on platelet lysates from indicated groups of mice. Data are representative of three independent experiments. (C) Bar graph indicating numbers of neutrophils in blood in indicated mice (n = 6 mice/group). (D) The severity of arthritis was evaluated after administration of K/BxN serum in sPLA2-IIA−/−ALOX12+/+, sPLA2-IIATGNALOX12+/+, sPLA2-IIATGNALOX12−/−, and sPLA2-IIA−/−ALOX12−/− mice (n = 15 mice in each group). (E) Equivalent arthritis scores (Materials and Methods) were induced in the four groups of mice to assess MP internalization by neutrophils from arthritic ankles. Fluorescent MPs (ALOX12+/+) were injected i.v. into the mouse tail vein, and neutrophils were visualized by confocal microscopy analyses. Shown are representative confocal microscopy images of neutrophils identified using FITC-conjugated anti-GR1 and the presence of neutrophil-distinctive polymorphonuclei (cyan). The presence of MPs was determined by red fluorescence. (F) ALOX12−/− and ALOX12+/+ MPs were injected i.v. into sPLA2−/−ALOX12−/− and sPLA2-IIATGNALOX12−/− mice with equivalent arthritis scores. The presence of MPs was determined by red fluorescence. Red fluorescent MPs present intracellularly were quantified as specific MFI in 100 neutrophils per mice (repeated with six mice) after subtraction of the background fluorescence displayed by neutrophils from mice injected with diluent. Graph bar presents the percentage of specific MP internalization in each condition relative to specific MP internalization by neutrophils from sPLA2-IIATGNALOX12+/+ arthritic mice (serving as positive control mice) (n = 6). ***P < 0.0001 compared with sPLA2-IIATGNALOX12+/+, Mann–Whitney test.
We next used the K/BxN model of inflammatory arthritis to examine the roles of sPLA2-IIA and 12-LO in a relevant pathology in which both neutrophils and platelet MPs participate. In agreement with a previous study (28), we confirmed more marked development of arthritis in sPLA2-IIATGN mice compared with sPLA2-IIA–deficient mice (Fig. 6D). Moreover, the sPLA2-IIATGNALOX12−/− mice developed only modest arthritis, similar to sPLA2-IIA–deficient mice. Given that ablation of the ALOX12 gene in C57BL6 mice (deficient in sPLA2-IIA) had no impact on arthritis, these data establish that sPLA2-IIA and 12-LO work in concert to promote inflammation in vivo. Furthermore, i.v. injection of fluorescent ALOX12+/+ platelet MPs into these groups of mice showed preferential localization in neutrophils in the joints of sPLA2-IIATGNALOX12+/+ mice (Fig. 6E). The internalization of ALOX12+/+ MPs by neutrophils of sPLA2-IIATGNALOX12−/− mice was more efficient than that for ALOX12−/− MPs, suggesting that the former provide sufficient 12(S)-HETE for their internalization (Fig. 6F).
Discussion
The rich collection of components with apparently no or only modest roles in anucleated platelets, and the platelet’s proficiency to produce MPs under a variety of inflammatory conditions was the impetus for our study. Our results identify sPLA2-IIA as an enzyme working in concert with platelet MP 12-LO to promote internalization (Fig. 7 and SI Appendix, Fig. S8G). The fact that two enzymes are required for MP internalization demonstrates that the internalization process is tightly regulated, in agreement with the potential significance of this process. Because sPLA2-IIA is an extracellular enzyme induced only in inflammatory exudates, this ensures that MPs are not internalized in neutrophils, unless the neutrophils reach the inflammatory site. Furthermore, the fact that neutrophils require activation also points to an additional level of regulation. 12-LO is essentially present only in platelet-derived MPs and not in MPs from other cellular lineages, suggesting that the role of sPLA2-IIA and 12-LO is to mediate specific platelet component transfer into neutrophils, potentially to impact their function. Of clinical relevance, the concerted actions of sPLA2-IIA and platelet-type 12-LO mediate inflammation in a model of inflammatory arthritis.
Fig. 7.
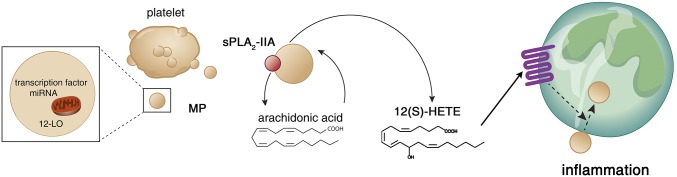
Schematic representation of the mechanism underlying MP internalization in neutrophils. Platelet MPs express 12-LO, and generate 12(S)-HETE on membrane phospholipid hydrolysis by sPLA2-IIA. 12(S)-HETE triggers MP internalization in neutrophils through BLT2 activation, thereby promoting platelet MP cargo transfer to neutrophils. The concerted actions of sPLA2-IIA and 12-LO enhance inflammation.
