Abstract
Acute kidney injury (AKI) has diverse causes and is associated with increased mortality and morbidity. In less developed countries (LDC), nephrotoxic AKI (ToxAKI) is common and mainly due to deliberate ingestion of nephrotoxic pesticides, toxic plants or to snake envenomation. ToxAKI shares some pathophysiological pathways with the much more intensively studied ischaemic AKI, but in contrast to ischaemic AKI, most victims are young, previously healthy adults. Diagnosis of AKI is currently based on a rise in serum creatinine. However this may delay diagnosis because of the kinetics of creatinine. Baseline creatinine values are also rarely available in LDC. Novel renal injury biomarkers offer a way forward because they usually increase more rapidly in AKI and are normally regarded as absent or very low in concentration, thereby reducing the need for a baseline estimate. This should increase sensitivity and speed of diagnosis. Specificity should also be increased for urine biomarkers since many originate from the renal tubular epithelium. Earlier diagnosis of ToxAKI should allow earlier initiation of appropriate therapy. However, translation of novel biomarkers of ToxAKI into clinical practice requires better understanding of non-renal factors in poisoning that alter biomarkers and the influence of dose of nephrotoxin on biomarker performance. Further issues are establishing LDC population-based normal ranges and assessing sampling and analytical parameters for low resource settings. The potential role of renal biomarkers in exploring ToxAKI aetiologies for chronic kidney disease of unknown origin (CKDu) is a high research priority in LDC. Therefore, developing more sensitive biomarkers for early diagnosis of nephrotoxicity is a critical step to making progress against AKI and CKDu in the developing world.
Keywords: acute kidney injury, biomarkers, envenomation, nephrotoxicity, pesticide poisoning, pharmacokinetics
Introduction
Nephrotoxic acute kidney injury (ToxAKI) is much more common in less developed countries (LDC) and is mainly due to poisoning with chemicals, plants and snake envenomation. ToxAKI associated with these toxic chemicals and toxins is an important clinical problem that predominantly affects healthy, economically active young adults 1–3. Many chemicals, drugs and toxins potentially cause nephrotoxic injury since kidneys often concentrate substances to much higher levels than seen in blood 4. Although ToxAKI may occur as an adverse event following therapeutic drug ingestion in LDC, this is under reported. Nephrotoxicity can be associated with commonly used therapeutic drugs and the current evidence for using renal biomarkers in such settings are described elsewhere 5. The most common causes of ToxAKI reported in LDC follow self-poisoning with pesticides [paraquat, glyphosate surfactant herbicide, propanil, 2-methyl-4-chlorophenoxyacetic acid (MCPA), aluminium phosphide (AlP)], other chemicals (paraphenylene-diamine dye, potassium permanganate and oxalic acid) and toxic plants (Gloriosa superba, Cleistanthus collinus, Callilepis laureola, poisonous mushrooms, herbal medicines and Euphorbia matabalensis) as well as snake envenomation (Russell's viper, hump-nosed pit viper, saw-scaled vipers, green pit viper, sea snakes, Bothrops and Crotalus species). These will be reviewed briefly before discussing potential roles of biomarkers of AKI in diagnosis, prognosis and monitoring of nephrotoxicity.
Typical causes of nephrotoxic AKI in LDC
The most common reported causes of ToxAKI in LDC are summarized in Table1. Deliberate self-poisoning with pesticides is common throughout the LDC of much of Asia and the Pacific region 1. Paraquat poisoning is very widely reported and has a mortality of 40–50% 6. Early AKI is very clearly linked to a poor prognosis and fatal outcome 7–9. Glyphosate herbicide poisoning is also common with an estimated mortality of 2% to 30% 10,11 with renal toxicity and acidosis the most prominent clinical features 10,11. Selective herbicides such as propanil and chlorphenoxy-herbicides [e.g. 2-methyl-4-chlorophenoxyacetic acid (MCPA)] are less widely reported but also commonly induce AKI with a reported case fatality of 11% 12 and 5% 13–15, respectively. The surfactants used in all herbicide preparations may be an important contributory cause for ToxAKI. ToxAKI is a less prominent feature of most insecticide poisonings (these generally have neurotoxic actions). However, aluminium phosphide ingestion is common throughout Northern India and Iran and this commonly leads to ToxAKI which also predicts a fatal outcome 16,17. There are geographically constrained clusters of many other plant and chemical poisonings, where a local culture of ingesting specific agents has developed and many of these cause ToxAKI. For example, over the last decade deliberate ingestion of a washing powder containing potassium permanganate and oxalic acid has been common in southern Sri Lanka and the majority ingesting these compounds develop AKI 18. Deliberate self-poisoning with poisonous plants that cause ToxAKI also occurs in clusters, for example Gloriosa superba in parts of Southern India and Sri Lanka 19,20 and Cleistanthus collinus 21,22 in Southern India.
Common causes of ToxAKI following poisoning or snake envenomation in LDC
| Most common cause | Case fatality | AKI incidence | References |
|---|---|---|---|
| Paraquat | 40–50% | >50% | 6,8,9 |
| Glyphosate | 2–30% | 15–25%* | 10,11,77 |
| Propanil | 11% | 8%* | 12 |
| Chlorphenoxy herbicides (MCPA) | 5% | 4%* | 13–15, 159 |
| Paraphenylene-diamine dye | 20–30% | 10–50% | 26,160,161 |
| Aluminium phosphide | 10–40% | 15–25% | 17,162 |
| Oxalic acid + potassium permanganate | 5–25% | 55%* | 18 |
| Gloriosa superba | 6% | 25%* | |
| Cleistanthus collinus | 30% | 15% | 21,22 |
| Herbal medicines (Aloe capensis, Aristolochia clematitis, Callilepis laureola, Catha edulis, Colchicum autumnale, Euphorbia matabalensis, Securidacea longepedunculata) | –† | –† | 26,163 |
| Snake envenoming‡ | |||
| Russell's viper | 1–20 % | 10–40% | 23, 164–166 |
| Hump-nosed pit viper | <1% | 5–10% | 65,167,168 |
| Saw-scaled vipers | <1% | 2–4% | 25,169,170 |
| Bothrops | 1% | 1.6%–38.5% | 32,171 |
| Crotalus species | 2% | 10%–29% | 32 |
Unpublished data obtained from South Asian Clinical Toxicology Research Collaboration database.
Regional and species specific variation; only selected species with data on AKI incidence are listed.
ToxAKI is also common following envenomation in South and South East Asia and the Middle East. The most commonly reported snakes from this region causing ToxAKI are Russell's viper (Daboia species) 3,23, the hump-nosed pit viper (Hypnale species) 3,24, saw-scaled vipers (Echis species) 3,25, green pit vipers (Trimeresurus albolabris) 3 and sea snakes (Hydrophiinae species) 3.
Poisoning with pesticides, chemicals, toxic plants, herbal medicines and envenomation following snakes bites also contributes to the majority of AKI in the African region 26,27. Ingestion of a hair dye containing paraphenylene-diamine contributes to much of the ToxAKI in this region 26 with an estimated mortality of >30%. Interestingly, paraphenylene-diamine poisoning is also reported from the Middle-East region and in India 28. Ingestion of many toxic plants, namely Callilepis laureola, poisonous mushrooms, Euphorbia matabalensis and herbal medicines, also contributes to the high incidence of ToxAKI in Africa 26,29,30.
Nephrotoxicity following snake envenomation is a major causative factor for ToxAKI in the Latin American region 31,32. Bothrops and Crotalus species contribute to most ToxAKI incidents in this region 32 and AKI is a risk factor for death following envenomation 33. Less commonly, exposure to pesticides and plants causes ToxAKI in this region 31,34,35.
Pathophysiology of nephrotoxic AKI
Most of the detailed pathophysiological and biomarker studies on AKI in both animals and humans have examined ischaemia-reperfusion injury 36. It is important to highlight that the pathophysiology of ToxAKI following various nephrotoxic agents, is not only often poorly characterized, but will vary markedly in the extent to which they share similar pathways.
Many different pathophysiological mechanisms overlap to generate renal injury after ischaemia-reperfusion injury. Epithelial damage includes loss of apical brush border, loss of integrity of the F-actin cytoskeletal system, and cell–cell contact, detachment of cells from the extracellular matrix, translocation of Na+/K+-ATPase pumps, flattening and loss of epithelial cell polarity and epithelial cell death by necrosis or apoptosis 36,37. Tubular obstruction 38 and production of excessive reactive oxygen species (hydrogen peroxide and superoxide) leading to oxidative stress 36 are salient features in epithelial injury. Endothelial damage due to ischaemia is also common, resulting in microvascular dysregulation 36,39,40. Ischaemic AKI causes over-expression of a variety of endothelial molecules [adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1(VCAM), P and E selectin and β2-intergrins], that enhance leucocyte activity and provoke inflammation with increased release of reactive oxygen species, interleukins, tissue necrosis factor-α (TNF-α), chemokines, vasoconstrictors, prostaglandins, leukotrienes, thromboxanes, nitric oxide, endothelin and monocyte chemoattractant protein-1 (MCP-1) 36, 39–41. Microvascular congestion and trapping of red blood cells contribute to early reduction in renal blood flow and glomerular filtration rate (GFR). An increase in microvascular permeability also contributes to injury 39.
The same overlapping mechanisms are involved in ToxAKI following exposure to common nephrotoxins 41 although the primary insult may reflect altered renal haemodynamics, direct injury to the tubular cells, tubular obstruction to urine flow, inflammation of the interstitium and thrombotic microangiopathy 4. Mechanisms of nephrotoxicity associated with the most common chemicals and natural toxins are summarized in Table2.
Table 2.
Suggested primary mechanisms of nephrotoxicity for common nephrotoxins in LDC
| Primary mechanisms | Agent | Other mechanisms |
|---|---|---|
| Oxidative stress (generation of ROS) | Paraquat 42,43 | inflammation, depletion of NADPH, mitochondrial damage, macromolecular oxidation |
| Aluminum phosphide 16 | lipid peroxidation, mitochondrial toxicity, tissue hypoxia | |
| Potassium permanganate 45,46 | methaemoglobinaemia | |
| Uncoupling of oxidative phosphorylation | Glyphosate 10, 48–50 | mitochondrial toxicity, decreased protein synthesis, caspase 3 activation, lipid peroxidation, DNA fragmentation. |
| MCPA 13,51 | cell membrane damage, increased coagulation. | |
| Methaemoglobinaemia | Propanil 54,55 | direct cytotoxicity |
| Rhabdomyolysis | Paraphenylene-diamine dye 56 | direct nephrotoxicity, hypoxia, hypovolaemia |
| Sea-snake (Hydrophiinae) 3,67,68 | direct nephrotoxicity, renal vasoconstriction | |
| Crotalus species 32,33 | renal vasoconstriction, direct nephrotoxicity, sepsis, ischaemia, intravascular coagulation | |
| Inhibition of mitosis and cell division | Glory lily (Gloriosa superba) 60,61 | rhabdomyolysis |
| Inhibition of vacuolar H+ATPase | Cleistanthus collinus 62 | depletion of glutathione and ATPase |
| Ischaemia-hypoperfusion | Russell's viper (Daboia russelii) 63,64 | disseminated intravascular coagulation, and haemolysis, direct nephrotoxicity, mitochondrial damage |
| Hump-nosed viper (Hypnale hypnale) 24,65,66 | direct nephrotoxicity, disseminated intravascular coagulation | |
| Bothrops species 32,33 | haemoglobinuria, coagulation, direct nephrotoxicity, thrombotic microangiopathy |
Pesticides and natural toxins have a very diverse range of dose-dependent mechanisms leading to ToxAKI. To illustrate this, we will discuss in more detail the primary mechanisms behind renal toxicity from the most thoroughly studied agents (Table2 and Figure1). Paraquat is an exemplar of agents causing oxidative stress; it directly generates reactive oxygen species and depletes NADPH. This secondarily leads to mitochondrial damage, lipid peroxidation, oxidation of proteins, carbohydrates, DNA and sulphide groups, and inflammation 42,43. Paraquat-induced tubular damage mostly involves proximal tubules, with loss of brush border, thickening of the basement membrane, coagulation of epithelial cells, necrosis of different nephron segments, interstitial haemorrhage and deposition of collagen in the interstitial space 44. Potassium permanganate similarly causes oxidative stress by inducing ROS production catalyzed by the manganate anion, but may also induce tubular hypoxia secondary to methaemoglobinaemia 45,46.
