Abstract
Aims
The P2Y12 inhibitor prasugrel is a prodrug, which is activated after its initial hydrolysis partly by cytochrome P450 (CYP) 3A4. Grapefruit juice, a strong inactivator of intestinal CYP3A4, greatly reduces the activation and antiplatelet effects of clopidogrel. The aim of this study was to investigate the effects of grapefruit juice on prasugrel.
Methods
In a randomized crossover study, seven healthy volunteers ingested 200 ml of grapefruit juice or water three times daily for 4 days. On day 3, they ingested a single 10 mg dose of prasugrel with an additional 200 ml of grapefruit juice or water. Plasma concentrations of prasugrel metabolites and the antiplatelet effect were measured.
Results
Grapefruit juice increased the geometric mean area under the plasma concentration–time curve (AUC0–∞) of the primary, inactive metabolite of prasugrel to 164% of the control value (95% confidence interval 122–220%, P = 0.008), without a significant effect on its peak plasma concentration (Cmax). The Cmax and AUC0–∞ of the secondary, active metabolite were decreased to 51% (95% confidence interval 32–84%, P = 0.017) and 74% of the control value (95% confidence interval 60–91%, P = 0.014) by grapefruit juice (P < 0.05). The average platelet inhibition, assessed with the VerifyNow® method at 0–24 h after prasugrel intake, was 5 percentage points (95% confidence interval 1–10 percentage points) lower in the grapefruit juice phase than in the water phase (P = 0.034).
Conclusions
Grapefruit juice reduces the bioactivation of prasugrel, but this has only a limited effect on the antiplatelet effect of prasugrel.
Keywords: cytochrome P450 3A4, drug interaction, grapefruit juice, prasugrel
What is Already Known about this Subject
Metabolic activation, in which cytochrome P450 (CYP) 3A4 has an important role, is required for the antiplatelet effect of prasugrel.
Grapefruit juice is an inhibitor of intestinal CYP3A4, but the role of the intestine and the effect of grapefruit juice in the extensive first-pass metabolism and bioactivation of prasugrel are unknown.
What this Study Adds
Intestinal first-pass metabolism via CYP3A4 participates in, but is not crucial for the bioactivation of prasugrel.
Daily use of grapefruit juice has a modest inhibitory effect on prasugrel bioactivation, but the antiplatelet efficacy is only reduced slightly.
Introduction
Thienopyridine drugs, such as clopidogrel and prasugrel, which target the platelet P2Y12 adenosine diphosphate (ADP) receptor, are used to improve prognosis in acute coronary syndrome. Compared with clopidogrel, the newer antiplatelet drug prasugrel offers improvement in terms of efficacy 1. The response to clopidogrel treatment varies considerably among individuals, for example due to pharmacogenetic factors and drug–drug interactions 2. Prasugrel has a more consistent pharmacodynamic profile and can be used to overcome high on-treatment platelet reactivity seen in some patients treated with clopidogrel [3, 4].
Similar to clopidogrel, prasugrel is a prodrug that requires metabolic activation in vivo for its inhibitory effect on platelet aggregation (Figure1) 5,6. Cytochrome P450 (CYP) 3A4, CYP2B6 and, to a lesser extent, CYP2C9 and CYP2C19, participate in the formation of the active metabolite of prasugrel, R-138727, which binds irreversibly to the P2Y12 receptor 7. However, the antiplatelet effect and clinical efficacy of prasugrel do not seem to be as susceptible as those of clopidogrel to variability in CYP activity 8. Ritonavir, a potent CYP3A4 inhibitor, decreases the total exposure to the active metabolite of prasugrel, whereas ketoconazole only modestly decreases its peak plasma concentration (Cmax) and has no effect on platelet inhibitory activity 9,10. In contrast, CYP induction by rifampicin has no effect on the pharmacokinetics of the active metabolite of prasugrel 11.
Figure 1.
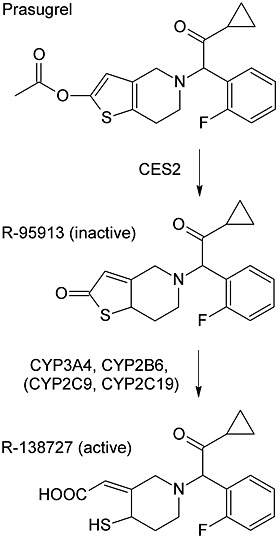
Bioactivation pathway of prasugrel, as described previously 7. Following oral administration, prasugrel is rapidly and completely hydrolysed to its inactive thiolactone metabolite, R-95913
The furanocoumarin constituents of grapefruit juice inhibit intestinal CYP3A4, and clinically relevant pharmacokinetic interactions have been observed with several drugs metabolized by CYP3A4 12–15. Recently, grapefruit juice was shown to inhibit the first-pass metabolism of ticagrelor, another new P2Y12 receptor antagonist, and to increase ticagrelor exposure in humans, enhancing the platelet inhibitory activity 16. In contrast to ticagrelor, the bioactivation of clopidogrel was strongly inhibited by grapefruit juice, which resulted in greatly impaired inhibition of platelet aggregation by clopidogrel 17.