Among the sPLA2s, sPLA2-IIA is by far the most abundantly expressed in inflammatory fluids and enhances inflammation in models of atherosclerosis and arthritis, conditions that involve platelet MPs (17, 21, 27). Whereas the AA liberated by MPs can be metabolized into eicosanoids by enzymes expressed by other cells in the vicinity (30), our lipidomic approach shows that the action of sPLA2-IIA on MPs generates eicosanoids, a process requiring the activity of enzymes packaged inside MPs, consistent with our proteomic analyses and those reported by other investigators (51, 52). Thus, with their membrane composition and content of functional enzymes, platelet MPs represent an extraordinary source of lipid mediators implicated in a vast range of physio(patho)logical functions when bathed in an environment rich in sPLA2-IIA. Although other sPLA2 groups other than sPLA2-IIA might potentially use MPs as a substrate if present in sufficient quantities, our findings support the proposal that a physiological role for sPLA2-IIA is the promotion of platelet MP functional activities, such as internalization.
Whereas sPLA2-IIA can use platelet MPs to generate proinflammatory 12(S)-HETE, other eicosanoids, produced by platelets and other cell lineages, also make significant contributions to arthritis. It was previously shown using the K/BxN serum transfer model of autoimmune arthritis that platelet COX-1 could generate large quantities of extracellular prostaglandin H2, which itself was metabolized transcellularly by the prostaclycin synthase expressed by fibroblast-like synoviocytes (53). The generation of prostacyclin by fibroblast-like synoviocytes amplifies inflammation, and, accordingly, the ablation of the gene coding for prostacyclin receptor reduces arthritis in vivo (54). The eicosanoid LTB4 is also an important lipid mediator implicated in arthritis, and mice deficient in 5-lipoxygenase (the enzyme regulating its biosynthesis) and in BLT1 (the high-affinity receptor for LTB4) are resistant to arthritogenic K/BxN serum (26, 55). Herein we shed light on the role of platelet-type 12-LO in inflammatory arthritis. Consistent with this is the observation that arthritis is also attenuated in mice deficient in BLT2, a high-affinity receptor for 12(S)-HETE (and 12-HHTrE) (56). Moreover, the contribution of platelet-type 12-LO could be determined only in mice expressing sPLA2-IIA, providing further support for the coupling between sPLA2-IIA and platelet-type 12-LO.
The internalization process revealed in this study occurs independently of COX-1, ruling out the involvement of other major lipid mediators produced by platelets, such as thromboxane and 12-HHTrE. Interestingly, collagen-induced platelet MPs dominantly produced 12(S)-HETE, consistent with the reported activation of 12-LO through the immunoreceptor-based activation motif-containing the FcRγ chain involved in collagen signaling (57). 12(S)-HETE is a too-often neglected mediator, and its exact clinical significance remains a matter of debate (58). It is thought to be involved in the reorganization of the actin cytoskeleton (59), hypertension (60), angiogenesis, and cancer (61). On the other hand, BLT2 is itself implicated in atherosclerosis (62, 63), cancer (64), and inflammation (65). Thus, it is probable that MP internalization might take place in a broad range of conditions, considering that it also might occur in BLT2-expressing cells other than neutrophils, such as mast cells, endothelial cells, and fibroblast-like synoviocytes (42, 66, 67).
MPs lacking 12-LO were not internalized, even in the presence of exogenous 12(S)-HETE. Furthermore, the S enantiomer, but not the R enantiomer, of 12-HETE triggered MP internalization, suggesting that MPs from cell lineages other than platelets might engage distinct mechanisms that have yet to be identified. In humans, the S enantiomer of 12-HETE is produced primarily by platelet-type 12-LO, which might be transferred from platelets to other cells so that they too produce 12(S)-HETE. Indeed, studies have identified platelet-type 12-LO in skin fibroblasts and in fibroblast-like synoviocytes from patients with psoriasis and RA (68, 69), suggesting that MPs derived from these cells also might be capable of conveying 12-LO and of using 12(S)-HETE to promote their internalization. Because both platelet MPs and sPLA2-IIA are present in inflamed SF (21, 28), one might ask whether 12(S)-HETE is found in RA SF. Of interest is that 5,12(S)-diHETE, which is produced only through the coordinated action of leukocyte 5-lipoxygenase and platelet 12-LO, is the most abundant eicosanoid in SF of patients with RA (70), thus suggesting the potential for platelet MP internalization in neutrophils. Future studies will undoubtedly uncover the role of 5,12(S)-diHETE in inflammation.
