Abstract
The objective of the present study was to review the available pharmacokinetic evidence for the utility of cystatin C (CysC) as a marker of renal function to predict the dose of renally excreted drugs.The bibliographic search used PubMed and EMBASE databases, from its inception through to January 2014, with the following keywords ‘pharmacokinetics’ and ‘cystatin C’.Sixteen pharmacokinetic publications were identified and seven drugs primarily excreted by the kidney were studied. Among them, only one study was performed in children, the others were performed in adults and/or elderly subjects, either healthy volunteers or patients with variable clinical conditions, such as cystic fibrosis and cancer. Most of studies (n = 13/16) demonstrated that CysC was better correlated with clearance/trough concentration of evaluated drugs compared with creatinine.Our review supports that CysC is a good marker of renal function to predict dose of renally excreted drugs. Efforts should be made to evaluate the impact of CysC in special populations in order to define its clinical value in dosing optimization.
Keywords: cystatin C, renal function, renal elimination, pharmacokinetics, clearance, glomerular filtration rate, dose regimen
Introduction
Renal function has a major impact on the pharmacokinetics and dose of predominantly renally excreted drugs. Quantification of renal function is central for dosage adjustment in patients with impaired renal function (i.e. in critically ill patients, the elderly) or in patients with renal immaturity (i.e. neonates particularly if premature), as renal function fluctuates considerably in such conditions 1,2.
Renal elimination is a drug-dependent process. Glomerular filtration rate (GFR) is, in general, accepted as the best overall measure of renal function and used for dosage adjustment. In clinical practice, the most common method to determine GFR is based on serum creatinine concentrations, allowing the calculation of creatinine clearance. However, the use of creatinine as a marker of GFR has its own limitation. Creatinine is not only filtered, but also secreted by the renal proximal tubules. The calculated creatinine clearance value may overestimate the true GFR, in particular for patients with decreased renal function 3–6 and be inaccurate in neonates 7,8. Additional methods, which used exogenous compounds (iohexol, inulin, sinistrin, radiolabelled isotope, aminoglycosides) 9–11, exist to estimate/predict GFR, but mainly for research purposes, as they are labour intensive, time consuming, expensive to perform and require a strict procedure of administration, making them difficult to use in routine clinical practice 12–16.
An alternative biomarker of GFR would be of great interest and many studies have been conducted in recent years to evaluate cystatin C (CysC) 17–21. CysC is a non-glycosylated basic protein with a low molecular weight of 13 kDa. It is produced at a constant rate by all nucleated cells 22 and not bound to plasma proteins. CysC is freely filtered through the renal glomerulus and subsequently reabsorbed and catabolized in proximal renal tubules 23–25. The results from previous studies have shown that serum CysC was an adequate marker of GFR and significantly outperformed serum creatinine for the detection of impaired GFR in critically ill patients 26. Meta-analyses also indicated that CysC was superior to serum creatinine in the determination of GFR injury 27–29.
Despite these results, the use of CysC for drug dosage adjustment remains limited. The purpose of the present study was to review the available pharmacokinetic evidence for the utility of CysC as a marker of renal function to predict dose of renally excreted drugs.
Methods
Search strategy, study selection and validation
Relevant publications were identified through electronic searches using PubMed and EMBASE databases up to January 2014. The following keywords ‘Pharmacokinetics’ AND ‘cystatin C’ with limitation to ‘human’ were used.
Studies were eligible if 1) they were pharmacokinetic studies and 2) CysC was used as a marker of renal function. All publications were screened on title, abstract and then full text independently by two investigators.
Data extraction
All data from eligible studies were independently extracted by two investigators using a standardized extraction form with the following information: year of publication and journal, studied patients’ characteristics (number of patients, age, weight and clinical condition), analytical method for creatinine (enzymatic or Jaffé method) and CysC, drug analytical method for determination of drug concentration and pharmacokinetic parameters.
Results
The electronic search based on the screening of title and abstract yielded a total of 165 reports from PubMed and 297 from EMBASE. The study screening process is presented in Figure1. After assessing the full text articles for eligibility, 16 articles were identified. They were published between 2004 and 2014 and conducted in three therapeutic classes: antimicrobials (vancomycin 30–35, amikacin 36, cefuroxime 37 and arbekacin 38), anti-cancer drugs (topotecan 39, carboplatin) 40 and cardiovascular drug (digoxin 41–43). Most studies were conducted with vancomycin (44%, n = 7). Corresponding study characteristics are presented in Tables3. All drugs are renally excreted. The percentage of renal clearance ranged from 50% (topotecan) to 96 % (carboplatin) 44–50.