Current data suggest that prasugrel pharmacokinetics is not as sensitive to CYP-mediated drug–drug interactions as clopidogrel and that a more consistent and predictable pharmacodynamic profile is achieved in patients treated with prasugrel. However, the extensive first-pass metabolism of prasugrel and the central role of CYP3A4 in its active metabolite formation 7 suggest that activation of prasugrel could be affected by inhibition of intestinal metabolism, in a similar manner to clopidogrel. We therefore found it important to investigate the effect of inhibition of intestinal CYP3A4 by grapefruit juice on the pharmacokinetics and pharmacodynamics of prasugrel.
Methods
Subjects
Ten healthy volunteers were enrolled in the study after giving written informed consent. Medical history, clinical examination and laboratory tests were used to confirm their health. All participants had normal blood platelet counts and haematocrit values and none was on any continuous medication or was a tobacco smoker. Two participants withdrew from the study before first administration of prasugrel and one was excluded from analysis due to noncompliance with the prohibition of the use of other drugs within 1 week before prasugrel administration. The mean ± SD age of the remaining seven volunteers (one woman and six men) was 21 ± 1 years (range 19–22 years), height 179 ± 5 cm (171–187 cm) and weight 73 ± 5 kg (65–79 kg).
Study design
The study protocol was approved by the Finnish National Committee on Medical Research Ethics TUKIJA (record number 62/06.00.01/2013) and by the Finnish Medicines Agency, Fimea. A randomized crossover study with two phases and a 2 week washout period was carried out. The participants ingested either 200 ml of normal strength grapefruit juice (Tropicana Golden Grapefruit; Tropicana Looza, Hermes, France) or water three times a day at 08.00, 12.00 and 20.00 h for 4 days. The grapefruit juice was stored refrigerated and used before the expiration date. On day 3, after an overnight fast, the participants ingested a single 10 mg dose of prasugrel (Efient®; Eli Lilly, Nederland BV, Houten, The Netherlands) with an additional 200 ml of grapefruit juice or water at 09.00 h. Standardized meals were served at 4, 7 and 10 h after prasugrel ingestion. The participants were prohibited from using grapefruit products, apple and orange juice and alcohol during the study and all other drugs for 1 week before prasugrel administration and at least 72 h thereafter.
Timed 9 ml ethylenediaminetetraacetic acid (EDTA) and 2.7 ml citrate (109 mmol l−1 sodium citrate, 3.2%) blood samples were collected before and up to 48 h after prasugrel administration. Plasma was separated within 30 min in the EDTA tubes and stored at −70 °C until analysis of drug concentrations. Whole-blood platelet function tests were performed from fresh sodium citrate samples within 2 h after sampling.
Determination of plasma prasugrel metabolite concentrations
Plasma concentrations of R-95913 and R-138727 were determined after derivatizing the sample with 2-bromo-3-methoxyacetophenone immediately after blood collection as previously described 18, with minor modifications. Liquid chromatography–tandem mass spectrometry measurements were conducted using a Shimadzu Nexera LC system (Shimadzu, Kyoto, Japan) coupled to a 5500 Qtrap mass spectrometer (AB Sciex, Toronto, ON, Canada) equipped with a TurboIonSpray ionization interface. The mobile phase consisted of 0.1% formic acid (channel A) and acetonitrile (channel B), and the chromatographic separation was achieved on a Kinetex C18 column (100 mm × 2.1 mm i.d., 2.6 µm; Phenomenex, Torrance, CA, USA) using gradient elution. The mobile phase gradient programme was set as follows: linear increase from 40% B to 55% B over 2.7 min, followed by 1 min at 90% B and equilibration at 40% B. The mass spectrometer was operated in positive ion mode, and measurements were performed using multiple reaction monitoring (MRM) of the m/z 498 to 248 and m/z 332 to 109 transitions for the methoxyphenacyl (MP) derivate of R-138727 and R-95913, respectively. Commercially available reference compounds R-95913 and R-138727-MP and deuterium-labelled internal standards R-95913-D4 and R-138727-MP-D3 (Clearsynth Labs Ltd, Mumbai, India) were used for quantification. The lower limits of quantification of R-95913 and R-138727 were 0.2 and 0.1 ng ml−1, respectively, and the day-to-day coefficients of variation were below 15% at relevant concentrations for both analytes.