We report an extensive set of transcription factors and nucleic acids expressed by platelet MPs, which frequently localize near the nucleus and organelles once internalized by neutrophils. Given that miRNAs are recognized as potent modulators of mRNA expression, their transfer to the recipient cell through EVs has received considerable attention (16); however, we emphasize that the MP cargo is far more extensive and contains other modulators besides miRNAs, including mRNAs, ncRNAs, transcription factors, active enzymes (such as 12-LO), cytokines, unique lipids, and even organelles such as mitochondria (11), all of which are potentially capable of contributing to reprogramming of the recipient cell. Accordingly, MPs could regulate transcripts on internalization by neutrophils (SI Appendix, Fig. S11 A and B and Dataset S4), thereby potentially modulating the biological processes and primary functions of these recipient cells. Although these observations suggest that the internalization of MPs may alter neutrophil functions, a feature seen in RA (24), definitively identifying the actual contribution of each individual MP component to the recipient cell is premature. The present study serves to highlight the complexity of the platelet MP cargo and, most importantly, to reveal how platelet MP transfer occurs.
The EV content is highly diversified, with different cellular lineages producing EVs. Furthermore, depending on the biological context, distinct cellular recipients might require specific EV cargoes for their functions and might be specifically targeted by particular EVs. Consistent with this idea, we have demonstrated that platelet MPs are not passively internalized by recipient cells. Specific transfer of the extensive platelet MP cargo to target cells is regulated by a lipid mediator that is unique to and produced by MPs (Fig. 7).
Materials and Methods
More details are provided in SI Appendix, SI Materials and Methods.
Patients.
SF was obtained from the affected knees of six patients with RA, including four with positive rheumatoid factors (RFs) and two with no detectable RFs, with their informed consent under the approval from the Centre Hospitalier Universitaire de Québec’s Ethics Committee. The patients (five females and one male, aged 20–60 y) were not treated with any medications before SF collection.
Mice.
Guidelines of the Canadian Council on Animal Care were followed for all our studies in a protocol approved by the Animal Welfare Committee at Laval University. Eight-week-old male C57BL/6J (sPLA2-IIA−/−), transgenic human sPLA2-IIA (sPLA2-IIATGN) (28, 44), and ALOX12−/− (71) mice backcrossed 10 times in a C57BL/6J background were obtained from The Jackson Laboratory. sPLA2-IIA−/−ALOX12−/− mice in a C57BL/6J background were crossed with sPLA2-IIATGNALOX12+/+ mice in a C57BL/6J background (sPLA2-IIATGN hemizygous). sPLA2-IIATGN12LO−/+ mice obtained from the progeny were crossed again with sPLA2-IIA−/−ALOX12−/− mice to generate the desired genotype sPLA2-IIATGNALOX12−/− identified by genotyping.
Arthritis Induction in Mice.
Induction of arthritis was performed using arthritogenic K/BxN serum (100 µL) transferred by i.p. injection to recipient sPLA2-IIA−/−ALOX12+/+, sPLA2-IIATGNALOX12+/+, sPLA2-IIATGNALOX12−/−, and sPLA2-IIA−/−ALOX12−/− mice. The development of arthritis was monitored daily by measuring the thickness of the ankles at the malleoli as described previously (28).
Experimental Design for MP Localization in Vivo.
To avoid any bias in quantitative analyses, each group of mice with comparable arthritis scores was used to assess MP localization in neutrophils in vivo. Preliminary experiments determined the volume of K/BxN serum needed to induce comparable levels of disease in all groups of mice (i.e., a clinical index plateau of 10 on a scale of 12, reached at day 5). At days 0 and 2, 150 µL of K/BxN serum was injected into sPLA2-IIA−/−ALOX12+/+, sPLA2-IIA−/−ALOX12−/−, and sPLA2-IIATGNALOX12−/− mice, and 75 µL of K/BxN serum was injected into sPLA2-IIATGNALOX12+/+ mice. At day 5 after K/BxN serum transfer, 1.5 × 108 CMPTX ALOX12−/− or ALOX12+/+ MPs were injected into the tail veins of arthritic mice from the four groups. The mice were killed at 3 h after MP injection. The ankles were digested for 3 h at 37 °C with collagenase IV (Worthington; 1 mg/mL in white RPMI medium). Digestion products were sifted through a filter (70-µm cell strainer). Under these conditions, the number of neutrophils in digested ankles remained similar in each group of mice, and 40 ± 6% of total Hoechst+ cells were GR1+ and displayed polylobed nuclei. Cells were washed twice with RPMI medium and centrifuged at 1300 × g for 5 min at room temperature (RT). Pellets were resuspended in 1X HBSS and fixed with paraformaldehyde (PFA) 2% (vol/vol) (final concentration) during 15 min at RT. Fixed cells were cytospun for 3 min at 500 rpm for confocal microscopy investigation.