Figure 1.
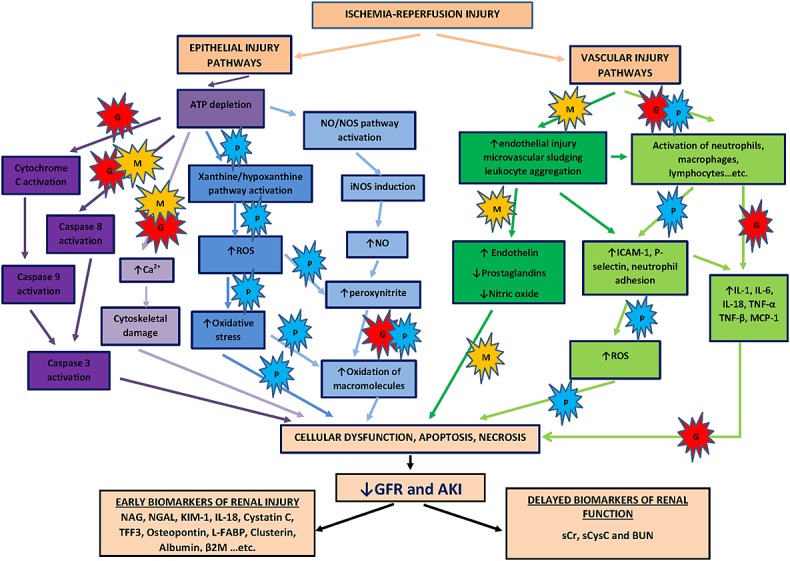
Overlapping mechanisms of pesticide and ischaemia-reperfusion renal injury. Ischaemia-reperfusion induces multiple injury pathways in renal epithelial and endothelial cells as well as inflammation leading ultimately to AKI 36–40. Novel biomarkers appear earlier than functional markers in response to mild injury. Nephrotoxic pesticides cause toxicity through a similarly diverse range of injury pathways which may overlap with those of the ischaemia-reperfusion injury cascade. This figure suggests speculative primary targets for the commonly studied pesticides, paraquat (P), glyphosate (G) and MCPA (M). The herbicide, MCPA, induces caspase pathway as well as increase thromboxane A2 activity which subsequently leads to microvascular congestion and endothelial injury in addition to uncoupling of oxidative phosphorylation 13,51. ToxAKI following paraquat is mainly attributed to increase ROS generation and inflammation 42,43,52, while glyphosate surfactant causes ATP depletion via uncoupling of oxidative phosphorylation, cytochrome C activation and macromolecular oxidation 10, 48–50. ROS, reactive oxygen species; NO, nitric oxide; NOS, nitric oxide synthase; iNOS, inducible nitric oxide synthase
Glyphosate surfactant herbicide is a good example of an agent that uncouples mitochondrial oxidative phosphorylation 10, causing ATP depletion and cell death. The amine surfactants are believed largely responsible for damaging the mitochondrial membrane. This leads to increased but inefficient utilization of energy substrates as the proton gradient required for efficient ATP production in mitochondria is lost. The surfactant used in glyphosate formulation also causes cytotoxicity by decreasing protein synthesis and disrupting other cellular metabolic pathways 47. Other possible mechanisms of toxicity investigated in in vitro and in vivo models include induction of apoptosis by inducing caspase 3 48, DNA damage 49 and lipid peroxidation 50. Chlorphenoxy herbicides such as MCPA toxicity also cause dose-dependent membrane damage 13 and uncoupling of oxidative phosphorylation 51, but also inhibition of platelet aggregation and thromboxane production 52.
Propanil poisoning causes tissue hypoxia, secondary to severe methaemoglobinaemia and thus might be expected to resemble closely ischaemic injury 53. However, direct renal cell cytotoxicity due to propanil has also been demonstrated in in vitro studies 54,55.
Many agents have complex poorly understood mechanisms. Aluminium phosphide inhibits cytochrome C oxidase and cellular respiration, but also increases formation of reactive oxygen species and causes lipid peroxidation 16. Paraphenylene-diamine dyes cause both severe rhabdomyolysis and direct nephrotoxicity 56.
There are many and varied co-formulants (mostly surface active agents, solvents, colourants etc.), which lack pesticide activity but are constituted in pesticide formulations for adjunctive agricultural purposes (e.g. to enhance mixing or spread). However, these agents can have considerable toxicity when ingested. Unfortunately, toxicity of these co-formulants is much less studied and is not generally part of toxicity classification. This is often highlighted for glyphosate surfactant formulations10,57 but it is apparent that toxicity of other herbicides 58 and insecticides may also be altered by co-formulants 59.
Colchicine in Gloriosa inhibits cell division and proliferation by inhibiting spindle formation and disrupting microtubule self-assembly by binding to tubulin. Colchicine may also induce rhabdomyolysis, shock and hypoxia which further aggravate AKI 60,61. Glycosides in Cleistanthus inhibit vacuolar H+ATPase in the renal tubular brush border membrane. These glycosides also deplete glutathione and ATPases in renal cells which may cause oxidation of thiol groups resulting in cellular injury 21,62.
Snake venoms contain hundreds of active compounds and the mechanisms of ToxAKI from snake bite are as a rule complex and poorly defined. For example, Russell's viper can cause direct venom-induced nephrotoxicity, but also renal hypoperfusion, intravascular haemolysis, rhabdomyolysis, myoglobinuria and disseminated intravascular coagulation (DIC) 63,64. Similarly, the hump-nosed viper (Hypnale species) causes thrombotic microangiopathy, direct nephrotoxicity, intravascular coagulation, hypotension and haemolysis 65,66. Rhabdomyolysis, direct nephrotoxicity and hypoxia are also described mechanisms of renal toxicity following Hydrophiinae 3,67,68, Bothrops 32,33 and Crotalus 32,33 snake envenomation.
Biomarkers in ToxAKI: general considerations
ToxAKI may be detected by biomarkers of renal function or renal cellular damage or by some combinations of both. Change in glomerular filtration rate (GFR) is usually detected indirectly by an increase in serum creatinine, which is thus the key functional biomarker in current consensus definitions. A significant increase in creatinine usually only occurs after substantial renal damage and can be due to non-renal factors. Any reduction in GFR leads to an increase in creatinine that requires three half-lives to reach a new equilibrium from which GFR can be estimated. Since the half-life of serum creatinine is approximately 4 h when GFR is normal, reliance on serum creatinine delays diagnosis in practice by up to 72 h particularly after modest injury 69. The delay, low sensitivity and specificity, and the need for a baseline value, all limit diagnostic utility in clinical practice and clinical trial recruitment. These in turn have hampered the development of new treatment strategies for AKI 70,71.
Because of these inherent limitations of serum creatinine, alternative biomarkers are being sought to detect AKI. Many of the novel biomarkers of renal cell injury that have been discovered are usually absent or in very low concentration, which should improve sensitivity, allow more rapid diagnosis and eliminate uncertainty when there is no baseline value. Specificity is improved for biomarkers that arise only from the renal tubular epithelium, and this has stimulated research on urinary biomarkers. The potential for novel biomarkers to facilitate early detection of AKI stimulated the Food and Drug Administration (FDA), European Medicines Agency (EMA) and the pharmaceutical industry to form a coalition to develop strategies for validating biomarkers in preclinical drug evaluation and translational clinical studies 72. A panel of urinary biomarkers including kidney injury molecule-1 (uKIM-1), albumin (uAlb), β2-miroglobulin (uβ2M), cystatin C (uCysC), clusterin (uClu) and trefoil factor-3 (uTFF3) have been qualified for assessment of nephrotoxicity in pre-clinical studies 72 but have rarely been studied following clinical ToxAKI.
Biomarkers of cellular damage usually appear earlier than change is detected by serum creatinine. Damage biomarkers may be localized to a specific nephron segment or may identify a mechanism of injury. For example, proximal tubular brush border proteins, such as gamma glutamyl transpeptidase, will be released upon injury to that segment, while pi-glutathione-s-transferase will be released following damage to the distal tubule 73. Alternatively, injury biomarkers may reflect mechanisms of injury such as oxidative stress, inflammation, apoptosis and necrosis, which are not segment or cell specific. For example, the product of caspase-1, interleukin-18 (IL-18), will increase with inflammation as well as apoptotic renal cell injury 40,70. Many novel injury urinary biomarkers including urinary neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), IL-18, liver-type fatty acid binding protein (L-FABP), clusterin and cystatin C have all shown high specificity and sensitivity in diagnosing AKI earlier than serum creatinine 70,71. However, this has been evaluated largely in the setting of ischaemic AKI, with application in ToxAKI limited to a few clinical studies and a few novel biomarkers 4,5,71 (Table3].
Table 3.
| Biomarker | Site of injury | Preclinical models | Human studies |
|---|---|---|---|
| N-acetyl-β-D-glucosaminidase (NAG) | Proximal tubular injury (brush boarder enzyme) | Cadmium, cisplatin, puromycin, aminonucleoside, gentamicin carboplatin, cyclosporine, doxorubicin | Russell's viper bite 75, tobramycin 172,173, gentamicin 174,175 |
| Albumin | Proximal tubular injury (reduced reabsorption); glomerular injury (leakage) | Cisplatin, gentamicin, carbapenem, cyclosporine A, adriamycin, puromycin aminonucleoside, carboplatin, doxorubicin, bromoethylamine, paraquat 176, MCPA 177, glyphosate 178 | Cisplatin 179, ifosfamide, methotrexate, gentamicin 175,180, contrast medium 181 |
| β2-microglobulin | Proximal tubular injury (reduced reabsorption); glomerular injury (leakage) | Cadmium, cisplatin, puromycin aminonucleoside, carboplatin, gentamicin, cyclosporine, doxorubicin, bromoethylamine, contrast medium, paraquat 176, MCPA 177, glyphosate 178 | Gentamicin and other aminoglycosides 172,182, vancomycin, contrast medium |
| Cystatin C | Proximal tubular injury (reduced reabsorption); glomerular injury (leakage) | Cisplatin, puromycin aminonucleoside, carboplatin, gentamicin, cyclosporine, doxorubicin, bromoethylamine, paraquat, MCPA 177, glyphosate 178 | Gentamicin 180, cisplatin 179,183,184, contrast 185,186, paraquat poisoning 9 |
| Interleukin-18 (IL-18) | Inflammatory cytokine in proximal tubular injury. | Contrast medium, cisplatin | Contrast medium 187, cisplatin 184 |
| Kidney injury molecule (KIM-1) | Specific to proximal tubular injury; secreted in large quantities by dedifferentiated tubular cells | Cisplatin, cyclosporine, gentamicin, cadmium, contrast medium, vancomycin carboplatin, doxorubicin, bromoethylamine, paraquat 176, MCPA 177, glyphosate 178 | Paraquat poisoning 7, gentamicin 174,180, cisplatin 151,184 |
| Neutrophil gelatinase-associated lipocalin (NGAL) | Both proximal and distal tubular injury. Inflammatory biomarker and scavengers of catalytic ions. | Cisplatin, contrast medium, puromycin aminonucleoside, carboplatin, gentamicin, ciclosporin, doxorubicin, bromoethylamine, paraquat 176, MCPA 177, glyphosate 178 | Paraquat poisoning 7,9, contrast medium 185,188, gentamicin 174,180, cisplatin 179,184,189 |
| Liver-type fatty acid binding protein (L-FABP) | Proximal tubular injury | Contrast medium, cisplatin | Contrast medium 190, aminoglycosides 191, |
| Clusterin | Both proximal and distal tubular injury | Cisplatin, gentamicin, puromycin aminonucleoside, carboplatin, cyclosporine, doxorubicin, bromoethylamine | Cisplatin 184 |
Only references related to clinical nephrotoxicity are listed in the table. The few clinical studies on biomarkers for agents relevant to LDC are shown in bold.