Figure 1.
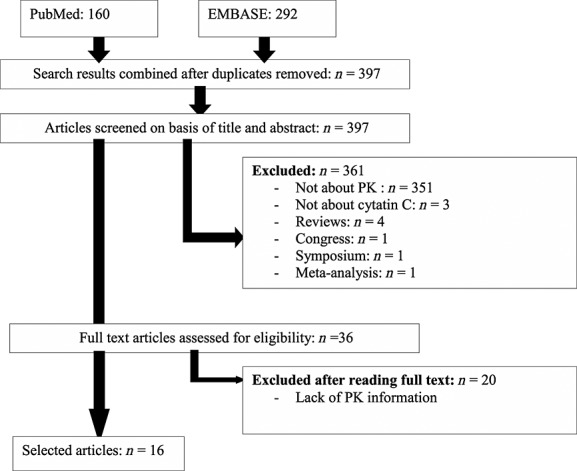
Study screening process
Table 3.
Cardiovascular and anticancer drugs cystatin C pharmacokinetic study
| Drug class | Drug | Study | Population | Detection method | Renal function | Renal function-PK | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Patients | Age (years) | Creatinine | CysC | Drug | Creatinine | CysC | Significant correlation between CysC and drug clearance (r2) | Significant correlation between CysC and drug C0 (r2) | CysC is better correlated with PK compared to creatinine (r2) | |||
| Cardiac drug | Digoxin | Hallberg et al. 41 | 149 | Adult and elderly patients | 55–106 | Jaffé | PENIA | FPIA | Concentration | Concentration | ― | Yes (0.20) | Yes (0.14) |
| Garcia et al. 42 | 61 | Adult and elderly patients with cardiac insufficiency | 24–92 | Jaffé | PENIA | FPIA | CG | Larsson | Yes (0.25) | Yes | Yes (0.16) | ||
| O'Riordan et al. 43 | 18 | Elderly healthy patients | 67–86 | Jaffé | PENIA | FPIA | Concentration | Concentration | ― | No (0.09) | No (0.16) | ||
| Anticancer | Carboplatin | Thomas et al. 40 | 45 | Adult and elderly patients with cancer | 21–79 | Jaffé | PENIA | FAAS | CG | Concentration | Yes | ― | Yes |
| Schmitt et al. 61 | 357 | Adult and elderly patients with cancer | 21–87 | Jaffé | PENIA | FAAS | Concentration | Concentration | Yes | ― | ― | ||
| Topotecan | Hoppe et al. 39 | 59 | Adult and elderly patients with cancer | 18–76 | Jaffé | PENIA | HPLC | Concentration | Concentration | Yes (0.43) | ― | Yes (0.23) | |
CG, Cockcroft–Gault equation
CysC, cystatin C
FAAS, flameless atomic absorption spectrophotometric analysis
FPIA, fluorescence polarization immunoassay
HPLC, high-performance liquid chromatography
PENIA, particle-enhanced nephelometric immunoassay.
Table 1.
Summary of seven evaluated drugs
| Drug class | Drugs | Number of studies | Renal elimination (%) |
|---|---|---|---|
| Antimicrobials | Vancomycin | 7 | >80 |
| Arbekacin | 1 | ∼50 | |
| Amikacin | 1 | 68–80 | |
| Cefuroxim | 1 | >90 | |
| Anticancer drugs | Carboplatin | 2 | 96 |
| Topotecan | 1 | ∼50 | |
| Cardiovascular drug | Digoxin | 3 | 79–83 |
Among these 16 studies, only one study was performed in children 36 and the others were performed in adults and/or elderly patients (>65 years). The particle-enhanced turbidimetric immunoassay (PETIA) (n = 4) and particle-enhanced nephelometric immunoassay (PENIA) (n = 12) were used to measure the serum concentrations of CysC. The Jaffe method (n = 7) and immune-enzymatic method (n = 7) were used to measure serum concentrations of creatinine.
Different equations were used to quantify renal function (Table2). For creatinine-based formulae, the Cockcroft & Gault (CG) equation (n = 10), modification of diet in renal disease equation (MDRD) (n = 1) or Schwartz formula (n = 1) were used. One study used creatinine clearance determined with serum and urine creatinine concentrations and five studies used only serum creatinine concentration. For CysC-based formulae, Hoek (n = 4), Rules (n = 1), Larsson (n = 3), Foldin (n = 1), Grubb (n = 1) and Söstrom (n = 1) formulae were used. Seven studies used directly serum CysC concentrations.