Cells and Microparticles.
Platelet MPs.
Human and mouse platelets were obtained from citrated blood of healthy human donors under an Institutional Review Board-approved protocol (Centre de Recherche du Centre Hospitalier Universitaire de Québec and Université Laval) (31) and healthy 12- to 15-wk-old mice, respectively. Platelets were isolated after centrifugation of blood (282 × g for 10 min at RT), after which the supernatant (platelet-rich plasma) was centrifuged at 600 × g for 5 min at RT. The supernatant was then centrifuged at 1,300 × g for 5 min at RT, and the pellet containing platelets was resuspended in Tyrode’s buffer (pH 7.4) containing 5 mM calcium. Platelets were counted (Cellometer AutoM10; Nexcelom Bioscience) and adjusted to a density of 100 × 106 cells/mL before stimulation with collagen (0.5 µg/mL; Takeda Austria) for 18 h. When required, platelets were prelabeled with 1 µM CMFDA (green fluorescent) or CMPTX (red fluorescent) (Invitrogen) for 15 min at 37 °C in the dark before stimulation. Contaminating remnant platelets were removed by centrifugation at 1300 × g for 5 min at RT, performed twice. Supernatants containing platelet MPs were centrifuged at 18,000 × g for 90 min at 18 °C. Pellets containing MPs were resuspended in Tyrode’s buffer (pH 7.4) with 5 mM calcium and quantified by flow cytometry using a FACSCanto II equipped with a small particle option (BD Biosciences) as described previously (31). The chosen parameters were optimal to detect polystyrene particles from 100 to 3,500 nm simultaneously on the forward scatter channel coupled to a photomultiplier tube, and all MP preparations were confirmed to contain no trace of platelets (SI Appendix, Fig. S1B).
Human Primary Neutrophils.
Polymorphonuclear neutrophils were isolated from citrated blood of healthy adult volunteers as described previously (72). Cells (density of 5 × 106 cells/mL) were kept in Mg2+-free 1X HBSS with Ca2+ and left unstimulated (native) or primed with TNF (100 U/mL) and GM-CSF (10 ng/mL) (Peprotech) (72), and activated using immune complexes (heat-aggregated IgG, 1 mg/mL final concentration) prepared by heat aggregation of human IgG (25 mg/mL; Sigma-Aldrich) for 1 h at 63 °C. Human neutrophils (2.5 × 105) were labeled with 1 µM CMPTX for 15 min at 37 °C (when required) and then incubated for 2 h at 37 °C with 17.5 × 106 fluorescent MPs (equivalent to 2 µg of proteins) or 70 MPs/neutrophil in a final volume of 50 µL. Thus, MPs (350,000 MPs/µL) were incubated with neutrophils (5 × 106cells/mL) in 50 µL. When the role of sPLA2-IIA on internalization was assessed, the recombinant enzyme (73) or its inactive mutant was added (0.1 µg/mL final concentration) for 1 h on MPs (on ice, to permit association of the enzyme with MPs) before the addition of MPs to neutrophils. In some experiments, the BLT1 receptor antagonist (CP105696, 10 nM; Pfizer Global Research and Development, a generous gift from Dr. Pierre Borgeat, Centre Hospitalier Universitaire de Québec), the BLT2 receptor antagonist (LY255283, 100 nM; Cayman) and lipid mediators 12(S)-HETE (0.1–10 µM), 12(R)-HETE (0.1–10 µM), 12-HHTrE (1 µM), thromboxane B2 (1 µM), and thromboxane A2c (1 µM) (all from Cayman) were added to neutrophils before the addition of MP.
Neutrophil-Like Cell Line.
PLB-985 cells (Deutsche Sammlung von Mikroorganismen und Zellkulturen) were kept at 0.2 × 106 cells/mL in RPMI medium containing 10% FBS and then differentiated into neutrophil-like cells by the addition of 0.3 mM dibutyryl-cAMP (dbAMPc; Sigma-Aldrich) over 3 d. Cells, at a density of 5 × 106 cells/mL, were kept in Mg2+-free 1X HBSS with Ca2+, and labeled with 1 µM CMPTX for 15 min at 37 °C in the dark when indicated. Neutrophil-like cells were primed and activated as described for primary neutrophils.