Biomarker studies following chemical poisoning and snake envenomation
Most studies examining new renal biomarkers in ToxAKI have focused on aminoglycosides, iodinated contrast agents and cisplatin-induced nephrotoxicity (Table3). The few studies assessing the utility of biomarkers after LDC-relevant chemical/toxin ingestion have only measured serum creatinine, plasma cystatin C, urinary NGAL and KIM-1 in small cohorts of patients and with few samples to allow assessment of biomarker profiles after potential renal injury 7–9, 74–75.
While limited in scope, these few prospective cohort studies have highlighted concerns that the biomarkers developed from ischaemia-reperfusion AKI studies might rise later and have lower sensitivity and specificity in some ToxAKI settings. Specifically, both urinary NGAL and KIM-1 were elevated between 24 and 48 h following paraquat poisoning irrespective of AKI status 7, although urinary NGAL levels were higher in patients with AKI. While this study had insufficient power to assess formally the diagnostic and prognostic utility of these two biomarkers, it raised questions about the assumptions discussed above favouring damage biomarkers over functional markers in the early diagnosis of AKI. Similarly in other studies, the functional biomarkers serum cystatin C and creatinine concentrations predicted death in severe paraquat poisoning 8,9,74 while the injury biomarker serum NGAL did not 9.
In contrast, following Russell's viper envenomation, an increase in urinary N-acetyl-β-D-glucosaminidase (NAG) occurred within 24 h of snake bite 75 and predicted AKI earlier than creatinine increase. Albuminuria has also been a useful predictor of AKI following Russell's viper envenomation and other unidentified snakes 76. There are no clinical data on the utility of other novel renal biomarkers following the remaining common causes of ToxAKI in LDC such as glyphosate, MCPA, aluminium phosphide, plant poisonings or most snake envenomation 77–79. It is therefore unclear whether the concerns about specific biomarkers highlighted above are applicable to other biomarkers. Clearly further systematic research on a broad range of agents and biomarkers is needed to examine the potential for novel biomarkers to improve the early diagnosis and monitoring of ToxAKI.
Strategies for selecting biomarkers in different types of nephrotoxic injury
The general characteristics of an ideal renal biomarker include high sensitivity and specificity, rapid detection of injury (rapid meaning earlier than creatinine) and concentrations proportional to severity of renal injury 69,70,80. Additional desirable features include the ability to identify the site and mechanism of injury, track progression or recovery and prognostic utility. A useful biomarker should also be rapidly quantifiable in accessible biological fluids (urine and/or blood) and the results should be reliable and reproducible with any given injury and at comparable cost to existing conventional biomarkers 70,80. An ideal biomarker for ToxAKI in LDC should have the same attributes with added emphasis on minimal cost and ease of assay in settings where access to sophisticated hospital laboratory infrastructure is limited. Clearly, no biomarker fulfils all of these criteria in any setting!
The mechanism and target site of injury of a nephrotoxin should provide a starting point for renal biomarker selection. As noted, nephrotoxins produce renal injury through different mechanisms. The range spans diverse pathophysiological processes (e.g. oxidative stress, inhibition of specific transporters, oxidation of macromolecules, inflammation, stimulation of coagulation cascade, vascular endothelial changes) and all major functional units within the kidney (vasculature, glomerulus, proximal and distal tubules, collecting ducts) are affected to varying degrees depending on the toxin 10,22,42,81. If the toxin ingested is unknown, multiple biomarkers may be required for diagnosis and to identify specific mechanisms, target sites and to follow the evolution of injury. However, as many nephrotoxins target the proximal tubule, especially the S3 segment, selecting a biomarker specific for the proximal tubules will often be most helpful 41. Several urinary biomarkers have high sensitivity and specificity for proximal tubular injury in animal ToxAKI studies. These include KIM-1, NGAL (also a distal tubular marker), cystatin C, IL-18, clusterin and L-FABP 70 (Table3). Recently, cell cycle arrest markers such as insulin-like growth factor-binding protein 7 (IGFBP7) and tissue inhibitor of metalloproteinases-2 (TIMP-2) have been shown to be superior to other novel injury biomarkers in diagnosing moderate to severe AKI within 12–30 h 82. These biomarkers have not been explored in LDC or ToxAKI to evaluate their role in early diagnosis and risk stratification 82.
Structural biomarkers are mostly studied in urine samples, but some biomarkers are also elevated in plasma 83,84. NGAL is filtered by nephrons, reabsorbed by megalin-cubulin in proximal tubules and also appears in the urine. Subsequently, increased plasma NGAL may reflect both glomerular and tubular injury 85,86. Plasma cystatin C and serum uric acid are functional markers that may also have a role in early detection of AKI 87. Urine sample collection is non-invasive and requires no training. However, urine sampling has some limitations. Patients with severe AKI early may develop oliguria 43 and hence urine sample collection is difficult. Further, urinary biomarker concentrations are altered by fluid intake and diuretic therapy 87,88. Therefore, plasma biomarkers may be preferred in some situations and should be included when selecting biomarkers for ToxAKI studies 89.
Combining damage biomarkers with functional markers
Recently, two large AKI studies (critical care settings) utilizing novel biomarkers (one of urinary and plasma NGAL and the other also including urinary KIM-1, L-FABP, IL-18 and cystatin C) identified a group of patients, who were damage biomarker-positive but AKI negative (creatinine-negative). These subjects had the same long term increased risk of requiring dialysis and of death as AKI positive biomarker negative subjects 90,91. Similarly, two recent studies of critically ill patients with apparent pre-renal AKI, defined as transient AKI (less than 48 h) with preserved tubular function, detected urinary biomarkers at concentrations intermediate between patients without AKI and those where AKI became established for more than 48 h 92,93. The latter observations suggested that what has been described as ‘pre-renal AKI’ is simply the mild end of a continuum of renal injury, rather than a reversible functional form of AKI without cellular damage. Consequently, the international acute dialysis quality initiative group (ADQI) has recommended adding biomarker estimation to the definition, staging and differential diagnosis of AKI to complement the KDIGO criteria 94. The ADQI also recommended that the pathophysiological terms ‘functional change’ and ‘kidney damage’ be used in preference to the anatomical classification terms ‘pre-renal, renal and post-renal’ AKI. Future revisions of the definition of AKI will need to include both biomarkers of function and damage, leading to three categories of AKI, namely functional, damage and combined functional and damage AKI. While there are currently insufficient quantitative biomarker data for AKI staging, nephrology societies have supported incorporation of biomarkers into the AKI definition once sufficient studies have determined the context specific biomarker thresholds for AKI of different aetiologies 95.
These considerations also apply to ToxAKI in LDC. Combining damage biomarkers with functional markers such as plasma albumin, cystatin C and perhaps β2-microglobulin might provide additional insight into the differential timing of glomerular and proximal tubular injury as well as facilitating early and definitive diagnosis. The few cases of paraquat-induced ToxAKI already cited 7,9 suggest that this toxin may transiently induce an AKI positive, damage biomarker negative state that is associated with high mortality.
However, there are insufficient human data on these and other damage biomarkers following paraquat. Thus while, novel biomarkers such as cystatin C, NGAL, KIM-1 and IL-18 appear promising 80, the large scale clinical studies required for validation and to establish the time dependent profile of each biomarker in each specific context of injury are currently lacking for both toxic and non-toxic AKI 96.
Modulation of urinary biomarker levels by non-renal factors
Many non-renal factors might potentially affect renal biomarkers and limit utility. For example, it is well known that serum creatinine concentration and rate of change are determined not only by glomerular filtration but also rate of production, volume of distribution and renal tubular secretion 4,70,97,98. These in turn are influenced by age, body weight, race, gender, diet, muscle mass, muscle metabolism, muscle injury (in rhabdomyolysis) and disease state 99. There may also be interference with creatinine assays in the presence of some underlying diseases (ketoacidosis) and with some substances (nitromethane) 97. Similar considerations apply to damage biomarkers and other markers of function. Plasma cystatin C is a potential substitute for creatinine as a glomerular filtration biomarker. However, some non-renal factors also influence cystatin C 100. Cystatin C increases with increasing age 101,102 and is modified by smoking status, hypertension, high triglyceride concentrations, elevated C-reactive protein (CRP), alcohol consumption and body fat (higher body mass index) 98,100,103. Dose-dependent increases in cystatin C concentrations have been reported after glucocorticoids, mineralocorticoids 104 and nicotine 105 ingestion. Hypothyroidism decreases and hyperthyroidism increases the production of cystatin C 106,107. Dialysis and haemofiltration remove cystatin C 108.
Serum NGAL concentrations are higher in females and are also affected by age 109. There is a significant biological day to day variation in some populations 110. Plasma and urine NGAL may also be increased by variables such as chronic kidney disease, systemic bacterial infection, systemic inflammatory conditions and malignancies 80. NGAL expression is enhanced in the liver and lungs of patients with AKI 111. Increased urinary NGAL has been observed in apparently healthy volunteers with leukocyturia, consistent with leucocyte NGAL generation 112. Monomeric and dimeric forms of NGAL exist in urine 113,114. The monomeric form is secreted by damaged tubular cells 113, the dimeric form generally comprises a small proportion of total NGAL and is predominantly produced by neutrophils. Consequently the type of NGAL assay used may affect the interpretation.
The influence of factors other than AKI on many other urinary biomarkers has not been widely investigated but there is potential for modification. Albuminuria and proteinuria increase urinary excretion of cystatin C 115. Systemic inflammatory disorders and endotoxaemia may increase the urinary concentrations of IL-18 116. A study reported increased IL-18 in females compared with males 109. Increased KIM-1 concentrations are also reported in non-renal conditions such as in cancer and cisplatin-induced ototoxicity (with increased cochlear KIM-1) 117. High urinary KIM-1 has been reported in patients with CKD 118. Given the increasing prevalence of CKD of unknown type amongst young rural males in many LCD countries 119–121, elevated baseline biomarker concentrations may confound the diagnosis of AKI after nephrotoxin ingestion in some of these cases.