Table 2.
Antimicrobial cystatin C pharmacokinetic study
| Drug | Study | Population | Analytical methods | Renal function | Renal function-PK | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Patients | Age range (years) | Creatinine | CysC | Drug | GFR based on creatinine | GFR based on CysC | Significant correlation between CysC and drug clearances (r2) | Significant correlation between CysC and drug C0 (r2) | CysC is better correlated with PK compared to creatinine (r2) | ||
| Vancomycin | Chen et al. 30 | 65 | Adult and elderly patients | 19–78 | Enzymatic | PETIA | FPIA | Creatinine clearance* | Flodin† | Yes (0.85) | ― | Yes (0.64) |
| Tanaka et al. 54 | 60 | Adult patients | 16–64 | ― | PENIA | ― | CG¶¶ | Hoek‡ | ― | Yes (0.78) | Yes (0.54) | |
| 105 | Elderly patients | 66–95 | ― | PENIA | ― | CG | Hoek | ― | Yes (0.79) | Yes (0.18) | ||
| Kees et al. 31 | 25 | Critically ill adults and elderly patients | 31–82 | Enzymatic | PENIA | HPLC | CG | Hoek | Yes (0.70) | ― | Yes (0.37) | |
| Suzuki et al. 32 | 18 | Adult patients | ― | Enzymatic | PENIA | FPIA | CG/ MDRD§ | Rules¶/Hoek | ― | Yes (0.42/0.49) | Yes (0.13/0.19) | |
| Tanaka et al. 33 | 164 | Adult and elderly patients | 17–95 | ― | PENIA | FPIA | ― | Hoek | Yes (0.84) | ― | ― | |
| Okamoto et al. 34 | 24 | Elderly patients | 66–87 | Enzymatic | PETIA | FPIA | CG | Larsson** | Yes (0.78) | ― | Yes (0.47) | |
| Chung et al. 35 | 678 | Adult and elderly patients | 18–96 | Jaffé | PETIA | FPIA | CG | Concentration | Yes | ― | Yes | |
| Amikacin | Halacova et al. 36 | 71 | Adults and children with cystic fibrosis | 4–28 | Enzymatic | PETIA | FPIA | CG / Schwatz†† | Grubb‡‡/Larsson | Yes | ― | Yes |
| Cefuroxime | Viberg et al. 37 | 97 | Adult patients | 24–95 | Enzymatic | PENIA | HPLC | Concentration | Concentration | ― | Yes | Yes |
| Arbekacin | Otsuka et al. 38 | 95 | Adult and elderly patients | 73–16 | Enzymatic | PENIA | FPIA | CG | Sjöström§§ | ― | Yes (0.89) | Yes (0.64) |
CG, Cockcroft–Gault equation
CysC, cystatin C
FPIA, fluorescence polarization immunoassay
HPLC, high-performance liquid chromatography
MDRD, modification of diet in renal disease equation
PENIA, particle-enhanced nephelometric immunoassay
PETIA, particle-enhanced turbidimetric immunoassay.
Creatinine clearance = creatinine concentration in urine x urine flow rate / plasma creatinine concentration.
Flodin equation: eGFR = 79.901 × CysC–1.4389.
Hoek equation: eGFR = (80.35 / CysC) – 4.32.
MDRD equation: eGFR = 186.3 × (creatinine / 88.4) –1.154 × age–0.203 × 0.742 (if female) × 1.21 (if African).
Rules equation: eGFR = 66.8 × CysC –1,3.
Larsson equation: eGFR = 99.43 × CysC–1.5837.
Schwartz equation: eGFR = k × height / serum creatinine; the value of k varies as a function of age and gender being 0.33 in preterm infants, 0.45 in term infants, 0.55 in child or adolescent girls and 0.7 in adolescent boys.
Grubb equation: eGFR = 84.69 × CysC–1.68 × 1.384 (if a child <14 years).
Sjöström equation eGFR = (124 / CysC) – 22.3.
Cockroft–Gault: eGFR= k × weight × (140 – age) / creatinine; the value of k is 1.23 for men and 1.04 for women.
