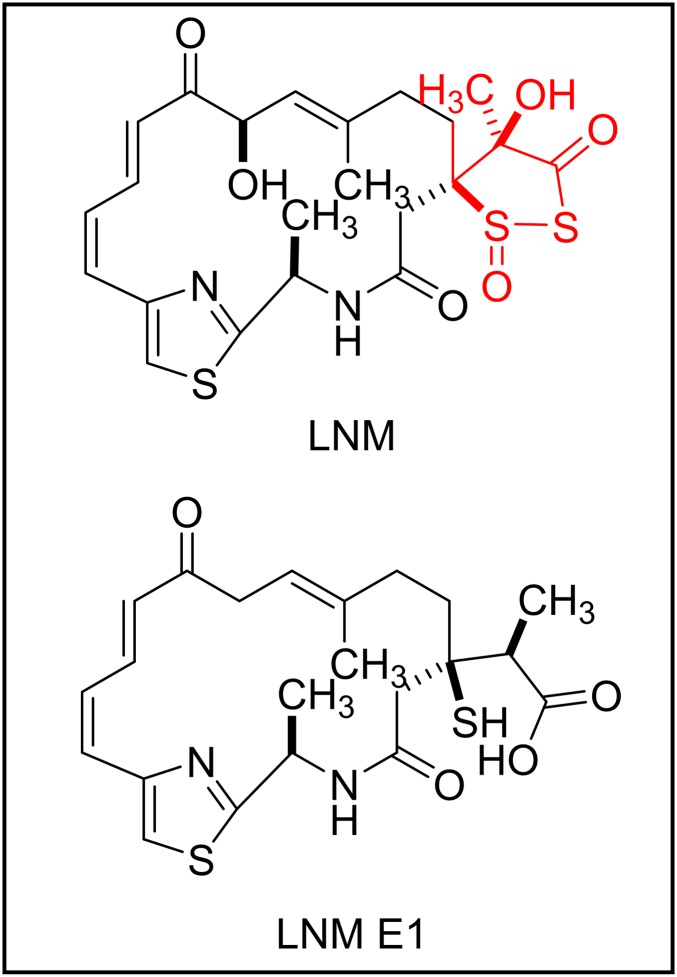
It would be an understatement to suggest that natural products, complex molecules derived from natural sources, have been influential to modern chemistry. Efforts to isolate and characterize these compounds have driven innovations in chromatography, crystallography, and NMR. Attempts to recreate the oftentimes beautiful chemical structures found in nature have been of particular importance to organic chemistry. One notable example is the total synthesis of vitamin B12 by Woodward and Eschenmoser, which beyond demonstrating nearly 100 chemical transformations that could be applied to other systems, also inspired the collaboration between Woodward and Hoffman that led to the formalization of the Woodward–Hoffman rules, allowing the prediction of stereospecificity of ring opening and closing reactions (1–3). Biological chemistry has greatly benefited from the isolation and characterization of toxins (e.g., like tetrodotoxin) and antibiotics (penicillin), as well as from natural products like rapamycin, which led to the discovery of its biological target, mammalian target of rapamycin, one of the most important regulatory protein complexes in mammalian cells (4, 5). Indeed, the Nobel prize for chemistry has often been awarded to natural products chemists, including the second ever award, granted in 1902 to Hermann Fischer for work on sugar and purine synthesis, the 1964 award, granted to Dorothy Hodgkin for her crystal structure of vitamin B12, and the 1990 award, granted to E. J. Corey, whose laboratory has pioneered the development of a number of chemical reactions, which are now being applied toward the pursuit of the total synthesis of assorted natural products (6). As part of this legacy of natural products science, Huang et al. (7) have recently published in PNAS work detailing part of the biosynthetic pathways that create the natural macrocycle leinamycin (LNM) (Fig. 1, Upper), as well as the properties of a natural precursor of LNM, which they call leinamycin E1 (LNM E1) (Fig. 1, Lower).
Fig. 1.
Chemical structures of LNM and LNM E1. The unique dithiolane ring of LNM is colored red.