Toxic substances may also influence renal biomarker concentrations independent of ToxAKI. For example, some pesticides 11,122 and snake venoms/toxins 3,123 cause rhabdomyolysis and myoglobinuria which subsequently leads to AKI. However, proteinuria may directly increase the urinary excretion of cystatin C 115. Many nephrotoxins also cause hepatotoxicity 43,124 and AKI may be secondary to or exacerbated by the hepatorenal syndrome 125. Hepatorenal syndrome is often associated with a reduction in glomerular filtration with little or no obvious tubular damage 125. Plasma biomarkers such as plasma NGAL and cystatin C may also change because hepatotoxicity may alter the de novo liver synthesis of these molecules 126,127.
Effect of timing and dose on biomarker levels
Urinary biomarkers have been most widely studied in hypoxic kidney injury following cardiopulmonary bypass surgery where timing of the insult is known and severity of injury is relatively well-defined. Similarly, nephrotoxic injury in animal models is predictable as experimental conditions are controlled and dosage and timing are well defined 128,129. In contrast, the bioavailable dose of nephrotoxin from human poisoning or envenomation is assumed to be highly variable but remains unknown, and the extent of exposure is further modified by varying elimination kinetics including those determined by baseline renal function. Typically, patients with poisoning or envenomation present to hospital 3–7 h later 6, although this will vary based on settings and clinical effects. To understand the impact of these variables would require clinical studies that examine changes in urinary biomarker levels over time and measure cumulative exposure and toxicokinetic parameters of a range of nephrotoxic agents. Future preclinical studies might also usefully consider toxicokinetic data as well as dose in interpretation of biomarker studies. This is particularly pertinent for renally excreted nephrotoxins since following nephrotoxicity, toxin-induced hypotension or hypothermia with hypoperfusion, there will be a progressive decrease in clearance and an increase in elimination half-life 130 and the ensuing accumulation may in turn worsen kidney injury. Increased accumulation of toxic substances may also result from down regulation or saturation of active transporters in proximal tubules 14,130. Many nephrotoxins also cause hepatotoxicity 43,124, which could reduce metabolic clearance and similarly increase exposure.
Importance of establishing normal ranges for biomarker levels in healthy populations
Creatinine-based definitions of AKI remain problematic because high creatinine values alone cannot distinguish acute from CKD and prior baseline creatinine values are absent in the majority of patients. The ADQI group suggested that a possible solution was calculation of a baseline serum creatinine by solving the Modification of Diet in Renal Disease (MDRD) formula for that person assuming a low normal GFR of 75 ml min–1 1.73 m–2 131. There are several major concerns with this approach in the LDC setting. The MDRD formula has not been validated widely in Asian and many other LDC populations, and is not accurate in healthy people or patients with low muscle mass 132. Further, back-calculation systematically misclassifies renal function and consequently the incidence of AKI 133. The RIFLE criteria rely on a baseline value. Although an absolute increase in serum creatinine can be used for diagnosis 134, severity staging in the Acute Kidney Injury Network (AKIN) criteria still utilizes a fold-change over baseline 135.
Urine biomarkers of kidney damage 94,136 do not need baseline values if these are only increased in AKI. However, most urine biomarkers may be present in low concentration under various conditions in the absence of AKI, such as in CKD. Thus it may still be useful to establish normal ranges of injury biomarkers in a variety of apparently healthy populations. Urinary biomarkers have a much wider ‘normal range’ than most serum biomarkers. A normal range for cystatin C has been defined in both healthy volunteers 101,102 and in intensive care unit (ICU) patients without AKI, with higher levels found in the ICU-based no-AKI patient control groups. The mean serum cystatin C concentration was 0.96 mg l–1 (range 0.57 to 1.79 mg l–1) in healthy donors 106, while mean urinary cystatin C was 95 ng ml–1 (range 33 to 290 ng ml–1) 109,106. Urinary NGAL in normal adults ranged from 12 to 400 ng ml–1 109,112,137. Similarly, urinary KIM-1 and IL-18 ranges in healthy populations were 31 to 1737 pg ml–1 and 30 to 311 pg ml–1, respectively 109.
Many ‘normal’ biomarker ranges are from patients; for example pre-cardiac procedure or surgery, or ICU or emergency department patients who did not develop AKI 91,138,139. Biomarker levels are generally higher in these patients than in healthy volunteers 109,138, although the latter are also generally younger. Using patients to define normal levels might be misleading if it is not recognized that co-morbidities might also include cases of sub-clinical AKI that might be diagnosed by new higher sensitivity damage biomarkers but missed by AKIN or RIFLE definitions that rely purely on functional change 138. The evolving concept of ‘pre-renal AKI’ also complicates interpretation. As discussed earlier, this is no longer regarded as a unique functional type of AKI, but rather the mild and reversible end of a continuum of injury. In ‘pre-renal AKI’, injury biomarkers are at lower levels than in patients satisfying the definition of AKI, but at higher levels than in those without any functional renal change 92,93. A further problem for determining normal ranges is that analytical methods, including sample timing and handling, are generally not well standardized or calibrated. Therefore, novel renal biomarker levels should ideally be obtained for normal healthy controls in age-matched ambulatory populations, for each study.
ToxAKI and CKD – potential roles for biomarkers
Renal toxicity biomarkers that have demonstrated relevance for humans are likely to be useful in assessing possible ToxAKI aetiologies for chronic kidney disease. A recent meta-analysis has shown that AKI increases the risk of CKD 140. For example in Sri Lanka, chronic kidney disease of unknown aetiology (CKDu) is a leading cause of death in the North Central province of Sri Lanka 119,141. There is much unconfirmed speculation about a range of toxicological causes including agrochemicals 142. CKDu is also common in other parts of Asia (attributed mostly to ToxAKI from herbal medicines 143–148) and in South America 120. The underlying toxic exposure in patients with CKDu may be intermittent and not linked to the time of clinical presentation with end-stage renal failure.
Markers that could reliably detect repeated acute toxic effects very shortly after exposure might allow causal links to be established. A diagnostic role for novel urinary biomarkers in CKDu has not been widely explored. However, a recent study showed an increase in urinary levels of α1-microglobulin and NAG in patients with CKDu 149. Serum cystatin C may also be a useful marker of diagnosing and risk prediction in CKD 150. A range of novel injury biomarkers needs to be evaluated in this context to determine which best predict AKI and CKD earlier than elevations in serum creatinine.
Translation of novel biomarkers into practice
An important aspect in selecting nephrotoxicity biomarkers is the feasibility of analyzing these biomarkers in the developing world where deliberate self-poisoning with chemicals and snake envenoming mostly occur and where laboratory facilities are limited and hospital wards are overcrowded. Bedside biomarker testing with real time or rapidly available results at low cost is ideal in such a setting to enable critical clinical decision making. A point of care device is available for the measurement of plasma NGAL (Triage® NGAL device, Biosite Incorporated, San Diego, CA, USA) and a rapid assay is available for urinary NGAL (ARCHITECT® analyzer, Abbott Diagnostics, IL, USA). These have been validated in many hypoxic injury settings 137. A rapid KIM-1 test (KIM-1 dipstick-RenaStick) has been developed recently 151 and a rapid GST assay (GST•Tag™) device has also become available. For many other biomarkers point of care tests are unlikely to be available in the near future and conventional immuno-assays will continue to be required.
Assays must be standardized to ensure analytical validity which includes, but is not limited to, sensitivity, specificity, linearity, detection limits, repeatability, reproducibility, inter- and intra-assay precision 152. Another challenge in translation of biomarker study findings into practice is biomarker stability across various storage conditions. Failure to recognize biomarker instability and analytical variation may lead to misinterpretation of research findings. Almost all published research on biomarkers of acute kidney injury has reported very good intra- and inter-assay precision across different settings. In addition, the most commonly studied biomarkers (KIM-1, NGAL, IL-18, CysC and L-FABP) are stable during short term (+4°C for 48 h) 137, 153–155 and long term (at −80°C for 6 months) 137, 153, 155–157 storage. Rapid processing of samples including centrifuging and adding protease inhibitors is not necessary to ensure biomarker stability. These findings make biomarker studies feasible in rural settings where –20°C freezers are available or biomarker assays can be performed within 48 h.
Furthermore, economic aspects of adaptation of these novel biomarkers need to be considered. For these tests to be considered cost effective in ToxAKI (particularly in LDC), it must be demonstrated that early diagnosis of AKI leads to early intervention and improved outcome. Generally, novel biomarkers initially cost much more than conventional renal function tests. Currently, validated bedside kits still remain more expensive. However, the costs associated with hospital care and productive life years lost associated with untreated, misdiagnosed or fatal AKI are high and may in some circumstances justify the increased cost of early biomarker detection using these biomarkers 158. Cost effectiveness varies with settings and the threshold for cost effectiveness in LDC may be harder to obtain.
Conclusions
ToxAKI is common following ingestion of chemicals, toxic plants and following snake envenomation in LDC. Earlier detection with novel biomarkers may allow exploration of strategies to prevent further injury. Nephrotoxicity may be a preventable cause of end-stage renal disease if detected early enough. Currently, the treatment of AKI is largely supportive and treatment of end stage renal disease is largely palliative, as in LDC, dialysis and renal transplantation are unaffordable for both the state and the individual. Early detection, correct attribution and prevention of further renal damage are plausible solutions to prevent further kidney damage and reduce other clinical complications associated with CKD. Thus, developing more sensitive biomarkers for early diagnosis of nephrotoxicity is a critical step to making progress against AKI and CKDu in the developing world.
Competing Interests
We declare that no support was received from any organization for the submitted work, there were no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years or any other relationships or activities that could appear to have influenced the submitted work.
Contributions
Fahim Mohamed prepared the first draft of the manuscript. Nicholas A. Buckley and Zoltan H. Endre revised, critically commented and contributed to the final version of the manuscript.