Platelet function testing
The antiplatelet activity of prasugrel was tested with turbidimetric optical detection (VerifyNow® P2Y12 test; Accumetrics, San Diego, CA, USA) in citrate-anticoagulated whole-blood samples collected at 0, 0.5, 1, 2, 4, 12, 24 and 48 h after dosing. The ADP-activated platelets aggregate in the test channel on fibrinogen-coated microbeads, and the resultant change in optical signal is measured and expressed as P2Y12 reaction units (PRUs). In the control channel, platelets are activated with thrombin receptor-activating peptides, and the baseline maximal aggregation is measured. The drug-dependent inhibition of P2Y12-mediated platelet aggregation is measured by comparing the aggregation in the two channels and expressed as percentage of maximal aggregation 19. The average values were calculated by dividing the area under the effect vs. time curve from time 0 to 24 h (dosing interval) or from 0 to 48 h by the corresponding time interval.
Pharmacokinetics
The following pharmacokinetic parameters were calculated with noncompartmental methods using MK-Model, version 5.0 (Biosoft, Cambridge, UK) for prasugrel metabolites R-95913 and R-138727: peak plasma concentration (Cmax), time to Cmax (tmax), elimination half-life (t½) and area under the plasma concentration–time curve from time 0 to infinity (AUC0–∞). The elimination rate constant (ke) was calculated by linear regression analysis of the terminal log-linear part of the plasma drug concentration–time curve. The t½ was calculated by the equation t½ = ln2/ke. The AUC0–∞ was calculated by a combination of the linear and log-linear trapezoidal rules, with extrapolation to infinity by division of the last measured concentration by ke.
Statistical analysis
The number of subjects was estimated to be sufficient to detect a 30% difference in the AUC0–∞ of prasugrel active metabolite between the water and grapefruit juice phases, with a power of 80% (α-level 5%). The Cmax, t½ and AUC0–∞ data were logarithmically transformed before statistical analysis. The data were analysed with SPSS 20 software (SPSS Inc., Chicago, IL, USA). For pharmacokinetic variables, the results are expressed as geometric means with geometric coefficients of variation, arithmetic means ± SD and geometric mean ratios (grapefruit juice phase/control) with 95% confidence intervals (CIs; for Cmax, t½ and AUC), or medians with range (tmax), or arithmetic means ± SEM in Figure 2. For pharmacodynamic variables, the results are expressed as arithmetic means with SD. The grapefruit juice and water phases were statistically compared by repeated-measures anova with treatment phase and treatment sequence as factors. The tmax data were analysed with the Wilcoxon signed rank test. Differences were considered statistically significant when P < 0.05.