Principal investigator Ben Shen and his laboratory and collaborators have been engaged in research surrounding LNM for over a decade, and have been instrumental in characterizing the proteins responsible for LNM biosynthesis, clearly demonstrating their great interest in the compound. In considering what makes this molecule important, the first two lines of Huang et al.’s introductory paragraphs are instructive: “Leinamycin (LNM) … is an antitumor antibiotic produced by Streptomyces atroolivaceus S-140. It features an unusual 1,3-dioxo-1,2-dithiolane moiety that is spiro-fused to a thiazole-containing 18-membered lactam ring, a molecular architecture not found to date in any other natural product” (7). That is to say: LNM simultaneously satisfies a number of the previously considered benefits of natural products research. It not only has a biological activity, but a potentially very useful one: it is an anticancer agent. In fact, LNM has been shown to inhibit the proliferation of cancers that are resistant to drugs currently used in the clinic, such as cisplatin, making it a valuable lead drug compound. It is a potent antibiotic as well. In addition, LNM is chemically beautiful and features a spirocycle not currently known anywhere else in nature. This uniqueness suggests the potential not only to develop new synthetic methodology, but also to investigate functionality that might be of use in other pharmacological applications. Importantly, the molecule is made in Streptomyces atroolivaceus, a bacterium. Although a number of valuable natural products have been isolated from plants and animals, which require considerable efforts and resources to grow and harvest, bacteria are easily handled and can be cultured in huge quantities in relatively small spaces.
Huang et al. (7) did not investigate LNM via typical organic synthesis approaches. Rather, the researchers have been working to characterize the biosynthetic apparatus responsible for LNM, and thus identified a gene cluster responsible for LNM synthesis composed of 27 ORFs (8, 9). Of these ORFs, they have characterized the roles of 9 encoded proteins, leaving up to 18 potential proteins with functions that cannot be easily predicted. Critically, the nine characterized proteins alone could not account fully for LNM synthesis. As such, the group took the approach of systematically inactivating each of the 18 uncharacterized ORFs, and analyzing the resulting levels of LNM metabolites. The deletion of one of these genes, lnmE, resulted in the accumulation of LNM E1 and turned out to prevent the cyclization of the unique 1,3-dioxo-1,2-dithiolane moiety of LNM. The precursor LNM E1 also requires oxidation at two carbon centers to install the hydroxyl groups present in LNM.
The discovery and preliminary characterization of lnmE alone is, in itself, likely of great interest. Here, we have a 10th critical protein involved in the manufacture of a single natural product (LNM), and the strong possibility that it is a gatekeeper to future synthetic steps and that additional proteins are directly involved in LNM biosynthesis (consider that this is already a full 10% of the steps needed by Woodward and Eschenmoser to prepare vitamin B12 without the aid of exquisitely fine-tuned enzymatic machinery, or compare this to the citric acid cycle, which uses just eight enzymes to form the core of cellular metabolism). Given the nature of the chemistry, such that a unique ring system is no longer being formed, it is possible that this 10th protein has a function never before observed in nature. However, beyond this, and serendipitously, LNM E1 turned out to have a very interesting property: it is less toxic to healthy cells—but potentially more toxic to cancerous cells—than the parent compound, LNM. LNM undergoes reductive activation when exposed to thiols, which breaks open its dithiolane ring to generate a reactive center, resulting in an episulfonium ion-generated DNA alkylation, with the resulting DNA damage eventually killing the cell. LNM E1 needs to form a similar reactive center to damage DNA, but it lacks the dithiolane ring, and as such cannot use the same activation mechanism. Huang et al. (7) show that, instead, LNM E1 is activated oxidatively upon exposure to reactive oxygen species (ROS). Beyond the curiosity of the precursor being activated via an opposite but analogous mechanism to the final product, why is this important? Cancer cells often have very high levels of ROS relative to healthy cells, and indeed Huang et al. were able to show that LNM E1 was most toxic to cancer cell lines that are being stressed with high amounts of ROS. Thus, unlike the parent compound, LNM E1 is effectively a targeted therapy; although it would be expected to access all cells, it should only activate in cells with high ROS, such as cancer cells.