Confocal Microscopy.
Cell Preparation.
Cells were fixed with 2% (vol/vol) PFA (final concentration) for 15 min at RT and then centrifuged using a cytospin protocol (500 rpm for 3 min at RT). For intracellular CD41 and COUP TF-I detection, cells were permeabilized with 0.5% saponine (Sigma-Aldrich) in 1X PBS twice for 5 min at RT. They were then treated with saturation solution (0.05% saponine, 5% FBS, and 5% horse serum) for 20 min at RT. Fluorescently conjugated markers were used to discriminate surface and intracellular compartments and to distinguish neutrophils and platelet MPs. Neutrophil surfaces were labeled with FITC-conjugated anti-CD66b (1 µg/mL; Beckman Coulter), cytoplasm was labeled with 1 µM CMPTX, and nuclei were labeled with either 1 µg/mL Hoechst 33342 (Invitrogen) or DRAQ5 (1/100; Cell Signaling Technology). When murine neutrophils were isolated from arthritic joints, fixed cells were labeled with Alexa Fluor 488-conjugated anti-GR1 (1.66 µg/µL; BD Bioscience) for 1 h. For experiments using CD66b+ cells purified with magnetic beads (Stemcell Technologies) from the SF of patients with RA, PE-conjugated anti-CD41 (20 µg/mL, (clone M148; Abcam) and FITC-conjugated anti-CD66b were used to label MPs and neutrophils, respectively. The COUP-TF I expression in human cells was determined using antibody against COUP-TF I (1 µg/mL, clone H8132; R&D Systems).
Image Analyses.
To quantitatively assess the localization of fluorescent MPs in vitro, images were processed after cropping individual neutrophils in a XYZ mode. Each MP (green) was analyzed for localization within the neutrophil cytoplasm (red), counted, and classified in either intracellular or surface groups. MP internalization in 100 neutrophils per condition was quantified and repeated at least three times using neutrophils and MPs from different blood donors.
To quantitatively assess localization of fluorescent MPs in vivo and ex vivo (i.e., in arthritic joints), images were also processed after cropping individual neutrophils in XYZ mode. Because MPs internalized in vivo do not display punctate signals (possibly owing to membrane metabolism in the recipient), intracellular fluorescent signals were quantified slightly differently. The fluorescence corresponding to red MPs injected in mice, inside the boundary given by the GR1 membrane labeling, was quantified using velocity software as mean fluorescence intensity (MFI). The specific MP internalization was calculated after substracting the MFI of neutrophils from mice injected with control diluent (PBS).
Lipidomics.
MPs (350,000 MPs/µL, or a total of 2.1 × 108 MPs in 600 μL, equivalent to 23 μg) were incubated in Tyrode’s buffer (without BSA) at 37 °C for 30 min and 6 h in the presence or absence of human recombinant sPLA2-IIA (0.1 µg/mL and 1 µg/mL). EGTA (20 mM) was added to stop the reaction. Then 200 µL of the reaction mixture was mixed with 800 µL of chloroform/methanol (2:1), followed by the addition of 15 µL of internal deuterated standard mixture. Lysophospholipid, fatty acid and eicosanoid analysis by combined liquid chromatography/tandem mass spectrometry was performed as described previously (74, 75).
Statistics.
The number of replicates (n) indicates the number of replicated experiments using cells from n different blood donors. Statistical analyses (t test, ANOVA, Mann–Whitney) were performed using GraphPad Prism version 5.
Supplementary Material
Acknowledgments
We are grateful to Richard Janvier from the microscopy core facility at Laval University. We thank Dr. Maria Fernandes for providing access to her confocal microscopy equipment, and Dr. Caroline Gilbert and Audrey Hubert for providing access to the Zetasizer Nano S. This work was supported by funds from the Canadian Institutes of Health Research (to E.B.), Arthritis Society (to E.B.), Canadian Arthritis Network (to E.B., L.H.B., and A.-C.D.), Fonds de Recherche du Québec en Santé (to E.B. and L.H.B.), Fonds de Recherche des maladies Rhumatismales de l’Université Laval (to A.-C.D.), Canadian Blood Services (to P.P., E.B., and B.L.), CNRS (to G.L.), French National Research Agency (Investments for the Future, Labex SIGNALIFE NR-11-LABX-0028-01, to G.L.), and National Institutes of Health (Grant R37 HL36235, to M.H.G.). E.B. is a recipient of a Canadian Institutes of Health Research New Investigator Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507905112/-/DCSupplemental.
