Abstract
Aims
Adverse drug events are an important cause of emergency department visits, unplanned admissions and prolonged hospital stays. Our objective was to synthesize the evidence on the effect of early in-hospital pharmacist-led medication review on patient-oriented outcomes based on observed data.
Methods
We systematically searched eight bibliographic reference databases, electronic grey literature, medical journals, conference proceedings, trial registries and bibliographies of relevant papers. We included studies that employed random or quasi-random methods to allocate subjects to pharmacist-led medication review or control. Medication review had to include, at a minimum, obtaining a best possible medication history and reviewing medications for appropriateness and adverse drug events. The intervention had to be initiated within 24 h of emergency department presentation or 72 h of admission. We extracted data in duplicate and pooled outcomes from clinically homogeneous studies of the same design using random effects meta-analysis.
Results
We retrieved 4549 titles of which seven were included, reporting the outcomes of 3292 patients. We pooled data from studies of the same design, and found no significant differences in length of hospital admission (weighted mean difference [WMD] –0.04 days, 95% confidence interval [CI] –1.63, 1.55), mortality (odds ratio [OR] 1.09, 95% CI 0.69, 1.72), readmissions (OR 1.15, 95% CI 0.81, 1.63) or emergency department revisits at 3 months (OR 0.60, 95% CI 0.27, 1.32). Two large studies reporting reductions in readmissions could not be included in our pooled estimates due to differences in study design.
Conclusions
Wide confidence intervals suggest that additional research is likely to influence the effect size estimates and clarify the effect of medication review on patient-oriented outcomes. This systematic review failed to identify an effect of pharmacist-led medication review on health outcomes.
Keywords: adverse drug events, clinical pharmacist, clinical pharmacy, drug related problems, medication history, medication review
Introduction
Prescription and over the counter medications account for 19% of healthcare spending in countries belonging to the Organization for Economic Co-operation and Development (OECD), with the United States reporting the highest per capita spending at US$ 1010 per year 1. In addition to the direct costs of medications, indirect costs occur when patients suffer from adverse drug events, their unintended and harmful effects. Adverse drug events are a leading cause of unplanned admissions and prolonged hospital stays, and increase healthcare costs 2–7. Identifying effective drug use interventions to optimize the treatment benefit of medications while minimizing their potential for harm is a public health priority 8,9.
Medication review, a structured and critical examination of an individual patient's medications by a qualified healthcare provider aims to accomplish exactly these goals 10. Medication review is performed by a qualified healthcare provider, usually a pharmacist, and includes establishing an individualized treatment plan, obtaining an accurate medication history, identifying and discontinuing any inappropriate or harmful drugs, and ensuring that indicated medications are taken correctly to optimize their effectiveness 10. An evolving body of evidence has linked a variety of medication review interventions to improved process outcomes, including reductions in the number of medications and reduced medication errors 10–12. The value of medication reviews is generally accepted among clinicians, despite lack of robust research evidence demonstrating clinical or cost effectiveness compared with usual care which remains a barrier to more widespread implementation of this costly intervention. The only quantitative systematic review on the effect of in-hospital medication review on patient-oriented outcomes excluded non-randomized studies, did not evaluate its effect on the length of admission and extrapolated 12 month outcomes for all but one of the included studies 13. Our objective was therefore to summarize the available evidence on the effect of pharmacist-led medication review initiated early within a patient's hospital course on the length of hospital stay, and on 3 month mortality, hospital readmissions and emergency department revisits based on observed data.
Methods
Study design
This was a systematic review to determine the effect of early in-hospital pharmacist-led medication review on health outcomes. Ethics approval was not required because the study did not involve the use of human subjects. We registered the study protocol with PROSPERO 14.
Data searches and sources
We developed a systematic search strategy with a professional medical librarian (MMDW). The search concepts included ((pharmacists AND medication review) OR pharmaceutical services) AND (emergency department OR acute care OR intensive care). We developed the search in MEDLINE (OvidSP) and included Medical Subject Headings terms for each concept (Appendix 1). We reviewed scope notes for each term for alternate and previous indexing terms and added keywords to increase the sensitivity of our search. We did not use any language or age restrictions. We adapted and applied our MEDLINE search to: Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Database of Abstracts of Reviews of Effects, International Pharmaceutical Abstracts, the Cumulative Index to Nursing and Allied Health Literature and Web of Science. All searches were from 1990 to March 2013, as medication review is a relatively recent intervention developed as a result of changes in pharmacists' scope of practice 15.