Acknowledgments
Our research on ToxAKI has been funded by an NHMRC project grant 1011772. Fahim Mohamed is recipient of an Australian Government Endeavour Postgraduate Scholarship (2241_2011)
References
- 1.Gunnell D, Eddleston M, Phillips MR, Konradsen F. The global distribution of fatal pesticide self-poisoning: systematic review. BMC Public Health. 2007;7:357. doi: 10.1186/1471-2458-7-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasturiratne A, Wickremasinghe AR, de Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, Savioli L, Lalloo DG, de Silva HJ. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanjanabuch T, Sitprija V. Snakebite nephrotoxicity in Asia. Semin Nephrol. 2008;28:363–72. doi: 10.1016/j.semnephrol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008;245:182–93. doi: 10.1016/j.tox.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pianta TJ, Buckley NA, Peake PW, Endre ZH. Clinical use of biomarkers for toxicant-induced acute kidney injury. Biomark Med. 2013;7:441–56. doi: 10.2217/bmm.13.51. [DOI] [PubMed] [Google Scholar]
- 6.Dawson AH, Eddleston M, Senarathna L, Mohamed F, Gawarammana I, Bowe SJ, Manuweera G, Buckley NA. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Med. 2010;7:e1000357. doi: 10.1371/journal.pmed.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gil HW, Yang JO, Lee EY, Hong SY. Clinical implication of urinary neutrophil gelatinase-associated lipocalin and kidney injury molecule-1 in patients with acute paraquat intoxication. Clin Toxicol (Phila) 2009;47:870–5. doi: 10.3109/15563650903306651. [DOI] [PubMed] [Google Scholar]
- 8.Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. The clinical features of acute kidney injury in patients with acute paraquat intoxication. Nephrol Dial Transplant. 2009;24:1226–32. doi: 10.1093/ndt/gfn615. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DM, Wilks MF, Roberts MS, Swaminathan R, Mohamed F, Dawson AH, Buckley NA. Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett. 2011;202:69–74. doi: 10.1016/j.toxlet.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradberry SM, Proudfoot AT, Vale JA. Glyphosate poisoning. Toxicol Rev. 2004;23:159–67. doi: 10.2165/00139709-200423030-00003. [DOI] [PubMed] [Google Scholar]
- 11.Roberts DM, Buckley NA, Mohamed F, Eddleston M, Goldstein DA, Mehrsheikh A, Bleeke MS, Dawson AH. A prospective observational study of the clinical toxicology of glyphosate-containing herbicides in adults with acute self-poisoning. Clin Toxicol (Phila) 2010;48:129–36. doi: 10.3109/15563650903476491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts DM, Heilmair R, Buckley NA, Dawson AH, Fahim M, Eddleston M, Eyer P. Clinical outcomes and kinetics of propanil following acute self-poisoning: a prospective case series. BMC Clin Pharmacol. 2009;9:3. doi: 10.1186/1472-6904-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradberry SM, Watt BE, Proudfoot AT, Vale JA. Mechanisms of toxicity, clinical features, and management of acute chlorophenoxy herbicide poisoning: a review. J Toxicol Clin Toxicol. 2000;38:111–22. doi: 10.1081/clt-100100925. [DOI] [PubMed] [Google Scholar]
- 14.Roberts DM, Dawson AH, Senarathna L, Mohamed F, Cheng R, Eaglesham G, Buckley NA. Toxicokinetics, including saturable protein binding, of 4-chloro-2-methyl phenoxyacetic acid (MCPA) in patients with acute poisoning. Toxicol Lett. 2011;201:270–6. doi: 10.1016/j.toxlet.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flanagan RJ, Meredith TJ, Ruprah M, Onyon LJ, Liddle A. Alkaline diuresis for acute poisoning with chlorophenoxy herbicides and ioxynil. Lancet. 1990;335:454–8. doi: 10.1016/0140-6736(90)90677-w. [DOI] [PubMed] [Google Scholar]
- 16.Gurjar M, Baronia AK, Azim A, Sharma K. Managing aluminum phosphide poisonings. J Emerg Trauma Shock. 2011;4:378–84. doi: 10.4103/0974-2700.83868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murali R, Bhalla A, Singh D, Singh S. Acute pesticide poisoning: 15 years experience of a large North-West Indian hospital. Clin Toxicol (Phila) 2009;47:35–8. doi: 10.1080/15563650701885807. [DOI] [PubMed] [Google Scholar]
- 18.Gawarammana IB, Ariyananda PL, Palangasinghe C, De Silva NG, Fernando K, Vidanapathirana M, Kuruppuarachchi MA, Munasinghe MA, Dawson AH. Emerging epidemic of fatal human self-poisoning with a washing powder in Southern Sri Lanka: a prospective observational study. Clin Toxicol (Phila) 2009;47:407–11. doi: 10.1080/15563650902915320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aleem HM. Gloriosa superba poisoning. J Assoc Physicians India. 1992;40:541–2. [PubMed] [Google Scholar]
- 20.Fernando R, Fernando DN. Poisoning with plants and mushrooms in Sri Lanka: a retrospective hospital based study. Vet Hum Toxicol. 1990;32:579–81. [PubMed] [Google Scholar]
- 21.Chrispal A. Cleistanthus collinus poisoning. J Emerg Trauma Shock. 2012;5:160–6. doi: 10.4103/0974-2700.96486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nampoothiri K, Chrispal A, Begum A, Jasmine S, Gopinath KG, Zachariah A. A clinical study of renal tubular dysfunction in Cleistanthus collinus (Oduvanthalai) poisoning. Clin Toxicol (Phila) 2010;48:193–7. doi: 10.3109/15563651003641786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kularatne SA. Epidemiology and clinical picture of the Russell's viper (Daboia russelii russelii) bite in Anuradhapura, Sri Lanka: a prospective study of 336 patients. Southeast Asian J Trop Med Public Health. 2003;34:855–62. [PubMed] [Google Scholar]
- 24.Karunarathne S, Udayakumara Y, Govindapala D, Fernando H. Type IV renal tubular acidosis following resolution of acute kidney injury and disseminated intravascular coagulation due to hump-nosed viper bite. Indian J Nephrol. 2013;23:294–6. doi: 10.4103/0971-4065.114476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kularatne SA, Sivansuthan S, Medagedara SC, Maduwage K, de Silva A. Revisiting saw-scaled viper (Echis carinatus) bites in the Jaffna Peninsula of Sri Lanka: distribution, epidemiology and clinical manifestations. Trans R Soc Trop Med Hyg. 2011;105:591–7. doi: 10.1016/j.trstmh.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Naicker S, Aboud O, Gharbi MB. Epidemiology of acute kidney injury in Africa. Semin Nephrol. 2008;28:348–53. doi: 10.1016/j.semnephrol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Okunola O, Akinsola A, Ayodele O. Kidney diseases in Africa: aetiological considerations, peculiarities and burden. Afr J Med Med Sci. 2012;41:119–33. [PubMed] [Google Scholar]
- 28.Elevli M, Civilibal M, Ersoy O, Demirkol D, Gedik AH. Paraphenylene diamine hair dye poisoning: an uncommon cause of rhabdomyolysis. Indian J Pediatr. 2014;81:709–11. doi: 10.1007/s12098-013-1074-z. [DOI] [PubMed] [Google Scholar]
- 29.Lowenthal MN, Jones IG, Mohelsky V. Acute renal failure in Zambian women using traditional herbal remedies. J Trop Med Hyg. 1974;77:190–2. [PubMed] [Google Scholar]
- 30.Jha V, Rathi M. Natural medicines causing acute kidney injury. Semin Nephrol. 2008;28:416–28. doi: 10.1016/j.semnephrol.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Lombardi R, Yu L, Younes-Ibrahim M, Schor N, Burdmann EA. Epidemiology of acute kidney injury in Latin America. Semin Nephrol. 2008;28:320–9. doi: 10.1016/j.semnephrol.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Pinho FM, Yu L, Burdmann EA. Snakebite-induced acute kidney injury in Latin America. Semin Nephrol. 2008;28:354–62. doi: 10.1016/j.semnephrol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Albuquerque PL, Jacinto CN, Silva Junior GB, Lima JB, Veras Mdo S, Daher EF. Acute kidney injury caused by Crotalus and Bothrops snake venom: a review of epidemiology, clinical manifestations and treatment. Rev Inst Med Trop Sao Paulo. 2013;55:295–301. doi: 10.1590/S0036-46652013000500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedroso JA, Silva CA. The nephrologist as a consultant for acute poisoning: epidemiology of severe poisonings in the State of Rio Grande do Sul and techniques to enhance renal elimination. J Bras Nefrol. 2010;32:340–8. [PubMed] [Google Scholar]
- 35.de Silva A, Wijekoon AS, Jayasena L, Abeysekera CK, Bao CX, Hutton RA, Warrell DA. Haemostatic dysfunction and acute renal failure following envenoming by Merrem's hump-nosed viper (Hypnale hypnale) in Sri Lanka: first authenticated case. Trans R Soc Trop Med Hyg. 1994;88:209–12. doi: 10.1016/0035-9203(94)90301-8. [DOI] [PubMed] [Google Scholar]
- 36.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–20. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 37.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 38.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sutton TA. Alteration of microvascular permeability in acute kidney injury. Microvasc Res. 2009;77:4–7. doi: 10.1016/j.mvr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endre ZH, Walker RJ. Cellular Mechanisms of Drug Nephrotoxicity. In: Alpern R, Caplan M, Moe O, editors. Seldin and Giebisch's The Kidney: Physiology & Pathophysiology. 5th edition. Vol. 2. Oxford, UK: Academic Press; 2012. pp. 2889–2932. [Google Scholar]
- 42.Dinis-Oliveira RJ, Duarte JA, Sanchez-Navarro A, Remiao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 43.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72:745–57. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinis-Oliveira RJ, Pontes H, Bastos ML, Remiao F, Duarte JA, Carvalho F. An effective antidote for paraquat poisonings: the treatment with lysine acetylsalicylate. Toxicology. 2009;255:187–93. doi: 10.1016/j.tox.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Mahomedy MC, Mahomedy YH, Canham PA, Downing JW, Jeal DE. Methaemoglobinaemia following treatment dispensed by witch doctors. Two cases of potassium permanganate poisoning. Anaesthesia. 1975;30:190–3. doi: 10.1111/j.1365-2044.1975.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 46.Middleton SJ, Jacyna M, McClaren D, Robinson R, Thomas HC. Haemorrhagic pancreatitis--a cause of death in severe potassium permanganate poisoning. Postgrad Med J. 1990;66:657–8. doi: 10.1136/pgmj.66.778.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song HY, Kim YH, Seok SJ, Gil HW, Yang JO, Lee EY, Hong SY. Cellular toxicity of surfactants used as herbicide additives. J Korean Med Sci. 2012;27:3–9. doi: 10.3346/jkms.2012.27.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Culbreth ME, Harrill JA, Freudenrich TM, Mundy WR, Shafer TJ. Comparison of chemical-induced changes in proliferation and apoptosis in human and mouse neuroprogenitor cells. Neurotoxicology. 2012;33:1499–510. doi: 10.1016/j.neuro.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Guilherme S, Gaivao I, Santos MA, Pacheco M. DNA damage in fish (Anguilla anguilla) exposed to a glyphosate-based herbicide - elucidation of organ-specificity and the role of oxidative stress. Mutat Res. 2012;743:1–9. doi: 10.1016/j.mrgentox.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Mensah PK, Palmer CG, Muller WJ. Lipid peroxidation in the freshwater shrimp Caridina nilotica as a biomarker of Roundup((R)) herbicide pollution of freshwater systems in South Africa. Water Sci Technol. 2012;65:1660–6. doi: 10.2166/wst.2012.060. [DOI] [PubMed] [Google Scholar]