References
- 1.Buzas EI, György B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10(6):356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 2.György B, et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 5.Italiano JE, Jr, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010;17(6):578–584. doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akbiyik F, et al. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104(5):1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- 7.Spinelli SL, et al. Platelets and megakaryocytes contain functional nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2010;30(3):591–598. doi: 10.1161/ATVBAHA.109.197343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis MM, et al. Escaping the nuclear confines: Signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122(3):379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth GJ, Hickey MJ, Chung DW, Hickstein DD. Circulating human blood platelets retain appreciable amounts of poly (A)+ RNA. Biochem Biophys Res Commun. 1989;160(2):705–710. doi: 10.1016/0006-291x(89)92490-x. [DOI] [PubMed] [Google Scholar]
- 10.Landry P, et al. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16(9):961–966. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudreau LH, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14):2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falati S, et al. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197(11):1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arraud N, et al. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12(5):614–627. doi: 10.1111/jth.12554. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta SK, et al. Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009;113(6):1332–1339. doi: 10.1182/blood-2008-07-167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dasgupta SK, Le A, Chavakis T, Rumbaut RE, Thiagarajan P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation. 2012;125(13):1664–1672. doi: 10.1161/CIRCULATIONAHA.111.068833. [DOI] [PubMed] [Google Scholar]
- 16.Laffont B, et al. Activated platelets can deliver mRNA regulatory Ago2•microRNA complexes to endothelial cells via microparticles. Blood. 2013;122(2):253–261. doi: 10.1182/blood-2013-03-492801. [DOI] [PubMed] [Google Scholar]
- 17.Boilard E, Blanco P, Nigrovic PA. Platelets: Active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol. 2012;8(9):534–542. doi: 10.1038/nrrheum.2012.118. [DOI] [PubMed] [Google Scholar]
- 18.Morrell CN, Aggrey AA, Chapman LM, Modjeski KL. Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123(18):2759–2767. doi: 10.1182/blood-2013-11-462432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herter JM, Rossaint J, Zarbock A. Platelets in inflammation and immunity. J Thromb Haemost. 2014;12(11):1764–1775. doi: 10.1111/jth.12730. [DOI] [PubMed] [Google Scholar]
- 20.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 21.Boilard E, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 23.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124(5):710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 24.Wright HL, Moots RJ, Edwards SW. The multifactorial role of neutrophils in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(10):593–601. doi: 10.1038/nrrheum.2014.80. [DOI] [PubMed] [Google Scholar]
- 25.Ottonello L, et al. Synovial fluid from patients with rheumatoid arthritis inhibits neutrophil apoptosis: role of adenosine and proinflammatory cytokines. Rheumatology (Oxford) 2002;41(11):1249–1260. doi: 10.1093/rheumatology/41.11.1249. [DOI] [PubMed] [Google Scholar]
- 26.Chen M, et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203(4):837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 28.Boilard E, et al. A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis. EMBO Mol Med. 2010;2(5):172–187. doi: 10.1002/emmm.201000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fourcade O, et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80(6):919–927. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- 30.Barry OP, Pratico D, Lawson JA, FitzGerald GA. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99(9):2118–2127. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cloutier N, et al. The exposure of autoantigens by microparticles underlies the formation of potent inflammatory components: The microparticle-associated immune complexes. EMBO Mol Med. 2013;5(2):235–249. doi: 10.1002/emmm.201201846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.György B, et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS ONE. 2012;7(11):e49726. doi: 10.1371/journal.pone.0049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rousseau M, et al. Detection and quantification of microparticles from different cellular lineages using flow cytometry: Evaluation of the impact of secreted phospholipase A2 on microparticle assessment. PLoS ONE. 2015;10(1):e0116812. doi: 10.1371/journal.pone.0116812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lotvall J, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boilard E, Bourgoin SG, Bernatchez C, Poubelle PE, Surette ME. Interaction of low molecular weight group IIA phospholipase A2 with apoptotic human T cells: Role of heparan sulfate proteoglycans. FASEB J. 2003;17(9):1068–1080. doi: 10.1096/fj.02-0938com. [DOI] [PubMed] [Google Scholar]