We searched 20 medical journals from 2000 to 2013, and the proceedings of 10 pharmacy conferences between 2008 and 2013 (Appendix 2). We used abstracts to identify manuscripts that were subsequently published, and contacted authors for the protocols and results of unpublished studies. We searched the following trial registries: Biomed, metaRegister of Current Controlled Trials, the NHA National Research Register, Clinical Practice Research Datalink and ClinicalTrials.gov. We completed grey literature searches using Google and relevant keywords from our bibliographic reference database searches. We hand searched the bibliographies of all relevant retrieved articles and contacted content experts for additional studies. During the course of the review, we completed periodic environmental scans of the literature to find newly published studies by searching Google using the key word ‘medication review’.
Study selection
We included randomized controlled trials and controlled clinical trials that used quasi-randomized, interrupted time series, and stepped wedge designs to study the effect of medication review in adults (>18 years) who presented to an acute care hospital for an unexpected illness. We excluded studies without comparator groups. We defined medication review as an intervention including (i) a best-possible medication history, and (ii) a review of a patient's medications to optimize medication use and identify and resolve medication-related problems including adverse drug events. The intervention had to target a broad group of patients (targeting patients with more than one diagnosis of interest), and not health professionals (i.e. not academic detailing). While other healthcare providers could obtain the medication history, pharmacists had to complete the medication review. Medication review had to be initiated within 24 h of emergency department presentation or within 72 h of an unplanned hospital admission. Medication reconciliation interventions were eligible if both of the required components of medication review had been completed. We excluded interventions conducted only over the phone or focusing on information technology. Studies had to report at least one outcome of interest and follow patients for at least 30 days.
Data extraction and quality assessment
Two authors independently reviewed all titles (CMH and SG) using standardized forms. Titles that either or both of the reviewers felt were potentially relevant underwent abstract review. Two authors independently reviewed all abstracts (CMH and EL) using standardized review forms. Abstracts that either or both reviewers felt were potentially relevant underwent full text review. Two pairs of authors independently reviewed all full texts (CMH and MLAS, CMH and JJP) for inclusion/exclusion criteria, and resolved disagreements by reaching consensus through discussion. The reviewers were not blinded to study title, authorship or journal of publication. Two reviewers (RH and MW) independently extracted data from included studies and extracted details on the design, setting, participants, intervention and outcomes of each study. We extracted outcome data according to intention-to-treat analysis. Disagreements were resolved by achieving consensus through discussion. We contacted study authors to clarify study methodology and results, and for patient-level data. Two reviewers (CMH and PB) independently assessed the quality of studies using the Risk of Bias quality assessment tool recommended by Cochrane and resolved disagreements though discussion 16.
Data synthesis and analysis
We decided a priori to pool data from studies of the same design, conducted in comparable patient populations, and that reported the same outcomes observed over the same period of follow-up. We analyzed data in an intention-to-treat analysis. Patients who died inhospital were retained for the analysis of mortality even if those patients had been excluded from the analysis of the trial. For continuous outcomes, we pooled results using a random effects model, and reported the weighted mean difference (WMD) with 95% confidence intervals (CIs). For dichotomous outcomes, we pooled results using random effects meta-analysis, and reported odds ratios (OR) with 95% CIs 17. We used Forest plots, the I2 statistic and Cochran's Q test to assess studies for heterogeneity 18,19. We used StatsDirect 2.8 for all analyses.
Results
Main results
Our search revealed 4549 titles of which 67 proceeded to full text review (Figure1). Seven studies met inclusion criteria and reported data on 3292 patients 20–26. Six studies were conducted in Europe 20–23,25,26 and one in Canada 24. Five studies were randomized controlled trials 20,22,23,25,26. We re-classified one study as quasi-randomized because the order of patient allocation was predictable 21. One study was unpublished. However, we obtained the protocol and patient-level data from the study authors 22.
Figure 1.