The reported correlations between renal function (determined with the different biomarkers and formulae) and clearance of renal excreted drugs were then analyzed. As demonstrated in Table2 for pharmacokinetic studies of antimicrobials, CysC was significantly correlated with the clearance/trough concentration of vancomycin (n = 7), amikacin (n = 1), cefuroxime (n = 1) and arbekacin (n = 1). In Table3, the pharmacokinetic studies of anticancer and cardiovascular drugs, CysC was significantly correlated with carboplatin (n = 2) and topotecan (n = 1) clearance. For digoxin studies (n = 3), two studies showed that CysC was significantly correlated with digoxin clearance/trough concentration, but one study found that neither CysC nor creatinine was significantly correlated with digoxin trough concentration. Among all the 16 published studies, 15 studies demonstrated the significant impact of CysC on clearance/trough concentration of evaluated drugs, except for one study conducted in elderly patients (see Table3).
In addition, 14 studies compared directly the impact of CysC and creatinine clearance on drug elimination. Thirteen of them showed that CysC was superior to creatinine to predict elimination of the evaluated drug.
Discussion
There is a need to optimize the evaluation of renal function, as it remains a central factor to predict accurately the dose of renally excreted drugs. Both creatinine and CysC are available in clinical practice as biomarkers, but creatinine determination is used in most cases. In the present work, 16 pharmacokinetic studies identified in the literature were used to compare two biomarkers of renal function. The comparison was based on different formulae to quantify GFR with creatinine or CysC, showing CysC was a better predictor of the elimination of predominantly renally excreted drugs.
Creatinine is produced from creatine, which is a component of muscle. It is filtered and secreted by proximal renal tubules. The calculated creatinine clearance values are known to overestimate GFR, in particular for patients with decreased renal function 3–6. In addition, for some special patients groups, such as neonates, the influence of residual maternal creatinine and interference with endogenous compounds and drugs used in sick patients (such as ketoacids, bilirubin, cephalosporins) may lead to inaccuracies in predicting GFR 7,8. CysC is freely filtered through the renal glomerulus and subsequently reabsorbed and catabolized in proximal renal tubules 23–25. CysC is a potential alternative marker to creatinine, as it is not affected by age, gender, diet or inflammation, making it an ideal endogenous marker of renal function 51,52.
Importantly, the consistent results were found in elderly patients. Renal function has a profound impact on dosage adjustment in this special population, as it is well known that drug elimination through the kidneys is impaired, due to reduced renal blood flow and GFR 53. Our results supported that CysC was well correlated with the elimination of renally excrelly drugs. This was in agreement with previous findings, showing that CysC was more precise to predict GFR than the creatinine-based Cockcroft–Gault equation in elderly patients 54. Only one study was conducted in children, showing that amikacin clearance was better correlated with CysC than serum creatinine 36. In addition, Neamatollah et al. also showed that CysC was more sensitive to detect acute kidney injury in critically ill children than creatinine 55. Given that renal maturation has a major impact in children, further studies are required to confirm the role of CysC to predict the dose of renally excreted drugs in this vulnerable population.
The underlying disease and mechanisms of renal impairment were variable in the present analysis, as the studies were conducted in healthy volunteers, cystic fibrosis and cancer patients. In patients with cystic fibrosis, amikacin clearance was better correlated with CysC than creatinine 36. These results are in accordance with the findings by Beringer et al. 56 who reported that the CysC formula demonstrated greater sensitivity and specificity to quantify GFR in cystic fibrosis patients compared with the equations with serum creatinine (Cockcroft–Gault; MDRD). For cancer patients, carboplatin and topotecan clearances were better correlated with CysC than creatinine clearance, in accordance with the findings of Barnfield et al. 57. Discordances were reported with digoxin [41–43. O'Riordan et al. reported that neither CysC nor creatinine was significantly correlated with digoxin trough concentration. This is probably related to the low number of patients (n = 18) and the limited alteration of renal function (serum CysC values of 0.7 to1.9 mg l–1, serum creatinine values of 70 – 154 µmol l–1).
According to differences between these markers in terms of renal handling, analytical methods, impact of physiological factors and origin of the formulae used, differences in the quantification of renal function are expected. Indeed, there is no clear consensus on the best CysC-based equation to predict the individual dose of a renally eliminated drug. Tanaka et al. showed that the Hoek formula was more accurate than the Grubb, Sjostrom, and Larson's formulae to predict vancomycin clearance and GFR 58. However, using arbekacin as test drug, Otsuka et al. reported that the Sjostrom equation was more accurate for determining the initial drug dose than the Hoek and Grubb equations in a Japanese population 38.