- 36.Boilard E, Bourgoin SG, Bernatchez C, Surette ME. Identification of an autoantigen on the surface of apoptotic human T cells as a new protein interacting with inflammatory group IIA phospholipase A2. Blood. 2003;102(8):2901–2909. doi: 10.1182/blood-2002-12-3702. [DOI] [PubMed] [Google Scholar]
- 37.Birts CN, Barton CH, Wilton DC. A catalytically independent physiological function for human acute phase protein group IIA phospholipase A2: Cellular uptake facilitates cell debris removal. J Biol Chem. 2008;283(8):5034–5045. doi: 10.1074/jbc.M708844200. [DOI] [PubMed] [Google Scholar]
- 38.FitzGerald GA. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2003;2(11):879–890. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- 39.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10(3):181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Donnell VB, Murphy RC, Watson SP. Platelet lipidomics: Modern day perspective on lipid discovery and characterization in platelets. Circ Res. 2014;114(7):1185–1203. doi: 10.1161/CIRCRESAHA.114.301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruf A, et al. Characterization of the thromboxane synthase pathway product 12-oxoheptadeca-5(Z)-8(E)-10(E)-trienoic acid as a thromboxane A2 receptor antagonist with minimal intrinsic activity. Br J Haematol. 1998;101(1):59–65. doi: 10.1046/j.1365-2141.1998.00669.x. [DOI] [PubMed] [Google Scholar]
- 42.Tager AM, Luster AD. BLT1 and BLT2: The leukotriene B(4) receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69(2-3):123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68-69:245–262. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 44.Grass DS, et al. Expression of human group II PLA2 in transgenic mice results in epidermal hyperplasia in the absence of inflammatory infiltrate. J Clin Invest. 1996;97(10):2233–2241. doi: 10.1172/JCI118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wingender E, Schoeps T, Dönitz J. TFClass: An expandable hierarchical classification of human transcription factors. Nucleic Acids Res. 2013;41(Database issue):D165–D170. doi: 10.1093/nar/gks1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou C, et al. The nuclear orphan receptor COUP-TFI is required for differentiation of subplate neurons and guidance of thalamocortical axons. Neuron. 1999;24(4):847–859. doi: 10.1016/s0896-6273(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 47.Risitano A, Beaulieu LM, Vitseva O, Freedman JE. Platelets and platelet-like particles mediate intercellular RNA transfer. Blood. 2012;119(26):6288–6295. doi: 10.1182/blood-2011-12-396440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheloufi S, Dos Santos CO, Chong MM, Hannon GJ. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 2010;465(7298):584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberger CM, et al. miR-451 regulates dendritic cell cytokine responses to influenza infection. J Immunol. 2012;189(12):5965–5975. doi: 10.4049/jimmunol.1201437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Laine VJ, Rajamäki A, Grass DS, Nevalainen TJ. Neutrophil response of transgenic mice expressing human group IIA phospholipase A2 in bacterial infections. Scand J Immunol. 2000;52(4):362–368. doi: 10.1046/j.1365-3083.2000.00797.x. [DOI] [PubMed] [Google Scholar]
- 51.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochim Biophys Acta. 2014;1841(1):108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Tang K, et al. Microparticles mediate enzyme transfer from platelets to mast cells: A new pathway for lipoxin A4 biosynthesis. Biochem Biophys Res Commun. 2010;400(3):432–436. doi: 10.1016/j.bbrc.2010.08.095. [DOI] [PubMed] [Google Scholar]
- 53.Boilard E, et al. Platelets participate in synovitis via Cox-1–dependent synthesis of prostacyclin independently of microparticle generation. J Immunol. 2011;186(7):4361–4366. doi: 10.4049/jimmunol.1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen M, et al. Predominance of cyclooxygenase 1 over cyclooxygenase 2 in the generation of proinflammatory prostaglandins in autoantibody-driven K/BxN serum-transfer arthritis. Arthritis Rheum. 2008;58(5):1354–1365. doi: 10.1002/art.23453. [DOI] [PubMed] [Google Scholar]