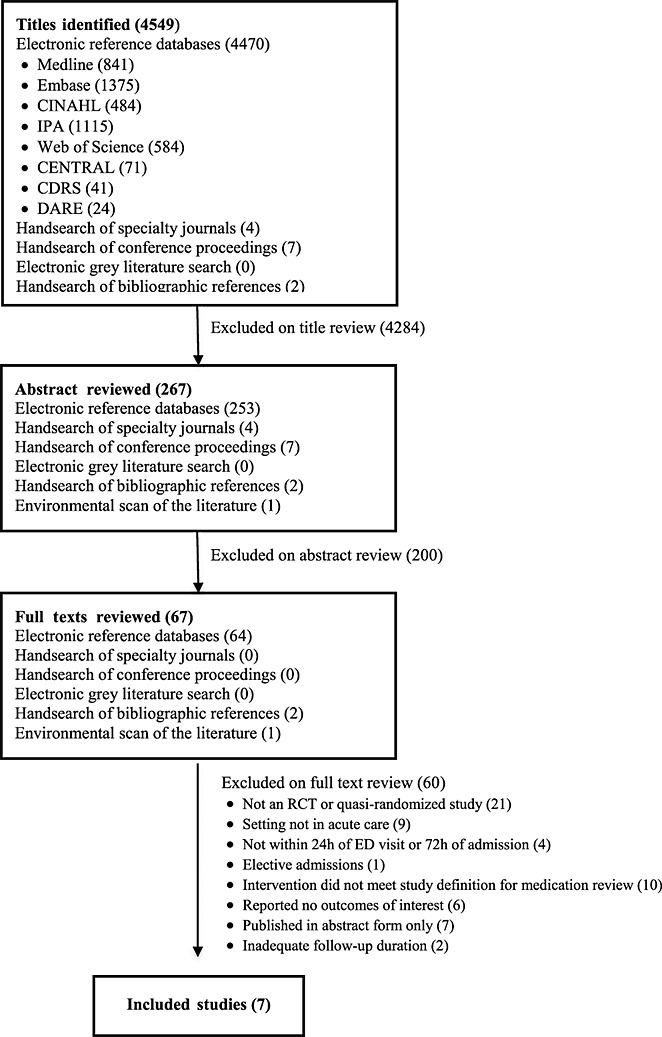
Flow diagram of included studies
All studies were conducted on hospital wards and none in the emergency department setting. The mean age of participants ranged from 70.1 to 86.6 years and the length of follow-up from 3 to 12 months. The number of pharmacists delivering the medication review interventions ranged from 1 to 10 per study, and most had postgraduate or residency training in pharmacy or pharmacology. Pharmacists were fully integrated into healthcare teams in five studies 20,21,24–26, yet were only able to enact their own recommendations independently in one study 20. Details of the characteristics of individual studies and the medication review interventions are described in Appendices 3 and 4.
Quality of included studies
Table1 summarizes the Risk of Bias assessments. All studies were felt to be of unclear or high risk of selection bias, as the methods of random sequence generation were inadequately described 22,23,26 or deemed high risk 20,25. Allocation concealment for randomized studies was unclear in all but one study, which was considered low risk 26. Blinding of participants and personnel was not feasible given the nature of the intervention. Thus, blinding of the outcomes assessment was judged highly relevant in this context, yet generally inadequately described, even for outcomes least susceptible to bias (i.e. mortality). In three studies, a high risk of bias was deemed to be present due to the risk of contamination between the intervention and control groups because the patients' physicians were the same in both groups 22,23,26. One study reported on differences in non-primary outcomes without adjusting for multiple comparisons 26 and one study was at high risk of multiple testing bias 22. Detection bias for the length of admission was at unclear or high risk due to unclear definitions of how the data were measured. Mortality, re-admission and emergency department revisit data were deemed at unclear or low risk of detection bias. All studies were at low risk of attrition bias.
Table 1.
Risk of bias in included studies for outcomes of the systematic review
| Study | Random sequence generation | Allocation concealment | Blinding: Participants | Blinding: Personnel | Blinding: Outcomes | Incomplete outcome assessment | Selective reporting | Other |
|---|---|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||||
| Bladh et al. 26 | Unclear | Low | High | High | Mortality: Unclear | Mortality: High | High | |
| LOA: Unclear | LOA: Low | |||||||
| Gillespie et al. 25 | High | Unclear | Unclear | Unclear | Mortality: Unclear | Mortality: Low | Low | Low |
| Re-admissions: Low | Re-admissions: Low | |||||||
| ED revisits: Low | ED revisits: Low | |||||||
| Lisby et al. 23 | Unclear | Unclear | Unclear | High | Mortality: Unclear | Mortality: Low | Low | |
| LOA: Unclear | LOA: Low | |||||||
| Re-admissions: Unclear | Re-admissions: Low | |||||||
| ED revisits: Unclear | ED revisits: Low | |||||||
| Lisby et al. 22 | Unclear | Unclear | Low | High | Mortality: Unclear | Mortality: Low | Low | High |
| LOA: High | LOA: Low | |||||||
| Re-admissions: Unclear | Re-admissions: Low | |||||||
| ED revisits: Unclear | ED revisits: Low | |||||||
| Scullin et al. 20 | High | Unclear | High | High | Mortality: NA | Mortality: Low | Low | NA |
| LOA: High | LOA: Unclear | |||||||
| Re-admissions: High | Re-admissions: Unclear | |||||||
| Controlled clinical trials | ||||||||
| Makowsky et al. 24 | High | Unclear | Low | High | LOA: High | LOA: Low | Low | High |
| Re-admissions: Unclear | Re-admissions: Low | |||||||
| ED revisits: Unclear | ED revisits: Low | |||||||
| Spinewine et al. 21 | High | Unclear | Low | High | Mortality: Low | Mortality: High | Unclear | NA |
| Re-admissions: High | Re-admissions: High | |||||||
| ED re-visits: High | ED re-visits: High | |||||||
CCT, controlled clinical trial;
HRQL, health-related quality of life;
LOA, length of admission;
NA, not applicable;
RCT, randomized controlled trial.