The impact of biomarker analytical methods on pharmacokinetics should also be analyzed carefully. Recently, we have reported that vancomycin population pharmacokinetic models in neonates cannot be transferred from the initial to different clinical settings because of inter-centre differences in the laboratory methods to measure serum creatinine 59. The same caution should be taken into consideration to interpret serum CysC concentrations, as analytical methods (namely PENIA, PETIA and ELISA) are used indifferently. It was proposed that CysC ELISA values required normalization by a factor 0.66 to correct the difference with PENIA and PETIA methods 60, although additional data are required for validation.
Conclusion
Our review supports that CysC is a good marker of renal function and can be used to adjust the dose of renally eliminated drugs in adult and elderly patients. Efforts should be made to evaluate the impact of CysC in special populations (e.g. paediatrics, critically ill patients), as renal function fluctuates considerably and a sensitive biomarker is required for dosage optimisation in these patients.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare this work was supported by the GRIP (Global Research in Paediatrics, European Commission FP7 project, grant agreement number 261060) and ‘The Fundamental Research Funds of Shandong University’, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Chan JC, Williams DM, Roth KS. Kidney failure in infants and children. Pediatr Rev. 2002;23:47–60. doi: 10.1542/pir.23-2-47. [DOI] [PubMed] [Google Scholar]
- 2.Ataei N, Bazargani B, Ameli S, Madani A, Javadilarijani F, Moghtaderi M, Abbasi A, Shams S, Ataei F. Early detection of acute kidney injury by serum cystatin C in critically ill children. Pediatr Nephrol. 2014;29:133–8. doi: 10.1007/s00467-013-2586-5. [DOI] [PubMed] [Google Scholar]
- 3.Urakami Y, Kimura N, Okuda M, Masuda S, Katsura T, Inui K. Transcellular transport of creatinine in renal tubular epithelial cell line LLC-PK1. Drug Metab Pharmacokinet. 2005;20:200–5. doi: 10.2133/dmpk.20.200. [DOI] [PubMed] [Google Scholar]
- 4.Sansoè G, Ferrari A, Castellana CN, Bonardi L, Villa E, Manenti F. Cimetidine administration and tubular creatinine secretion in patients with compensated cirrhosis. Clin Sci (Lond) 2002;102:91–8. [PubMed] [Google Scholar]
- 5.Kemperman FA, Surachno J, Krediet RT, Arisz L. Cimetidine improves prediction of the glomerular filtration rate by the Cockcroft–Gault formula in renal transplant recipients. Transplantation. 2002;73:770–4. doi: 10.1097/00007890-200203150-00020. [DOI] [PubMed] [Google Scholar]
- 6.Branten AJ, Vervoort G, Wetzels JF. Serum creatinine is a poor marker of GFR in nephrotic syndrome. Nephrol Dial Transplant. 2005;20:707–11. doi: 10.1093/ndt/gfh719. [DOI] [PubMed] [Google Scholar]
- 7.Van den Anker JN. Renal function in preterm infants. Eur J Pediatr. 1997;156:583–4. [PubMed] [Google Scholar]
- 8.Peake M, Whiting M. Measurement of serum creatinine--current status and future goals. Clin Biochem Rev. 2006;27:173–84. [PMC free article] [PubMed] [Google Scholar]
- 9.Rosner MH, Bolton WK. Renal function testing. Am J Kidney Dis Off J Natl Kidney Found. 2006;47:174–83. doi: 10.1053/j.ajkd.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005;89:457–73. doi: 10.1016/j.mcna.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W, Biran V, Jacqz-Aigrain E. Amikacin maturation model as a marker of renal maturation to predict glomerular filtration rate and vancomycin clearance in neonates. Clin Pharmacokinet. 2013;52:1127–34. doi: 10.1007/s40262-013-0101-6. [DOI] [PubMed] [Google Scholar]
- 12.Kazama JJ, Kutsuwada K, Ataka K, Maruyama H, Gejyo F. Serum cystatin C reliably detects renal dysfunction in patients with various renal diseases. Nephron. 2002;91:13–20. doi: 10.1159/000057599. [DOI] [PubMed] [Google Scholar]