- 51.Zychlinski L, Zolnierowicz S. Comparison of uncoupling activities of chlorophenoxy herbicides in rat liver mitochondria. Toxicol Lett. 1990;52:25–34. doi: 10.1016/0378-4274(90)90162-f. [DOI] [PubMed] [Google Scholar]
- 52.Elo HA, Luoma T, Ylitalo P. Inhibition of human and rabbit platelet aggregation by chlorophenoxyacid herbicides. Arch Toxicol. 1991;65:140–4. doi: 10.1007/BF02034941. [DOI] [PubMed] [Google Scholar]
- 53.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646–56. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- 54.Valentovic MA, Ball JG, Sun H, Rankin GO. Characterization of 2-amino-4,5-dichlorophenol (2A45CP) in vitro toxicity in renal cortical slices from male Fischer 344 rats. Toxicology. 2002;172:113–23. doi: 10.1016/s0300-483x(01)00597-2. [DOI] [PubMed] [Google Scholar]
- 55.Rankin GO, Racine C, Sweeney A, Kraynie A, Anestis DK, Barnett JB. In vitro nephrotoxicity induced by propanil. Environ Toxicol. 2008;23:435–42. doi: 10.1002/tox.20353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sampathkumar K, Yesudas S. Hair dye poisoning and the developing world. J Emerg Trauma Shock. 2009;2:129–31. doi: 10.4103/0974-2700.50749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seok SJ, Park JS, Hong JR, Gil HW, Yang JO, Lee EY, Song HY, Hong SY. Surfactant volume is an essential element in human toxicity in acute glyphosate herbicide intoxication. Clin Toxicol (Phila) 2011;49:892–9. doi: 10.3109/15563650.2011.626422. [DOI] [PubMed] [Google Scholar]
- 58.Wilks MF, Tomenson JA, Fernando R, Ariyananda PL, Berry DJ, Buckley NA, Gawarammana IB, Jayamanne S, Gunnell D, Dawson A. Formulation changes and time trends in outcome following paraquat ingestion in Sri Lanka. Clin Toxicol (Phila) 2011;49:21–8. doi: 10.3109/15563650.2010.544658. [DOI] [PubMed] [Google Scholar]
- 59.Eddleston M, Street JM, Self I, Thompson A, King T, Williams N, Naredo G, Dissanayake K, Yu LM, Worek F, John H, Smith S, Thiermann H, Harris JB, Eddie CluttonR. A role for solvents in the toxicity of agricultural organophosphorus pesticides. Toxicology. 2012;294:94–103. doi: 10.1016/j.tox.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang WH, Hsu CW, Yu CC. Colchicine overdose-induced acute renal failure and electrolyte imbalance. Ren Fail. 2007;29:367–70. doi: 10.1080/08860220601166644. [DOI] [PubMed] [Google Scholar]
- 61.Molad Y. Update on colchicine and its mechanism of action. Curr Rheumatol Rep. 2002;4:252–6. doi: 10.1007/s11926-002-0073-2. [DOI] [PubMed] [Google Scholar]
- 62.Kettimuthu KP, Lourthuraj AA, Manickam AS, Subramani S, Ramachandran A. Mechanisms of toxicity of Cleistanthus collinus: vacuolar ATPases are a putative target. Clin Toxicol (Phila) 2011;49:457–63. doi: 10.3109/15563650.2011.590939. [DOI] [PubMed] [Google Scholar]
- 63.Warrell DA. Snake venoms in science and clinical medicine. 1. Russell's viper: biology, venom and treatment of bites. Trans R Soc Trop Med Hyg. 1989;83:732–40. doi: 10.1016/0035-9203(89)90311-8. [DOI] [PubMed] [Google Scholar]
- 64.Ratcliffe PJ, Pukrittayakamee S, Ledingham JG, Warrell DA. Direct nephrotoxicity of Russell's viper venom demonstrated in the isolated perfused rat kidney. Am J Trop Med Hyg. 1989;40:312–9. doi: 10.4269/ajtmh.1989.40.312. [DOI] [PubMed] [Google Scholar]
- 65.Herath N, Wazil A, Kularatne S, Ratnatunga N, Weerakoon K, Badurdeen S, Rajakrishna P, Nanayakkara N, Dharmagunawardane D. Thrombotic microangiopathy and acute kidney injury in hump-nosed viper (Hypnale species) envenoming: a descriptive study in Sri Lanka. Toxicon. 2012;60:61–5. doi: 10.1016/j.toxicon.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 66.Silva A, Gunawardena P, Weilgama D, Maduwage K, Gawarammana I. Comparative in-vivo toxicity of venoms from South Asian hump-nosed pit vipers (Viperidae: Crotalinae: Hypnale) BMC Res Notes. 2012;5:471. doi: 10.1186/1756-0500-5-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kularatne SA, Hettiarachchi R, Dalpathadu J, Mendis AS, Appuhamy PD, Zoysa HD, Maduwage K, Weerasinghe VS, de Silva A. Enhydrina schistosa (Elapidae: Hydrophiinae) the most dangerous sea snake in Sri Lanka: three case studies of severe envenoming. Toxicon. 2014;77:78–86. doi: 10.1016/j.toxicon.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 68.Ali SA, Alam JM, Abbasi A, Zaidi ZH, Stoeva S, Voelter W. Sea snake Hydrophis cyanocinctus venom. II. Histopathological changes, induced by a myotoxic phospholipase A2 (PLA2-H1) Toxicon. 2000;38:687–705. doi: 10.1016/s0041-0101(99)00184-1. [DOI] [PubMed] [Google Scholar]
- 69.Endre ZH, Pickering JW, Walker RJ. Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI) Am J Physiol Renal Physiol. 2011;301:F697–707. doi: 10.1152/ajprenal.00448.2010. [DOI] [PubMed] [Google Scholar]
- 70.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28:436–40. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–93. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, Sultana S, Gerhold DL, Phillips JA, Maurer G, Carl K, Laurie D, Harpur E, Sonee M, Ennulat D, Holder D, Andrews-Cleavenger D, Gu YZ, Thompson KL, Goering PL, Vidal JM, Abadie E, Maciulaitis R, Jacobson-Kram D, Defelice AF, Hausner EA, Blank M, Thompson A, Harlow P, Throckmorton D, Xiao S, Xu N, Taylor W, Vamvakas S, Flamion B, Lima BS, Kasper P, Pasanen M, Prasad K, Troth S, Bounous D, Robinson-Gravatt D, Betton G, Davis MA, Akunda J, McDuffie JE, Suter L, Obert L, Guffroy M, Pinches M, Jayadev S, Blomme EA, Beushausen SA, Barlow VG, Collins N, Waring J, Honor D, Snook S, Lee J, Rossi P, Walker E, Mattes W. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–62. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 73.Westhuyzen J, Endre ZH, Reece G, Reith DM, Saltissi D, Morgan TJ. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant. 2003;18:543–51. doi: 10.1093/ndt/18.3.543. [DOI] [PubMed] [Google Scholar]
- 74.Hantson P, Weynand B, Doyle I, Bernand A, Hermans C. Pneumoproteins as markers of paraquat lung injury: a clinical case. J Forensic Leg Med. 2008;15:48–52. doi: 10.1016/j.jcfm.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Aung W, Kyaw A, Win T, Kun S, Thin-Hlain T. Urinary NAG as an early indicator of renal damage in Russell's viper bite envenomation. Trans R Soc Trop Med Hyg. 1996;90:169–72. doi: 10.1016/s0035-9203(96)90125-x. [DOI] [PubMed] [Google Scholar]
- 76.Dharod MV, Patil TB, Deshpande AS, Gulhane RV, Patil MB, Bansod YV. Clinical predictors of acute kidney injury following snake bite envenomation. N Am J Med Sci. 2013;5:594–9. doi: 10.4103/1947-2714.120795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee CH, Shih CP, Hsu KH, Hung DZ, Lin CC. The early prognostic factors of glyphosate-surfactant intoxication. Am J Emerg Med. 2008;26:275–81. doi: 10.1016/j.ajem.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 78.Moon JM, Min YI, Chun BJ. Can early hemodialysis affect the outcome of the ingestion of glyphosate herbicide? Clin Toxicol (Phila) 2006;44:329–32. doi: 10.1080/15563650600584550. [DOI] [PubMed] [Google Scholar]
- 79.Moon JM, Chun BJ. Predicting acute complicated glyphosate intoxication in the emergency department. Clin Toxicol (Phila) 2010;48:718–24. doi: 10.3109/15563650.2010.488640. [DOI] [PubMed] [Google Scholar]
- 80.Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4:265–80. doi: 10.2217/bmm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jha V, Chugh CK. Nephropathy associated with animal, plant, and chemical toxins in the tropics. Semin Nephrol. 2003;23:49–65. doi: 10.1053/snep.2003.50003. [DOI] [PubMed] [Google Scholar]
- 82.Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, Davison DL, Feldkamp T, Forni LG, Gong MN, Gunnerson KJ, Haase M, Hackett J, Honore PM, Hoste EA, Joannes-Boyau O, Joannidis M, Kim P, Koyner JL, Laskowitz DT, Lissauer ME, Marx G, McCullough PA, Mullaney S, Ostermann M, Rimmele T, Shapiro NI, Shaw AD, Shi J, Sprague AM, Vincent JL, Vinsonneau C, Wagner L, Walker MG, Wilkerson RG, Zacharowski K, Kellum JA. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haase-Fielitz A, Bellomo R, Devarajan P, Bennett M, Story D, Matalanis G, Frei U, Dragun D, Haase M. The predictive performance of plasma neutrophil gelatinase-associated lipocalin (NGAL) increases with grade of acute kidney injury. Nephrol Dial Transplant. 2009;24:3349–54. doi: 10.1093/ndt/gfp234. [DOI] [PubMed] [Google Scholar]
- 84.Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–7. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 86.Pickering JW, Endre ZH. The clinical utility of plasma neutrophil gelatinase-associated lipocalin in acute kidney injury. Blood Purif. 2013;35:295–302. doi: 10.1159/000351542. [DOI] [PubMed] [Google Scholar]
- 87.Lisowska-Myjak B. Serum and urinary biomarkers of acute kidney injury. Blood Purif. 2010;29:357–65. doi: 10.1159/000309421. [DOI] [PubMed] [Google Scholar]
- 88.Ralib AM, Pickering JW, Shaw GM, Devarajan P, Edelstein CL, Bonventre JV, Endre ZH. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol. 2012;23:322–33. doi: 10.1681/ASN.2011040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carter JL, Lamb EJ. Evaluating new biomarkers for acute kidney injury: putting the horse before the cart. Am J Kidney Dis. 2014;63:543–6. doi: 10.1053/j.ajkd.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 90.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge P, Siew E, Ware LB, Ikizler TA, Mertens PR. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, Elger A, Maarouf O, Sola-Del Valle DA, O'Rourke M, Sherman E, Lee P, Geara A, Imus P, Guddati A, Polland A, Rahman W, Elitok S, Malik N, Giglio J, El-Sayegh S, Devarajan P, Hebbar S, Saggi SJ, Hahn B, Kettritz R, Luft FC, Barasch J. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–55. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nejat M, Pickering JW, Devarajan P, Bonventre JV, Edelstein CL, Walker RJ, Endre ZH. Some biomarkers of acute kidney injury are increased in pre-renal acute injury. Kidney Int. 2012;81:1254–62. doi: 10.1038/ki.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doi K, Katagiri D, Negishi K, Hasegawa S, Hamasaki Y, Fujita T, Matsubara T, Ishii T, Yahagi N, Sugaya T, Noiri E. Mild elevation of urinary biomarkers in prerenal acute kidney injury. Kidney Int. 2012;82:1114–20. doi: 10.1038/ki.2012.266. [DOI] [PubMed] [Google Scholar]
- 94.Murray PT, Mehta RL, Shaw A, Ronco C, Endre Z, Kellum JA, Chawla LS, Cruz D, Ince C, Okusa MD. Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int. 2014;85:513–21. doi: 10.1038/ki.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Langham RG, Bellomo R, D' Intini V, Endre Z, Hickey BB, McGuinness S, Phoon RK, Salamon K, Woods J, Gallagher MP. KHA-CARI guideline: KHA-CARI adaptation of the KDIGO Clinical Practice Guideline for Acute Kidney Injury. Nephrology. 2014;19:261–5. doi: 10.1111/nep.12220. [DOI] [PubMed] [Google Scholar]
- 96.Endre ZH, Pickering JW. Biomarkers and creatinine in AKI: the trough of disillusionment or the slope of enlightenment? Kidney Int. 2013;84:644–7. doi: 10.1038/ki.2013.168. [DOI] [PubMed] [Google Scholar]