Length of admission
Six studies reported on the length of hospital admission 20,22–26. However, only three reported it as a study outcome 20,22,23. Individual and pooled estimates for length of stay are shown in Figure2. When pooling data from five studies (n = 1737), the average length of stay was reduced by 0.04 days (95% CI –1.63, 1.55, P = 0.96) 20,22,23,25,26. There was substantial statistical heterogeneity (I2 = 61.0%, Cochran Q = 10.6, P = 0.03).
Figure 2.
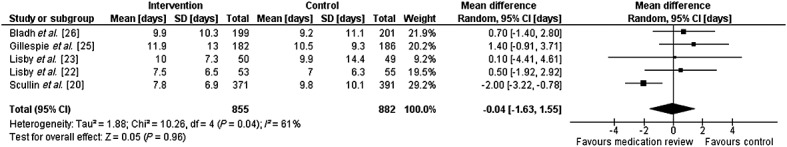
Forest plot of the effect of medication review on the length of admission. Length of stay data from Gillespie exclude patients who died during the index admission.
Mortality
Six studies reported data on mortality 20–23,25,26. However, only four analyzed it as an outcome 21–23,25 and only three randomized trials reported or released 3 month data for pooling (n = 607) 22,23,25. Individual and pooled estimates are shown in Figure3. The pooled odds ratio for mortality was 1.09 with medication review (95% CI 0.69, 1.72, P = 0.71), with low statistical heterogeneity (I2 = 0%, Cochran Q = 0.6, P = 0.75). Two studies not included in the meta-analysis because of missing 3 month data, reported no difference in mortality at 12 months, with Scullin et al. (n = 762) reporting 18.1% vs. 19.8% mortality 20 and Spinewine et al. (n = 186) reporting 22.1% vs. 30.1% in the intervention vs. control groups, respectively 21.
Figure 3.
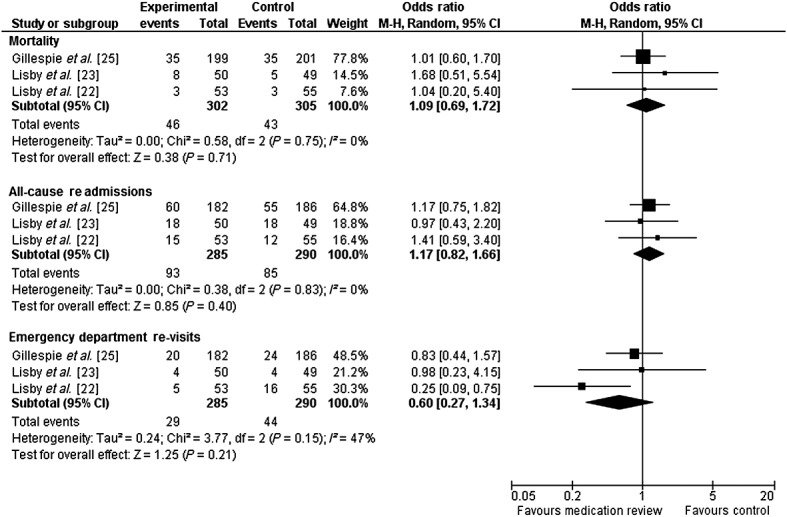
Forest plot of the effect of medication review on 3 month mortality, 3 month all cause re-admissions and 3 month emergency department revisits. Gillespie data obtained from the study authors include all randomized patients.
Re-admissions
Six studies reported data on re-admissions 20–25, two of which were not randomized 21,24. We pooled data from the three randomized studies for which 3 month outcomes were reported or released (n = 607) 22,23,25. The pooled odds ratio for re-admissions was 1.15 (95% CI 0.81, 1.63,P = 0.44) and is shown in Figure3. Statistical heterogeneity of the pooled estimate was low (I2 = 0%, Cochran Q = 0.38, P = 0.83). However, the largest randomized trial (n = 762) not included in the meta-analysis because of missing data, reported a significant decrease in re-admissions at 12 months (40.9% vs. 49.3%, P = 0.03) 20. One non-randomized study (n = 452) reported a reduced odds of being re-admitted at 3 months (OR 0.63, 95% CI 0.42, 0.94) 24, while the other reported no difference 21.