- 13.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function - measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 14.Coll E, Botey A, Alvarez L, Poch E, Quintó L, Saurina A, Vera M, Piera C, Darnell A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 15.Le Bricon T, Leblanc I, Benlakehal M, Gay-Bellile C, Erlich D, Boudaoud S. Evaluation of renal function in intensive care: plasma cystatin C vs. creatinine and derived glomerular filtration rate estimates. Clin Chem Lab Med. 2005;43:953–7. doi: 10.1515/CCLM.2005.163. [DOI] [PubMed] [Google Scholar]
- 16.Donadio C, Lucchesi A, Ardini M, Giordani R. Cystatin C, beta 2-microglobulin, and retinol-binding protein as indicators of glomerular filtration rate: comparison with plasma creatinine. J Pharm Biomed Anal. 2001;24:835–42. doi: 10.1016/s0731-7085(00)00550-1. [DOI] [PubMed] [Google Scholar]
- 17.Liu J. Evaluation of serum cystatin C for diagnosis of acute rejection after renal transplantation. Transplant Proc. 2012;44:1250–3. doi: 10.1016/j.transproceed.2012.01.138. [DOI] [PubMed] [Google Scholar]
- 18.Cai X, Long Z, Lin L, Feng Y, Zhou N, Mai Q. Serum cystatin C is an early biomarker for assessment of renal function in burn patients. Clin Chem Lab Med. 2012;50:667–71. doi: 10.1515/cclm-2011-0838. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy N, Arumugasamy K, Anand U, Anand CV, Aruna V, Venu G. Serum cystatin C levels in renal transplant recipients. Indian J Clin Biochem. 2011;26:120–4. doi: 10.1007/s12291-010-0084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumaresan R, Giri P. A comparison of serum cystatin C and creatinine with glomerular filtration rate in Indian patients with chronic kidney disease. Oman Med J. 2011;26:421–5. doi: 10.5001/omj.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yap M, Lamarche J, Peguero A, Courville C. Serum cystatin C versus serum creatinine in the estimation of glomerular filtration rate in rhabdomyolysis. J Renal Care. 2011;37:155–7. doi: 10.1111/j.1755-6686.2011.00228.x. [DOI] [PubMed] [Google Scholar]
- 22.Abrahamson M, Olafsson I, Palsdottir A, Ulvsbäck M, Lundwall A, Jensson O, Grubb A. Structure and expression of the human cystatin C gene. Biochem J. 1990;268:287–94. doi: 10.1042/bj2680287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56:409–14. doi: 10.3109/00365519609088795. [DOI] [PubMed] [Google Scholar]
- 24.Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol. 1992;38:20–7. [PubMed] [Google Scholar]
- 25.Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function - a review. Clin Chem Lab Med. 1999;37:389–95. doi: 10.1515/CCLM.1999.064. [DOI] [PubMed] [Google Scholar]
- 26.Delanaye P, Cavalier E, Morel J, Mehdi M, Maillard N, Claisse G, Lambermont B, Dubois BE, Damas P, Krzesinski J-M, Lautrette A, Mariat C. Detection of decreased glomerular filtration rate in intensive care units: serum cystatin C versus serum creatinine. BMC Nephrol. 2014;15:9. doi: 10.1186/1471-2369-15-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 28.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40:221–6. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 29.Sh orr AF, Combes A, Kollef MH, Chastre J. Methicillin-resistant Staphylococcus aureus prolongs intensive care unit stay in ventilator-associated pneumonia, despite initially appropriate antibiotic therapy. Crit Care Med. 2006;34:700–6. doi: 10.1097/01.CCM.0000201885.57697.21. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Feng J, Li B, Zhang L, Yang Y. Estimation of safe and effective dose of vancomycin in MRSA-infected patients using serum cystatin C concentrations. Int J Clin Pharmacol Ther. 2013;51:161–9. doi: 10.5414/CP201776. [DOI] [PubMed] [Google Scholar]