This work (7) poses as many new questions as it answers, some of which are more obvious than others. Most directly related to the study, we are left to wonder: What are the roles of the other proteins encoded in the lnm gene cluster? How many total proteins are involved in the biosynthesis of leinamycin? Are these proteins involved in any other biosynthetic pathways? How are they regulated? Do the antibiotic properties of LNM E1 differ from those of LNM? And, perhaps most importantly, is LNM E1 a good candidate for pharmaceutical intervention? Being produced by a bacterium, can it be harvested
Huang et al. have, through an elegant series of genetic manipulations, mass spectrometry studies, NMR spectra, and cellular growth assays, substantially expanded our understanding of the biology and chemistry surrounding LNM.
in sufficient quantity and purity to disseminate to a patient population? Only one total synthesis of LNM has been reported to date, by Kanda and Fukuyama, and it does not include the biological intermediate in its route (10). Can a total synthesis be adapted to create LNM E1? Moreover, will further testing of LNM E1 show that it is, in fact, comparable or superior to other anticancer drugs that have already undergone rigorous examination? LNM E1’s activity has been shown to be modulated by ROS, but an external agent was required to stimulate sufficient ROS production in the cancer cells tested to generate the active drug species. Will intact tumors, compared with established cell lines, generate sufficient ROS to activate the drug, or will cotreatment with additional agents continue to be required to make it functional in vivo?
Of course, perhaps the most interesting questions pertain to the uniqueness of LNM itself. Although it is not unheard of for a large number of proteins to be involved in a biosynthetic pathway, it is not necessarily common either. Indeed, enzymatic reactions are often shown to organic chemistry students alongside the rather more tedious and complex procedures they must follow themselves to achieve identical products from identical starting materials. Furthermore, LNM is unique, by virtue of its unusual dithiolane spirocycle, and it has only been found in Streptomyces bacteria species (11). Why has no other organism developed an antibiotic with this sort of scaffold? Given the other potent antibacterial natural products that have been discovered, none of which feature the critical ring, is it possible that LNM truly serves some other biological function, such that both the anticancer and antibacterial properties are mere byproducts?
Huang et al. (7) have, through an elegant series of genetic manipulations, mass spectrometry studies, NMR spectra, and cellular growth assays, substantially expanded our understanding of the biology and chemistry surrounding LNM. This has implications to both the basic and applied research communities, as well as the pharmaceutical industry. It will be especially interesting to see how the latter exploits this new information.
Footnotes
The authors declare no conflict of interest.
See companion article on page 8278.
References
- 1.Eschenmoser A, Wintner CE. Natural product synthesis and vitamin B12. Science. 1977;196(4297):1410–1420. doi: 10.1126/science.867037. [DOI] [PubMed] [Google Scholar]
- 2.Woodward RB. The total synthesis of vitamin B 12. Pure Appl Chem. 1973;33(1):145–177. doi: 10.1351/pac197333010145. [DOI] [PubMed] [Google Scholar]
- 3.Woodward RB, Hoffmann R. Stereochemistry of electrocyclic reactions. J Am Chem Soc. 1965;87(2):395–397. [Google Scholar]
- 4.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253(5022):905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 5.Vézina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot (Tokyo) 1975;28(10):721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- 6.nobelprize.org 2014 All Nobel Prizes in Chemistry. Available at: www.nobelprize.org/nobel_prizes/chemistry/laureates. Accessed May 26, 2015.
- 7.Huang S-X, et al. Leinamycin E1 acting as an anticancer prodrug activated by reactive oxygen species. Proc Natl Acad Sci USA. 2015;112:8278–8283. doi: 10.1073/pnas.1506761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang GL, Cheng YQ, Shen B. Leinamycin biosynthesis revealing unprecedented architectural complexity for a hybrid polyketide synthase and nonribosomal peptide synthetase. Chem Biol. 2004;11(1):33–45. doi: 10.1016/j.chembiol.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Cheng YQ, Tang GL, Shen B. Identification and localization of the gene cluster encoding biosynthesis of the antitumor macrolactam leinamycin in Streptomyces atroolivaceus S-140. J Bacteriol. 2002;184(24):7013–7024. doi: 10.1128/JB.184.24.7013-7024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanda Y, Fukuyama T. Total synthesis of (+)-leinamycin. J Am Chem Soc. 1993;115(18):8451–8452. [Google Scholar]
- 11.Hara M, et al. Leinamycin, a new antitumor antibiotic from Streptomyces: Producing organism, fermentation and isolation. J Antibiot (Tokyo) 1989;42(12):1768–1774. doi: 10.7164/antibiotics.42.1768. [DOI] [PubMed] [Google Scholar]