Emergency department revisits
Four studies reported data on emergency department revisits 21–23,25, of which three were randomized 22,23,25. The individual and pooled estimates from the three randomized studies (n = 607) are shown in Figure3 22,23,25. The odds ratio for emergency department revisits was 0.60 (95% CI 0.27, 1.32, P = 0.20) with medication review 22,23,25. Statistical heterogeneity was substantial, but not statistically significant (I2 = 46.3%, Cochran Q = 3.73, P = 0.16). The fourth (non-randomized) study reported a non-statistically significant reduction in emergency department revisits: 7.9% vs. 12.0% (P = 0.45) 21.
Discussion
This systematic review on in-hospital pharmacist-led medication review did not identify an effect on the length of hospital admission, mortality or re-admissions. Our pooled estimates on emergency department revisits was consistent with a 40% reduction, but was not statistically significant. However, limitations in the available evidence, including the number, size and quality of available studies precluded us from concluding that no effect exists.
Recent reviews on a variety medication review interventions have reported beneficial effects on outcomes related to process, including medication errors and the numbers of prescribed medications 10–13. While their effect on process outcomes is promising, medication review interventions incur substantial cost, and warrant rigorous evaluation on objective and sustained patient-oriented outcomes to ensure optimal health value for expenditure. Evidence should guide implementation strategies to ensure that qualified healthcare personnel target patient groups who are most likely to benefit and deliver effective components of the intervention.
Only one previous quantitative systematic review has examined the relationship between in-hospital medication review and patient outcomes 13. Christensen et al. pooled the results of studies involving physician- and pharmacist-led medication review, making it difficult to isolate the effects of interventions by pharmacists 13,27. They excluded non-randomized studies, and did not report its effect on the length of hospital admission, a crucial outcome measure of in-hospital medication review in an era marked by hospital crowding and bed shortages. Finally, the authors extrapolated 12 month outcomes from 3 and 6 month outcomes for three of four pooled studies, while little is known about the magnitude, duration and attenuation of any effect that medication review may have on these outcomes. It is possible that the effect of medication review is attenuated over time, and that Christensen et al. may have overestimated its effect by combining outcomes from 3, 6 and 12 month observation periods. Thus, our goal was to summarize the available evidence based on observed outcomes and primary data obtained from study authors, while including the results of non-randomized studies and reporting its effect on the length of admission.
Our review demonstrates an inconsistent effect on the length of admission. Four of five studies involving just over one thousand subjects reported no effect 22,23,25,26, while the remaining study of nearly 800 patients found a large decrease in the length of admission 20. Patients were younger in this study, suggesting that an effect in younger patients may be negated by no effect in the frail elderly whose length of stay may be determined by factors not amenable to medication review such as waiting for long term care placement. In this study, personnel delivering the interventions received dedicated training, delivered the intervention at each stage of the patients' hospital journey, and participated in discharge planning and dispensing. The latter aspects may have influenced the duration of admissions, by facilitating patient discharges. Finally, pharmacists were fully integrated into healthcare teams, and partly enacted their own recommendations 20. In all other studies pharmacists were unable to enact their recommendations 22,23,25,26, and in three studies, pharmacists had limited contact with patients and physicians, resulting in fewer than 50% of recommendations being adopted 22,23,26. Such differences in the delivery of the interventions could have diluted its effect.
Our review was limited by the quantity and quality of the available evidence. Only few studies have been published on the effect of pharmacist-led medication review in the hospital setting. We do not believe that selection or retrieval bias impacted on our results, as we used an exhaustive search and mitigated publication bias by soliciting data from unpublished trials. The quality of reporting of most studies was modest. Because blinding of patients and personnel to medication review is not feasible, it is essential that future randomized trials incorporate, and clearly disclose, mechanisms to blind the outcome assessments. Finally, our meta-analysis was limited by the variation in the follow-up periods between studies, precluding pooling of data from all randomized trials. Only three studies provided 3 month data on our secondary outcomes 22,23,25. We selected 3 months as being sufficiently long to identify a sustained difference in outcomes, yet short enough to be practical for an interventional trial.