- 97.Bagshaw SM, Gibney RT. Conventional markers of kidney function. Crit Care Med. 2008;36:S152–8. doi: 10.1097/CCM.0b013e318168c613. [DOI] [PubMed] [Google Scholar]
- 98.Chew-Harris JS, Florkowski CM, George PM, Elmslie JL, Endre ZH. The relative effects of fat versus muscle mass on cystatin C and estimates of renal function in healthy young men. Ann Clin Biochem. 2013;50:39–46. doi: 10.1258/acb.2012.011241. [DOI] [PubMed] [Google Scholar]
- 99.Pickering JW, Ralib AM, Endre ZH. Combining creatinine and volume kinetics identifies missed cases of acute kidney injury following cardiac arrest. Crit Care. 2013;17:R7. doi: 10.1186/cc11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bagshaw SM, Bellomo R. Cystatin C in acute kidney injury. Curr Opin Crit Care. 2010;16:533–99. doi: 10.1097/MCC.0b013e32833e8412. [DOI] [PubMed] [Google Scholar]
- 101.Uzun H, Ozmen Keles M, Ataman R, Aydin S, Kalender B, Uslu E, Simsek G, Halac M, Kaya S. Serum cystatin C level as a potentially good marker for impaired kidney function. Clin Biochem. 2005;38:792–8. doi: 10.1016/j.clinbiochem.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 102.Uhlmann EJ, Hock KG, Issitt C, Sneeringer MR, Cervelli DR, Gorman RT, Scott MG. Reference intervals for plasma cystatin C in healthy volunteers and renal patients, as measured by the Dade Behring BN II System, and correlation with creatinine. Clin Chem. 2001;47:2031–3. [PubMed] [Google Scholar]
- 103.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–21. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 104.Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47:2055–9. [PubMed] [Google Scholar]
- 105.Sjostrom PA, Jones IL, Tidman MA. Cystatin C as a filtration marker - haemodialysis patients expose its strengths and limitations. Scand J Clin Lab Invest. 2009;69:65–72. doi: 10.1080/00365510802326469. [DOI] [PubMed] [Google Scholar]
- 106.Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A. Cystatin C as a marker of GFR-history, indications, and future research. Clin Biochem. 2005;38:1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 107.Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–7. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 108.Kiers HD, de Sevaux R, Pickkers P. Cystatin C is not a reliable marker of residual glomerular filtration rate during continuous renal replacement therapy. Intensive Care Med. 2011;37:1893–4. doi: 10.1007/s00134-011-2346-6. author reply 97–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang X, Gibson B, Jr, Mori R, Snow-Lisy D, Yamaguchi Y, Campbell SC, Simmons MN, Daly TM. Analytical and biological validation of a multiplex immunoassay for acute kidney injury biomarkers. Clin Chim Acta. 2012;415C:88–93. doi: 10.1016/j.cca.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 110.Delanaye P, Rozet E, Krzesinski JM, Cavalier E. Urinary NGAL measurement: biological variation and ratio to creatinine. Clin Chim Acta. 2011;412:390. doi: 10.1016/j.cca.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 111.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–13. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 112.Cullen MR, Murray PT, Fitzgibbon MC. Establishment of a reference interval for urinary neutrophil gelatinase-associated lipocalin. Ann Clin Biochem. 2012;49:190–3. doi: 10.1258/acb.2011.011105. [DOI] [PubMed] [Google Scholar]
- 113.Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010;5:2229–35. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martensson J, Xu S, Bell M, Martling CR, Venge P. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clin Chim Acta. 2012;413:1661–7. doi: 10.1016/j.cca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 115.Nejat M, Hill JV, Pickering JW, Edelstein CL, Devarajan P, Endre ZH. Albuminuria increases cystatin C excretion: implications for urinary biomarkers. Nephrol Dial Transplant. 2012;27(3):iii96–103. doi: 10.1093/ndt/gfr222. [DOI] [PubMed] [Google Scholar]
- 116.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–24. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 117.Vaidya VS, Bonventre JV. Mechanistic biomarkers for cytotoxic acute kidney injury. Expert Opin Drug Metab Toxicol. 2006;2:697–713. doi: 10.1517/17425255.2.5.697. [DOI] [PubMed] [Google Scholar]
- 118.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–17. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 119.Jayatilake N, Mendis S, Maheepala P, Mehta FR. Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 2013;14:180. doi: 10.1186/1471-2369-14-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weiner DE, McClean MD, Kaufman JS, Brooks DR. The Central American epidemic of CKD. Clin J Am Soc Nephrol. 2013;8:504–11. doi: 10.2215/CJN.05050512. [DOI] [PubMed] [Google Scholar]
- 121.Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, Cicerchi C, Inaba S, Le M, Miyazaki M, Glaser J, Correa-Rotter R, Gonzalez MA, Aragon A, Wesseling C, Sanchez-Lozada LG, Johnson RJ. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int. 2014;86:294–302. doi: 10.1038/ki.2013.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weng SF, Hung DZ, Hu SY, Tsan YT, Wang LM. Rhabdomyolysis from an intramuscular injection of glyphosate-surfactant herbicide. Clin Toxicol (Phila) 2008;46:890–1. doi: 10.1080/15563650802286731. [DOI] [PubMed] [Google Scholar]
- 123.Warrell DA. Redi award lecture: Clinical studies of snake-bite in four tropical continents. Toxicon. 2013;69:3–13. doi: 10.1016/j.toxicon.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 124.Sribanditmongkol P, Jutavijittum P, Pongraveevongsa P, Wunnapuk K, Durongkadech P. Pathological and toxicological findings in glyphosate-surfactant herbicide fatality: a case report. Am J Forensic Med Pathol. 2012;33:234–7. doi: 10.1097/PAF.0b013e31824b936c. [DOI] [PubMed] [Google Scholar]
- 125.Venkat D, Venkat KK. Hepatorenal syndrome. South Med J. 2010;103:654–61. doi: 10.1097/SMJ.0b013e3181e07751. [DOI] [PubMed] [Google Scholar]
- 126.Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36:S159–65. doi: 10.1097/CCM.0b013e318168c652. [DOI] [PubMed] [Google Scholar]
- 127.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 128.Sasaki D, Yamada A, Umeno H, Kurihara H, Nakatsuji S, Fujihira S, Tsubota K, Ono M, Moriguchi A, Watanabe K, Seki J. Comparison of the course of biomarker changes and kidney injury in a rat model of drug-induced acute kidney injury. Biomarkers. 2011;16:553–66. doi: 10.3109/1354750X.2011.613123. [DOI] [PubMed] [Google Scholar]
- 129.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, End P, Staedtler F, Legay F, Carl K, Laurie D, Chibout SD, Vonderscher J, Maurer G. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28:463–9. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 130.Roberts DM, Buckley NA. Pharmacokinetic considerations in clinical toxicology: clinical applications. Clin Pharmacokinet. 2007;46:897–939. doi: 10.2165/00003088-200746110-00001. [DOI] [PubMed] [Google Scholar]
- 131.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14:2573–80. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 133.Pickering JW, Endre ZH. Back-calculating baseline creatinine with MDRD misclassifies acute kidney injury in the intensive care unit. Clin J Am Soc Nephrol. 2010;5:1165–73. doi: 10.2215/CJN.08531109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–9. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Endre ZH, Kellum JA, Di Somma S, Doi K, Goldstein SL, Koyner JL, Macedo E, Mehta RL, Murray PT. Differential diagnosis of AKI in clinical practice by functional and damage biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:30–44. doi: 10.1159/000349964. [DOI] [PubMed] [Google Scholar]
- 137.Grenier FC, Ali S, Syed H, Workman R, Martens F, Liao M, Wang Y, Wong PY. Evaluation of the ARCHITECT urine NGAL assay: assay performance, specimen handling requirements and biological variability. Clin Biochem. 2010;43:615–20. doi: 10.1016/j.clinbiochem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 138.Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, Gioules C, Bradwin G, Matsouaka R, Betensky RA, Curhan GC, Bonventre JV. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–8. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Royakkers AA, Korevaar JC, van Suijlen JD, Hofstra LS, Kuiper MA, Spronk PE, Schultz MJ, Bouman CS. Serum and urine cystatin C are poor biomarkers for acute kidney injury and renal replacement therapy. Intensive Care Med. 2011;37:493–501. doi: 10.1007/s00134-010-2087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int. 2012;81:442–8. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jayasekara JM, Dissanayake DM, Adhikari SB, Bandara P. Geographical distribution of chronic kidney disease of unknown origin in North Central Region of Sri Lanka. Ceylon Med J. 2013;58:6–10. doi: 10.4038/cmj.v58i1.5356. [DOI] [PubMed] [Google Scholar]
- 142.Redmon JH, Elledge MF, Womack DS, Wickremashinghe R, Wanigasuriya KP, Peiris-John RJ, Lunyera J, Smith K, Raymer JH, Levine KE. Additional perspectives on chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka-lessons learned from the WHO CKDu population prevalence study. BMC Nephrol. 2014;15:125. doi: 10.1186/1471-2369-15-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barsoum RS. Overview: end-stage renal disease in the developing world. Artif Organs. 2002;26:735–36. doi: 10.1046/j.1525-1594.2002.00916.x. [DOI] [PubMed] [Google Scholar]
- 144.Jha V. End-stage renal care in developing countries: the India experience. Ren Fail. 2004;26:201–8. doi: 10.1081/jdi-120039516. [DOI] [PubMed] [Google Scholar]
- 145.Naicker S. End-stage renal disease in sub-Saharan and South Africa. Kidney Int Suppl. 2003;83:S119–22. doi: 10.1046/j.1523-1755.63.s83.25.x. [DOI] [PubMed] [Google Scholar]
- 146.Wazaify M, Alawwa I, Yasein N, Al-Saleh A, Afifi FU. Complementary and alternative medicine (CAM) use among Jordanian patients with chronic diseases. Complement Ther Clin Pract. 2013;19:153–7. doi: 10.1016/j.ctcp.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 147.Lai MN, Lai JN, Chen PC, Tseng WL, Chen YY, Hwang JS, Wang JD. Increased risks of chronic kidney disease associated with prescribed Chinese herbal products suspected to contain aristolochic acid. Nephrology. 2009;14:227–34. doi: 10.1111/j.1440-1797.2008.01061.x. [DOI] [PubMed] [Google Scholar]