- 31.Kees MG, Hilpert JW, Gnewuch C, Kees F, Voegeler S. Clearance of vancomycin during continuous infusion in Intensive Care Unit patients: correlation with measured and estimated creatinine clearance and serum cystatin C. Int J Antimicrob Agents. 2010;36:545–8. doi: 10.1016/j.ijantimicag.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki A, Imanishi Y, Nakano S, Niwa T, Ohmori T, Shirai K, Yoshida S, Furuta N, Takemura M, Ito H, Ieiri I, Seishima M, Ogura S, Itoh Y. Usefulness of serum cystatin C to determine the dose of vancomycin in critically ill patients. J Pharm Pharmacol. 2010;62:901–7. doi: 10.1211/jpp.62.07.0011. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka A, Aiba T, Otsuka T, Suemaru K, Nishimiya T, Inoue T, Murase M, Kurosaki Y, Araki H. Population pharmacokinetic analysis of vancomycin using serum cystatin C as a marker of renal function. Antimicrob Agents Chemother. 2010;54:778–82. doi: 10.1128/AAC.00661-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamoto G, Sakamoto T, Kimura M, Ukishima Y, Sonoda A, Mori N, Kato Y, Maeda T, Kagawa Y. Serum cystatin C as a better marker of vancomycin clearance than serum creatinine in elderly patients. Clin Biochem. 2007;40:485–90. doi: 10.1016/j.clinbiochem.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Chung JY, Jin SJ, Yoon JH, Song YG. Serum cystatin C is a major predictor of vancomycin clearance in a population pharmacokinetic analysis of patients with normal serum creatinine concentrations. J Korean Med Sci. 2013;28:48–54. doi: 10.3346/jkms.2013.28.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halacova M, Kotaska K, Kukacka J, Vavrova V, Kuzelova M, Ticha J, Prusa R. Serum cystatin C level for better assessment of glomerular filtration rate in cystic fibrosis patients treated by amikacin. J Clin Pharm Ther. 2008;33:409–17. doi: 10.1111/j.1365-2710.2008.00932.x. [DOI] [PubMed] [Google Scholar]
- 37.Viberg A, Lannergård A, Larsson A, Cars O, Karlsson MO, Sandström M. A population pharmacokinetic model for cefuroxime using cystatin C as a marker of renal function. Br J Clin Pharmacol. 2006;62:297–303. doi: 10.1111/j.1365-2125.2006.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Otsuka T, Tanaka A, Suemaru K, Inoue T, Nishimiya T, Murase M, Araki H. Evaluation of the clinical application of cystatin C, a new marker of the glomerular filtration rate, for the initial dose-setting of arbekacin. J Clin Pharm Ther. 2008;33:227–35. doi: 10.1111/j.1365-2710.2008.00905.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoppe A, Séronie-Vivien S, Thomas F, Delord J-P, Malard L, Canal P, Chatelut E. Serum cystatin C is a better marker of topotecan clearance than serum creatinine. Clin Cancer Res. 2005;11:3038–44. doi: 10.1158/1078-0432.CCR-04-2086. [DOI] [PubMed] [Google Scholar]
- 40.Schmitt A, Gladieff L, Lansiaux A, Bobin-Dubigeon C, Etienne-Grimaldi M-C, Boisdron-Celle M, Serre-Debauvais F, Pinguet F, Floquet A, Billaud E, Le Guellec C, Penel N, Campone M, Largillier R, Capitain O, Fabbro M, Houede N, Medioni J, Bougnoux P, Lochon I, Chatelut E. A universal formula based on cystatin C to perform individual dosing of carboplatin in normal weight, underweight, and obese patients. Clin Cancer Res. 2009;15:3633–9. doi: 10.1158/1078-0432.CCR-09-0017. [DOI] [PubMed] [Google Scholar]
- 41.Hallberg P, Melhus H, Hansson L-O, Larsson A. Cystatin C vs. creatinine as markers of renal function in patients on digoxin treatment. Ups J Med Sci. 2004;109:247–53. doi: 10.3109/2000-1967-087. [DOI] [PubMed] [Google Scholar]
- 42.Garcia A, Hermida J, Tutor JC. Estimation of the glomerular filtration rate from serum creatinine and cystatin C with regard to therapeutic digoxin monitoring. J Clin Pharmacol. 2007;47:1450–5. doi: 10.1177/0091270007305503. [DOI] [PubMed] [Google Scholar]
- 43.O'Riordan S, Ouldred E, Brice S, Jackson SHD, Swift CG. Serum cystatin C is not a better marker of creatinine or digoxin clearance than serum creatinine. Br J Clin Pharmacol. 2002;53:398–402. doi: 10.1046/j.1365-2125.2002.01549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matzke GR, Zhanel GG, Guay DR. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet. 1986;11:257–82. doi: 10.2165/00003088-198611040-00001. [DOI] [PubMed] [Google Scholar]
- 45.Lanao JM, Pedraz JL, Navarro AS, Dominguez-Gil A. Influence of dose in the urinary excretion of amikacin. Int J Clin Pharmacol. 1984;22:538–42. [PubMed] [Google Scholar]
- 46.Fujii R, Fujita K, Sakata Y, Abe T, Tajima T, Terashima I, Meguro H, Watanabe N, Mikuni K, Sakai T. Clinical studies of arbekacin sulfate in the pediatric field. Jpn J Antibiot. 1994;47:57–83. [PubMed] [Google Scholar]