Given the wide confidence intervals of our estimates, the methodological flaws of individual studies, the variation in the medication review interventions studied, and missing data from positive studies that could not contribute to our pooled estimates, we could not reach a conclusive result for or against pharmacist-led medication review. In light of these limitations, it is likely that further high quality randomized trials will contribute to a better understanding of the effects of medication review. Such research is urgently needed to guide the costly and resource intensive implementation of medication review interventions in hospitals, and to inform future hospital accreditation standards to ensure that they are evidence-based.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare support from a Meetings, Planning and Dissemination Grant from the Canadian Institutes for Health Research for the submitted work [all authors], support from a New Investigator Grant from the Canadian Institutes of Health Research [CMH], no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Appendix 1 Medline search strategy

Appendix 2 List of medical journals and conference proceedings we searched
Medical Journals
Archives of Internal Medicine
Annals of Internal Medicine
The Journal of the American Medical Association
The British Medical Journal
The Canadian Medical Association Journal
BMC Clinical Pharmacology
Pharmacoepidemiology
Drug Safety
The American Journal of Health-System Pharmacy
Annals of Pharmacotherapy
Medical Care
BMC Health Services Research
BMJ Quality and Safety
Basic and Clinical Pharmacology and Toxicology
Clinical Pharmacology and Therapeutics
The British Journal of Clinical Pharmacology
The European Journal of Clinical Pharmacology
The Journal of the American Geriatrics Society
Age and Ageing
The Journal of Population Therapeutics and Clinical Pharmacology
Conference Proceedings
British Pharmaceutical Conference
The UK Clinical Pharmacy Association Conference
Health Services Research and Pharmacy Practice Conference
Pharmacy Care Network Europe Conference
Canadian Association of Health Services and Policy Research Conference
Agency for Healthcare Research and Quality Meeting
International Forum on Safety and Quality in Healthcare
Canadian Society of Hospital Pharmacists Conference
American Society of Health-System Pharmacists Conference
The International Society for Pharmacoepidemiology Conference
European Society of Clinical Pharmacy
European Association of Hospital Pharmacy
Nordic Networking Group of Clinical Pharmacy
International Conference on Emergency Medicine
Appendix 3 Characteristics of included studies
| Study | Country | Population | Age (years) | Patients | Outcomes (Primary bold) | Reported results (Intervention vs. Control) |
|---|---|---|---|---|---|---|
| Randomized controlled trials | ||||||
| Bladh et al. 26 | Sweden | Internal | Median 82 | Med review: 199 | 1. HRQL: Global Health | 3.0 vs. 2.8; P = 0.08 |
| medicine | Control: 201 | 2. Mean number PIP/pt | 0.34 vs. 0.38; P = 0.67 | |||
| patients | 3. Number of drug-related problems | Not reported quantitatively | ||||
| Gillespie et al. 25 | Sweden | Geriatric patients on internal medicine wards | Mean | Med review: 199 | 1. ED revisits and re-admissions | RR: 0.84 (95% CI 0.72, 0.99) |
| 86.6 | Control: 201 | 2. Drug-related re-admissions | RR: 0.20 (95% CI 0.10, 0.41) | |||
| 3. Cost, $ | −400 (95% CI −4000, 3200) | |||||
| 4.Survival | RR: 0.94 (95% CI 0.65, 1.34) | |||||
| Lisby et al. 23 | Denmark | Geriatric patients on internal medicine ward | Mean 78.2* | Med review: 50 | 1. Length of admission, days | 10 (95% CI 7.9, 12.1) vs. |
| 9.9 (95% CI 5.8, 14.2) | ||||||
| Control: 49 | 2. Mean number of re-admissions | 0.4 (0.3–0.6) vs. 0.5 (0.3–0.7) | ||||
| 3. Mortality | 16% (95% CI 7, 29) vs. | |||||
| 10%(95% CI 3, 22) | ||||||
| 4. Number of contacts with HCP | 8.8 (95% CI 7.2, 10.4) vs. | |||||
| 10.5 (95% CI 8.8, 12.3) | ||||||
| 5. HRQL (EQ-5D) | No statistically significant differences. | |||||
| Lisby et al. 22 | Denmark | Geriatric patients on orthopaedic ward | Mean | Med review: 53 | 1. Time to physician contact | No statistically significant differences. |
| 80.5* | Control: 55 | 2. Length of admission, days | 7.5 (95% CI 5.8, 9.4) vs. | |||
| 7.0 (95% CI 5.3, 8.7) | ||||||
| 3. Time to re-admission, days | 76 (95% CI 69, 84) vs. | |||||
| 78 (95% CI 71, 86) | ||||||
| 4. Mean number of re-admissions | 0.5 (95% CI 0.2, 0.9) vs. | |||||
| 0.3 (95% CI 0.1, 0.5) | ||||||
| 5. Mean number of ED revisits | 0.2 (95% CI 0.0, 0.3) vs. | |||||
| 0.4 (95% CI 0.2, 0.6), P = 0.01 | ||||||
| 6. Mortality | 5.6% (95% CI 1.1, 15.7) vs. | |||||
| 5.4 (95% CI 1.1, 15.1) | ||||||
| Scullin et al. 20 | Northern Ireland | Patients on medical or surgical wards | Mean 70.1 | Med review: 371 | 1. Length of admission, days | 7.8 (95% CI 7.1, 8.6) vs. |
| 9.8 (95% CI 8.8, 10.9) P = 0.003 | ||||||
| Control: 391 | 2. Proportion re-admitted | 40.8% vs. 49.3%; P = 0.03 | ||||
| 3. HCP satisfaction | Not reported quantitatively. | |||||
| 4. Mortality | 18.1% vs. 19.8%, P = 0.59 | |||||
| Controlled clinical trials | ||||||
| Makowsky et al. 24 | Canada | Internal medicine and hospitalist patients | Mean 74 | Med review: 221** | 1. Adherence to indicators | Difference: 10.4% (95% CI 5.0, 15.7) |
| Control: 231 | 2. Re-admissions (3 months) | OR 0.63 (95% CI 0.42, 0.94) | ||||
| 3. Re-admissions (6 months) | OR 0.78 (95% CI 0.53, 1.15) | |||||
| 4. Length of admission | Median ratio: 1.2 (95% CI 1.0, 1.3) | |||||
| Spinewine et al. 21 | Belgium | Geriatric patients | Mean 81.9 | Med review: 103 | 1.MAI improvement | OR 9.1 (95% CI 4.2, 21.6) |
| Control: 100 | 2. Mortality | 22.5% vs. 30.1%, P = 0.3 | ||||
| 3. Proportion re-admitted | 32.6% vs. 33.7%, P = 1.0 | |||||
| 4. Proportion with ED revisits | 7.9% vs. 12.0%, P = 0.45 | |||||
CI = confidence intervals
HRQL = health-related quality of life
PIP = potentially inappropriate prescriptions
pt = patient
ED = emergency department
HCP = healthcare provider
RR = risk ratio
OR = odds ratio
MAI = Medication Appropriateness Index.