- 148.Zhang J, Zhang L, Wang W, Wang H. Association between aristolochic acid and CKD: a cross-sectional survey in China. Am J Kidney Dis. 2013;61:918–22. doi: 10.1053/j.ajkd.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 149.Nanayakkara S, Senevirathna ST, Karunaratne U, Chandrajith R, Harada KH, Hitomi T, Watanabe T, Abeysekera T, Aturaliya TN, Koizumi A. Evidence of tubular damage in the very early stage of chronic kidney disease of uncertain etiology in the North Central Province of Sri Lanka: a cross-sectional study. Environ Health Prev Med. 2012;17:109–17. doi: 10.1007/s12199-011-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peralta CA, Shlipak MG, Judd S, Cushman M, McClellan W, Zakai NA, Safford MM, Zhang X, Muntner P, Warnock D. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–52. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vaidya VS, Ford GM, Waikar SS, Wang Y, Clement MB, Ramirez V, Glaab WE, Troth SP, Sistare FD, Prozialeck WC, Edwards JR, Bobadilla NA, Mefferd SC, Bonventre JV. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76:108–14. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mansfield E, O'Leary TJ, Gutman SI. Food and Drug Administration regulation of in vitro diagnostic devices. J Mol Diagn. 2005;7:2–7. doi: 10.1016/S1525-1578(10)60002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Parikh CR, Butrymowicz I, Yu A, Chinchilli VM, Park M, Hsu CY, Reeves WB, Devarajan P, Kimmel PL, Siew ED, Liu KD, Investigators A-AS. Urine stability studies for novel biomarkers of acute kidney injury. Am J Kidney Dis. 2014;63:567–72. doi: 10.1053/j.ajkd.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Herget-Rosenthal S, Feldkamp T, Volbracht L, Kribben A. Measurement of urinary cystatin C by particle-enhanced nephelometric immunoassay: precision, interferences, stability and reference range. Ann Clin Biochem. 2004;41:111–8. doi: 10.1258/000456304322879980. [DOI] [PubMed] [Google Scholar]
- 155.Han WK, Wagener G, Zhu Y, Wang S, Lee HT. Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2009;4:873–82. doi: 10.2215/CJN.04810908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.van de Vrie M, Deegens JK, van der Vlag J, Hilbrands LB. Effect of long-term storage of urine samples on measurement of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) Am J Kidney Dis. 2014;63:573–6. doi: 10.1053/j.ajkd.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 157.Pennemans V, Rigo JM, Penders J, Swennen Q. Collection and storage requirements for urinary kidney injury molecule-1 (KIM-1) measurements in humans. Clin Chem LabMed CCLM / FESCC. 2012;50:539–43. doi: 10.1515/CCLM.2011.796. [DOI] [PubMed] [Google Scholar]
- 158.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R. Acute kidney injury: an increasing global concern. Lancet. 2013;382:170–9. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 159.Roberts DM, Seneviratne R, Mohammed F, Patel R, Senarathna L, Hittarage A, Buckley NA, Dawson AH, Eddleston M. Intentional self-poisoning with the chlorophenoxy herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) Ann Emerg Med. 2005;46:275–84. doi: 10.1016/j.annemergmed.2005.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abdelraheem MB, El-Tigani MA, Hassan EG, Ali MA, Mohamed IA, Nazik AE. Acute renal failure owing to paraphenylene diamine hair dye poisoning in Sudanese children. Ann Trop Paediatr. 2009;29:191–6. doi: 10.1179/027249309X12467994693815. [DOI] [PubMed] [Google Scholar]
- 161.Jain PK, Sharma AK, Agarwal N, Sengar NS, Siddiqui MZ, Pawal P, Singh AK, Gaba RD. A prospective clinical study of myocarditis in cases of acute ingestion of paraphenylene diamine (hair dye) poisoning in northern India. J Assoc Physicians India. 2013;61:633–6. 644. [PubMed] [Google Scholar]
- 162.Arefi M, Taghaddosinejad F, Salamaty P, Soroosh D, Ashraf H, Ebrahimi M. Renal failure prevalence in poisoned patients. Nephrourol Mon. 2014;6:e11910. doi: 10.5812/numonthly.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Steenkamp V, Stewart MJ. Nephrotoxicity associated with exposure to plant toxins, with particular reference to Africa. Ther Drug Monit. 2005;27:270–7. doi: 10.1097/01.ftd.0000162229.86303.67. [DOI] [PubMed] [Google Scholar]
- 164.Kularatne SA, Silva A, Weerakoon K, Maduwage K, Walathara C, Paranagama R, Mendis S. Revisiting Russell's viper (Daboia russelii) bite in Sri Lanka: is abdominal pain an early feature of systemic envenoming? PLoS One. 2014;9:e90198. doi: 10.1371/journal.pone.0090198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Myint L, Warrell DA, Phillips RE, Tin Nu S, Tun P, Maung MaungL. Bites by Russell's viper (Vipera russelli siamensis) in Burma: haemostatic, vascular, and renal disturbances and response to treatment. Lancet. 1985;2:1259–64. doi: 10.1016/s0140-6736(85)91550-8. [DOI] [PubMed] [Google Scholar]
- 166.Thein T, Tin T, Hla P, Phillips RE, Myint L, Tin Nu S, Warrell DA. Development of renal function abnormalities following bites by Russell's vipers (Daboia russelii siamensis) in Myanmar. Trans R Soc Trop Med Hyg. 1991;85:404–9. doi: 10.1016/0035-9203(91)90307-k. [DOI] [PubMed] [Google Scholar]
- 167.Maduwage K, Kularatne K, Wazil A, Gawarammana I. Coagulopthy, acute kidney injury and death following Hypnale zara envenoming: the first case report from Sri Lanka. Toxicon. 2011;58:641–3. doi: 10.1016/j.toxicon.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 168.Maduwage K, Isbister GK, Silva A, Bowatta S, Mendis S, Gawarammana I. Epidemiology and clinical effects of hump-nosed pit viper (Genus: Hypnale) envenoming in Sri Lanka. Toxicon. 2013;61:11–5. doi: 10.1016/j.toxicon.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 169.Gnanathasan A, Rodrigo C, Peranantharajah T, Coonghe A. Saw-scaled viper bites in Sri Lanka: is it a different subspecies? Clinical evidence from an authenticated case series. Am J Trop Med Hyg. 2012;86:254–7. doi: 10.4269/ajtmh.2012.11-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kochar DK, Tanwar PD, Norris RL, Sabir M, Nayak KC, Agrawal TD, Purohit VP, Kochar A, Simpson ID. Rediscovery of severe saw-scaled viper (Echis sochureki) envenoming in the Thar desert region of Rajasthan, India. Wilderness Environ Med. 2007;18:75–85. doi: 10.1580/06-WEME-OR-078R.1. [DOI] [PubMed] [Google Scholar]
- 171.Albuquerque PL, Silva Junior GB, Jacinto CN, Lima JB, Lima CB, Amaral YS, Veras MD, Mota RM, Daher EF. Acute kidney injury after snakebite accident treated in a tertiary care center. Nephrology. 2014;19:764–70. doi: 10.1111/nep.12327. [DOI] [PubMed] [Google Scholar]
- 172.Ylitalo P, Morsky P, Parviainen MT, Koivula T. Nephrotoxicity of tobramycin. Value of examining various protein and enzyme markers. Methods Find Exp Clin Pharmacol. 1991;13:281–7. [PubMed] [Google Scholar]
- 173.Glass S, Plant ND, Spencer DA. The effects of intravenous tobramycin on renal tubular function in children with cystic fibrosis. J Cyst Fibros. 2005;4:221–5. doi: 10.1016/j.jcf.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 174.McWilliam SJ, Antoine DJ, Sabbisetti V, Turner MA, Farragher T, Bonventre JV, Park BK, Smyth RL, Pirmohamed M. Mechanism-based urinary biomarkers to identify the potential for aminoglycoside-induced nephrotoxicity in premature neonates: a proof-of-concept study. PLoS One. 2012;7:e43809. doi: 10.1371/journal.pone.0043809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mohammadi-Karakani A, Asgharzadeh-Haghighi S, Ghazi-Khansari M, Seyed-Ebrahimi A, Ghasemi A, Jabari E. Enzymuria determination in children treated with aminoglycosides drugs. Hum Exp Toxicol. 2008;27:879–82. doi: 10.1177/0960327108100417. [DOI] [PubMed] [Google Scholar]
- 176.Wunnapuk K, Liu X, Peake P, Gobe G, Endre Z, Grice JE, Roberts MS, Buckley NA. Renal biomarkers predict nephrotoxicity after paraquat. Toxicol Lett. 2013;222:280–8. doi: 10.1016/j.toxlet.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 177.Wunnapuk K, Liu X, Gobe GC, Endre ZH, Peake PW, Grice JE, Roberts MS, Buckley NA. Kidney biomarkers in MCPA-induced acute kidney injury in rats: Reduced clearance enhances early biomarker performance. Toxicol Lett. 2014;225:467–78. doi: 10.1016/j.toxlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 178.Wunnapuk K, Gobe G, Endre Z, Peake P, Grice JE, Roberts MS, Buckley NA, Liu X. Use of a glyphosate-based herbicide-induced nephrotoxicity model to investigate a panel of kidney injury biomarkers. Toxicol Lett. 2014;225:192–200. doi: 10.1016/j.toxlet.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 179.Lin HY, Lee SC, Lin SF, Hsiao HH, Liu YC, Yang WC, Hwang DY, Hung CC, Chen HC, Guh JY. Urinary neutrophil gelatinase-associated lipocalin levels predict cisplatin-induced acute kidney injury better than albuminuria or urinary cystatin C levels. Kaohsiung J Med Sci. 2013;29:304–11. doi: 10.1016/j.kjms.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Askenazi DJ, Koralkar R, Hundley HE, Montesanti A, Parwar P, Sonjara S, Ambalavanan N. Urine biomarkers predict acute kidney injury in newborns. J Pediatr. 2012;161:270. doi: 10.1016/j.jpeds.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Levin A, Pate GE, Shalansky S, Al-Shamari A, Webb JG, Buller CE, Humphries KH. N-acetylcysteine reduces urinary albumin excretion following contrast administration: evidence of biological effect. Nephrol Dial Transplant. 2007;22:2520–4. doi: 10.1093/ndt/gfl707. [DOI] [PubMed] [Google Scholar]
- 182.Schentag JJ, Plaut ME. Patterns of urinary beta 2-microglobulin excretion by patients treated with aminoglycosides. Kidney Int. 1980;17:654–61. doi: 10.1038/ki.1980.77. [DOI] [PubMed] [Google Scholar]
- 183.Benohr P, Grenz A, Hartmann JT, Muller GA, Blaschke S. Cystatin C - a marker for assessment of the glomerular filtration rate in patients with cisplatin chemotherapy. Kidney Blood Press Res. 2006;29:32–5. doi: 10.1159/000092485. [DOI] [PubMed] [Google Scholar]
- 184.Pianta TJ, Chin M, Peake P, Buckley N, Pickering JW, Endre Z. Annual Scientific Meeting of the Australian and New Zealand Society of Nephrology. 2013. Plasma cystatin c is elevated in the absence of acute kidney injury following cisplatin with contemporary antiemetics. In, Brisbane, Australia, ; 41.
- 185.Bachorzewska-Gajewska H, Malyszko J, Sitniewska E, Malyszko JS, Pawlak K, Mysliwiec M, Lawnicki S, Szmitkowski M, Dobrzycki S. Could neutrophil-gelatinase-associated lipocalin and cystatin C predict the development of contrast-induced nephropathy after percutaneous coronary interventions in patients with stable angina and normal serum creatinine values? Kidney Blood Press Res. 2007;30:408–15. doi: 10.1159/000109102. [DOI] [PubMed] [Google Scholar]
- 186.Briguori C, Visconti G, Rivera NV, Focaccio A, Golia B, Giannone R, Castaldo D, De Micco F, Ricciardelli B, Colombo A. Cystatin C and contrast-induced acute kidney injury. Circulation. 2010;121:2117–22. doi: 10.1161/CIRCULATIONAHA.109.919639. [DOI] [PubMed] [Google Scholar]
- 187.Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin Pract. 2008;108:c176–81. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- 188.McCullough PA, Williams FJ, Stivers DN, Cannon L, Dixon S, Alexander P, Runyan D, David S. Neutrophil gelatinase-associated lipocalin: a novel marker of contrast nephropathy risk. Am J Nephrol. 2012;35:509–14. doi: 10.1159/000339163. [DOI] [PubMed] [Google Scholar]
- 189.Gaspari F, Cravedi P, Mandala M, Perico N, de Leon FR, Stucchi N, Ferrari S, Labianca R, Remuzzi G, Ruggenenti P. Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: a pilot prospective case-control study. Nephron Clin Pract. 2010;115:c154–60. doi: 10.1159/000312879. [DOI] [PubMed] [Google Scholar]
- 190.Nakamura T, Sugaya T, Node K, Ueda Y, Koide H. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am J Kidney Dis. 2006;47:439–44. doi: 10.1053/j.ajkd.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 191.Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland KE, Gioules CJ, Bonventre JV. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2010;77:708–14. doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]