- 55.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203(4):829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathis SP, Jala VR, Lee DM, Haribabu B. Nonredundant roles for leukotriene B4 receptors BLT1 and BLT2 in inflammatory arthritis. J Immunol. 2010;185(5):3049–3056. doi: 10.4049/jimmunol.1001031. [DOI] [PubMed] [Google Scholar]
- 57.Coffey MJ, et al. Platelet 12-lipoxygenase activation via glycoprotein VI: Involvement of multiple signaling pathways in agonist control of H(P)ETE synthesis. Circ Res. 2004;94(12):1598–1605. doi: 10.1161/01.RES.0000132281.78948.65. [DOI] [PubMed] [Google Scholar]
- 58.Porro B, Songia P, Squellerio I, Tremoli E, Cavalca V. Analysis, physiological and clinical significance of 12-HETE: A neglected platelet-derived 12-lipoxygenase product. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;964:26–40. doi: 10.1016/j.jchromb.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 59.Tang DG, et al. The lipoxygenase metabolite, 12(S)-HETE, induces a protein kinase C-dependent cytoskeletal rearrangement and retraction of microvascular endothelial cells. Exp Cell Res. 1993;207(2):361–375. doi: 10.1006/excr.1993.1203. [DOI] [PubMed] [Google Scholar]
- 60.González-Núñez D, Claria J, Rivera F, Poch E. Increased levels of 12(S)-HETE in patients with essential hypertension. Hypertension. 2001;37(2):334–338. doi: 10.1161/01.hyp.37.2.334. [DOI] [PubMed] [Google Scholar]
- 61.Connolly JM, Rose DP. Enhanced angiogenesis and growth of 12-lipoxygenase gene-transfected MCF-7 human breast cancer cells in athymic nude mice. Cancer Lett. 1998;132(1-2):107–112. doi: 10.1016/s0304-3835(98)00171-2. [DOI] [PubMed] [Google Scholar]
- 62.Hoyer FF, Albrecht L, Nickenig G, Müller C. Selective inhibition of leukotriene receptor BLT-2 reduces vascular oxidative stress and improves endothelial function in ApoE−/− mice. Mol Cell Biochem. 2012;359(1-2):25–31. doi: 10.1007/s11010-011-0995-y. [DOI] [PubMed] [Google Scholar]
- 63.Sánchez-Galán E, et al. Leukotriene B4 enhances the activity of nuclear factor-kappaB pathway through BLT1 and BLT2 receptors in atherosclerosis. Cardiovasc Res. 2009;81(1):216–225. doi: 10.1093/cvr/cvn277. [DOI] [PubMed] [Google Scholar]
- 64.Cho NK, Joo YC, Wei JD, Park JI, Kim JH. BLT2 is a pro-tumorigenic mediator during cancer progression and a therapeutic target for anti-cancer drug development. Am J Cancer Res. 2013;3(4):347–355. [PMC free article] [PubMed] [Google Scholar]
- 65.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2: A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192(3):421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lundeen KA, Sun B, Karlsson L, Fourie AM. Leukotriene B4 receptors BLT1 and BLT2: Expression and function in human and murine mast cells. J Immunol. 2006;177(5):3439–3447. doi: 10.4049/jimmunol.177.5.3439. [DOI] [PubMed] [Google Scholar]
- 67.Qiu H, et al. Differential induction of BLT receptor expression on human endothelial cells by lipopolysaccharide, cytokines, and leukotriene B4. Proc Natl Acad Sci USA. 2006;103(18):6913–6918. doi: 10.1073/pnas.0602208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hussain H, et al. Epidermis contains platelet-type 12-lipoxygenase that is overexpressed in germinal layer keratinocytes in psoriasis. Am J Physiol. 1994;266(1 Pt 1):C243–C253. doi: 10.1152/ajpcell.1994.266.1.C243. [DOI] [PubMed] [Google Scholar]
- 69.Liagre B, Vergne P, Rigaud M, Beneytout JL. Expression of arachidonate platelet-type 12-lipoxygenase in human rheumatoid arthritis type B synoviocytes. FEBS Lett. 1997;414(1):159–164. doi: 10.1016/s0014-5793(97)00904-6. [DOI] [PubMed] [Google Scholar]
- 70.Giera M, et al. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim Biophys Acta. 2012;1821(11):1415–1424. doi: 10.1016/j.bbalip.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson EN, Brass LF, Funk CD. Increased platelet sensitivity to ADP in mice lacking platelet-type 12-lipoxygenase. Proc Natl Acad Sci USA. 1998;95(6):3100–3105. doi: 10.1073/pnas.95.6.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flamand N, Lefebvre J, Surette ME, Picard S, Borgeat P. Arachidonic acid regulates the translocation of 5-lipoxygenase to the nuclear membranes in human neutrophils. J Biol Chem. 2006;281(1):129–136. doi: 10.1074/jbc.M506513200. [DOI] [PubMed] [Google Scholar]
- 73.Singer AG, et al. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem. 2002;277(50):48535–48549. doi: 10.1074/jbc.M205855200. [DOI] [PubMed] [Google Scholar]
- 74.Bollinger JG, Ii H, Sadilek M, Gelb MH. Improved method for the quantification of lysophospholipids including enol ether species by liquid chromatography-tandem mass spectrometry. J Lipid Res. 2010;51(2):440–447. doi: 10.1194/jlr.D000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bollinger JG, et al. Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal Chem. 2010;82(16):6790–6796. doi: 10.1021/ac100720p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.