Mean age in control group.
221 patients in the Makowsky et al. study were randomized to the medication review group. However, one patient was excluded post-randomization.
Appendix 4 Characteristics of the medication review interventions in included studies
| BPMH component | Medication review | Communication | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Timing | HCP collecting BPMH | Information sources | Number of pharmacists delivering med review | Education/experience of involved pharmacist | Ability to enact recommendations independently | Pharmacist integration into team | With patients | With physicians | Additional components |
| Randomized controlled trials | ||||||||||
| Bladh et al. 26 | Continuous | NR | Medical record | 3 | Education NR; None/limited experience in clinical pharmacy) | Unable (41% recommendations adopted) | Partly integrated healthcare team | DC instructions, written medication report | Oral feedback on prescribing, written medica-tion report | HCP education |
| Gillespie et al. 25 | BMPH at admission, med review continuous | Clinical pharma-cist | Not reported | 3 | Post-graduate training (M.Sc. or courses), ward-based experience | Unable (75% of recommendations adopted) | Participated at rounds as team member | Education, DC counseling, 2-mo post-DC follow-up. | Face-to-face discussion about drug selection, dose, monitoring | HCP education |
| Lisby et al. 23 | Within 24–72 h of admission | Clinical pharma-cist | Pts, GPs, medical and medication records | 2 | Post-graduate training in clinical pharmacy or pharmacology | Unable (<50% recommendations adopted) | Not integrated | Only for BMPH | Through written notes, letters and fax only | HCP education |
| Lisby et al.22 | Within 24–72 h of admission | Clinical pharma-cist | Pt, GP, medical and medication records | 2 | Post-graduate training in clinical pharmacy or pharmacology | Unable (43% recommendations adopted) | Not integrated | Only for BMPH | Through written notes, letters and fax only | HCP education |
| Scullin et al. 20 | Continuous | Clinical pharma-cist | Pt, care provider, GP, med record, community pharmacist | 10 | Pairs of clinical pharmacists and technicians, training provided | Partly able to enact recommendations | Part of healthcare team | Direct contact with pts for education and DC counseling | Direct, face-to-face contact with team | HCP education, DC instructions, ward stock management |
| Controlled clinical trials | ||||||||||
| Makowsky et al. 24 | Continuous | Clinical pharma-cist | Pt | 2 | Pharmacy residency and prior experience in clinical pharmacy | Unable | Part of health-care team | DC counseling, written summary of changes | Face-to-face contact, bedside rounds partici-pation, GP contact | Med rec, HCP education, contact with community pharmacist |
| Spinewine et al. 21 | Continuous | Clinical pharma-cist | Unclear | 1 | Post-graduate training | Unable to enact recommendations | Part of health-care team | DC counseling | Direct, face-to-face contact | HCP education, GP contact |
NR = not reported
DC = discharge
BPMH = best possible medication history
HCP = healthcare provider
GP = general practitioner
Med rev = medication review
Med rec = medication reconciliation
Pts = patients
References
- 1.OECD. 2014; Pages. Available at OECD at http://www.oecd-ilibrary.org/social-issues-migration-health/pharmaceutical-expenditure-per-capita-2014-1_pharmexpcap-table 2014-1-en (last accessed 3 August 2014)
- 2.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–12. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 3.Hohl CM, Dankoff J, Colacone A, Afilalo M. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38:666–71. doi: 10.1067/mem.2001.119456. [DOI] [PubMed] [Google Scholar]
- 4.Hohl CM, Nosyk B, Zed P, Kuramoto L, Brubacher J, Abu-Laban RB, Sheps SB, Sobolev B. Outcomes of emergency department patients presenting with adverse drug events. Ann Emerg Med. 2011;58:270–9. doi: 10.1016/j.annemergmed.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Zed PJ, Abu-Laban RB, Balen RM, Loewen PS, Hohl CM, Brubacher JR, Wilbur K, Wiens MO, Samoy LJ, Lacaria K, Purssell RA. Incidence, severity and preventability of medication-related visits to the emergency department: a prospective study. Can Med Assoc J. 2008;178:1563–9. doi: 10.1503/cmaj.071594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazarou J, Pomeranz B, Corey P. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 7.Patel H, Bell D, Molokhia M, Srishanmuganathan J, Patel M, Car J, Majeed A. Trends in hospital admissions for adverse drug reactions in England: analysis of national hospital episode statistics 1998–2005. BMC Clin Pharmacol. 2007;7:1–11. doi: 10.1186/1472-6904-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamblyn R. 2001. Evidence-based utilization of prescription drugs: challenges and directions for the future in Canada.