- 47.Foord RD. Cefuroxime: human pharmacokinetics. Antimicrob Agents Chemother. 1976;9:741–747. doi: 10.1128/aac.9.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herben VM, ten Bokkel Huinink WW, Beijnen JH. Clinical pharmacokinetics of topotecan. Clin Pharmacokinet. 1996;31:85–102. doi: 10.2165/00003088-199631020-00001. [DOI] [PubMed] [Google Scholar]
- 49.Gaver RC, Deeb G. High-performance liquid chromatographic procedures for the analysis of carboplatin in human plasma and urine. Cancer Chemother Pharmacol. 1986;16:201–6. doi: 10.1007/BF00293978. [DOI] [PubMed] [Google Scholar]
- 50.Hinderling PH, Hartmann D. Pharmacokinetics of digoxin and main metabolites/derivatives in healthy humans. Ther Drug Monit. 1991;13:381–401. doi: 10.1097/00007691-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Zhang PP, Zhan JF, Xie HL, Li LS, Liu ZH. Evaluation of glomerular filtration rate using cystatin C in diabetic patients analysed by multiple factors including tubular function. J Int Med Res. 2010;38:473–83. doi: 10.1177/147323001003800211. [DOI] [PubMed] [Google Scholar]
- 52.Aksun SA, Ozmen D, Ozmen B, Parildar Z, Mutaf I, Turgan N, Habif S, Kumanlioğluc K, Bayindir O. Beta2-microglobulin and cystatin C in type 2 diabetes: assessment of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 2004;112:195–200. doi: 10.1055/s-2004-817933. [DOI] [PubMed] [Google Scholar]
- 53.Turnheim K. Drug therapy in the elderly. Exp Gerontol. 2004;39:1731–8. doi: 10.1016/j.exger.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka A, Suemaru K, Otsuka T, Ido K, Nishimiya T, Sakai I, Hasegawa H, Inoue T, Murase M, Yasukawa M, Araki H. Estimation of the initial dose setting of vancomycin therapy with use of cystatin C as a new marker of renal function. Ther Drug Monit. 2007;29:261–4. doi: 10.1097/FTD.0b013e31803bcfd2. [DOI] [PubMed] [Google Scholar]
- 55.Ataei N, Bazargani B, Ameli S, Madani A, Javadilarijani F, Moghtaderi M, Abbasi A, Shams S, Ataei F. Early detection of acute kidney injury by serum cystatin C in critically ill children. Pediatr Nephrol. 2014;29:133–8. doi: 10.1007/s00467-013-2586-5. [DOI] [PubMed] [Google Scholar]
- 56.Beringer PM, Hidayat L, Heed A, Zheng L, Owens H, Benitez D, Rao AP. GFR estimates using cystatin C are superior to serum creatinine in adult patients with cystic fibrosis. J Cyst Fibros. 2009;8:19–25. doi: 10.1016/j.jcf.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Barnfield MC, Burniston MT, Reid U, Graham AM, Henderson M, Picton SV. Cystatin C in assessment of glomerular filtration rate in children and young adults suffering from cancer. Nucl Med Commun. 2013;34:609–14. doi: 10.1097/MNM.0b013e328360d929. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka A, Suemaru K, Otsuka T, Ido K, Nishimiya T, Sakai I, Hasegawa H, Yasukawa M, Inoue T, Murase M, Araki H. Hoek's formula, a cystatin C-based prediction formula for determining the glomerular filtration rate, is the most effective method for original adjusting the dosage of vancomycin. Int J Clin Pharmacol Ther. 2007;45:592–7. doi: 10.5414/cpp45592. [DOI] [PubMed] [Google Scholar]
- 59.Zhao W, Kaguelidou F, Biran V, Zhang D, Allegaert K, Capparelli EV, Holford N, Kimura T, Lo YL, Peris JE, Thomson A, van den Anker JN, Fakhoury M, Jacqz-Aigrain E. External evaluation of population pharmacokinetic models of vancomycin in neonates: The transferability of published models to different clinical settings. Br J Clin Pharmacol. 2012;75:1068–80. doi: 10.1111/j.1365-2125.2012.04406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hossain MA, Emara M, El Moselhi H, Shoker A. Comparing measures of cystatin C in human serum by three methods. Am J Nephrol. 2009;29:381–91. doi: 10.1159/000168486. [DOI] [PubMed] [Google Scholar]
- 61.Thomas F, Séronie-Vivien S, Gladieff L, Dalenc F, Durrand V, Malard L, Lafont T, Poublanc M, Bugat R, Chatelut E. Cystatin C as a new covariate to predict renal elimination of drugs: application to carboplatin. Clin Pharmacokinet. 2005;44:1305–16. doi: 10.2165/00003088-200544120-00009. [DOI] [PubMed] [Google Scholar]