- 9.The WHO Research Priority Setting Working Group. 2008. Global Priorities for Research in Patient Safety (first edition): World Health Organization.
- 10.Blekinsopp A, Bond C, Raynor DK. Medication reviews. Br J Clin Pharmacol. 2012;74:573–80. doi: 10.1111/j.1365-2125.2012.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graabaek T, Kjeldsen LJ. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: a systematic review. Basic Clin Pharmacol Toxicol. 2013;113:359–73. doi: 10.1111/bcpt.12062. [DOI] [PubMed] [Google Scholar]
- 12.Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist-led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta-analysis. Br J Clin Pharmacol. 2007;65:303–16. doi: 10.1111/j.1365-2125.2007.03071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality (Review) Cochrane Lib. 2013;2 doi: 10.1002/14651858.CD008986.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Hohl CM, Garrison S, Lang E, Lexchin J, Sobolev B, Rowe B, Brasher P, Sivilotti MLA, Goodland H, Wanbon R, Perry JJ, Holland R. 2013. Pages. Available at University of York, Centre for Reviews and Dissemination at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013004298 (last accessed 9 April 2013)
- 15.Hepler C, Strand L. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47:533–43. [PubMed] [Google Scholar]
- 16.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scullin C, Scott MG, Hogg A, McElnay JC. An innovative approach to integrated medicines management. J Eval Clin Pract. 2007;13:781–8. doi: 10.1111/j.1365-2753.2006.00753.x. [DOI] [PubMed] [Google Scholar]
- 21.Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc. 2007;55:658–65. doi: 10.1111/j.1532-5415.2007.01132.x. [DOI] [PubMed] [Google Scholar]
- 22.Lisby M, Bonnerup D, Brock B, Gregersen P, Jensen J, Rungby J. Systematic medication review and clinically health-related outcome in elderly admitted acutely to an orthopaedic ward: a randomized controlled study. submitted.
- 23.Lisby M, Thomsen A, Nielsen L, Lyhne N, Breum-Leer C, Fredberg U, Jørgensen J, Brock B. The effect of systematic medication review in elderly patients admitted to an acute ward of internal medicine. Basic Clin Pharmacol Toxicol. 2010;106:422–7. doi: 10.1111/j.1742-7843.2009.00511.x. [DOI] [PubMed] [Google Scholar]
- 24.Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment, The COLLABORATE Study. Med Care. 2009;47:642–50. doi: 10.1097/MLR.0b013e3181926032. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, Kettis-Lindblad A, Melhus H, Mörlin C. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169:894–900. doi: 10.1001/archinternmed.2009.71. [DOI] [PubMed] [Google Scholar]
- 26.Bladh L, Ottosson E, Karlsson J, Klintberg L, Wallerstedt SM. Effects of a clinical pharmacist service on health-related quality of life and prescribing of drugs: a randomised controlled trial. BMJ Qual Saf. 2011;20:738e46. doi: 10.1136/bmjqs.2009.039693. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher PF, O'Connor MN, O'Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89:845–54. doi: 10.1038/clpt.2011.44. [DOI] [PubMed] [Google Scholar]
- 28.Canadian Pharmacists Assocation. 2006; Pages. Available at Canadian Pharmacists Association at https://www.e-therapeutics.ca/wps/portal/!ut/p/.scr/Login (last accessed 1 January 2007)
- 29.Pharmaceutical Services Division Ministry of Health. 2012. Medication Review Services - Policies, Procedures and Guidelines for Pharmacists. Victoria, BC;