Figure 2.
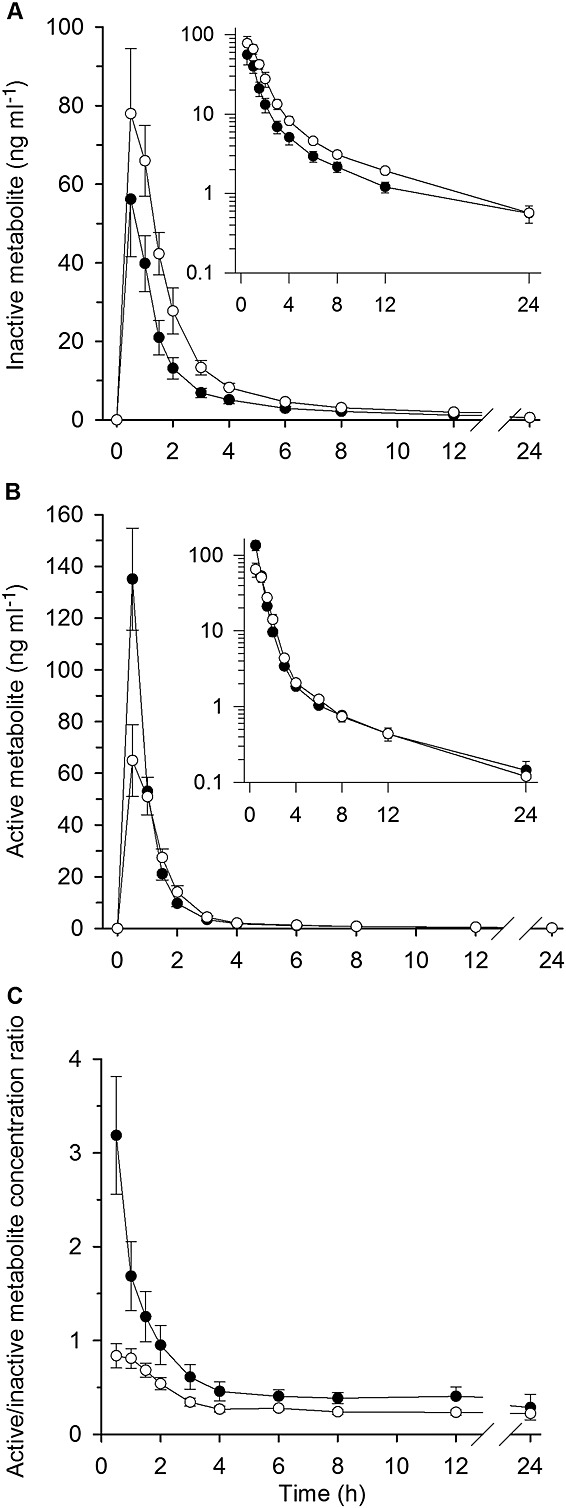
Arithmetic mean ± SEM plasma concentrations of prasugrel inactive R-95913 (A) and active R-138727 metabolite (B) and the ratio of active/inactive metabolite concentrations (C) in seven healthy volunteers. The volunteers ingested 200 ml of grapefruit juice or water three times a day for 4 days, and a single 10 mg dose of prasugrel on day 3 with an additional 200 ml of grapefruit juice or water. Some error bars have been omitted for clarity.  , water phase;
, water phase;  , grapefruit juice phase
, grapefruit juice phase
Results
Primary, inactive metabolite of prasugrel, R-95913
Grapefruit juice had no statistically significant effect on the Cmax of R-95913, but increased its AUC0–∞ to 164% of the control value (95% CI 122–220%, P = 0.008; Figure2, Table1). The tmax and t½ values of R-95913 remained unchanged by grapefruit juice.
Table 1.
Pharmacokinetic and pharmacodynamic variables of prasugrel active and inactive metabolites in seven healthy volunteers, who ingested 200 ml of grapefruit juice or water three times a day for 4 days, and a single 10 mg dose of prasugrel on day 3 with an additional 200 ml of grapefruit juice or water
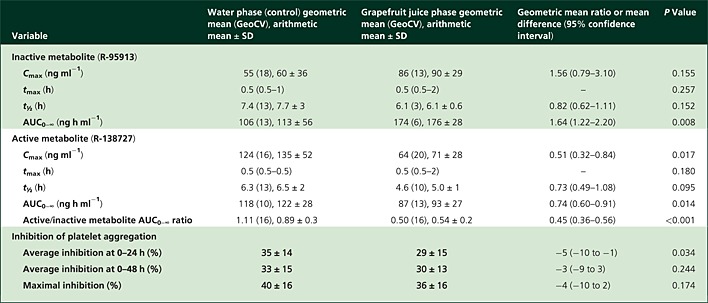 |
The Cmax, t½ and AUC data are given both as geometric means (GeoCV) and as arithmetic means ± SD, tmax as median (range), and average and maximal inhibition as arithmetic means ± SD. The geometric mean ratios (Cmax, t½ and AUC) and mean differences (average and maximal inhibition) between the phases are given with 95% confidence intervals. Abbreviations are as follows: AUC0–∞, area under the plasma concentration–time curve from time 0 to infinity; Cmax, peak plasma concentration; GeoCV, geometric coefficient of variation; tmax, time to Cmax; t½, elimination half-life.
Secondary, active metabolite of prasugrel, R-138727
Grapefruit juice significantly reduced the plasma concentrations of R-138727 (Figure2, Table1). The Cmax of R-138727 was reduced to 51% (95% CI 32–84%, P = 0.017) of the control value and its AUC0–∞ to 74% of the control value (95% CI 60–91%, P = 0.014) by grapefruit juice. The tmax and t½ of R-138727 remained unchanged by grapefruit juice. A decrease in active metabolite concentrations by grapefruit juice was observed in six of the seven individuals (Figure3). The active/inactive metabolite AUC0–∞ ratio was decreased to 45% (95% CI 36–56%, P < 0.001) of the control value by grapefruit juice (Figure2, Table1).
Figure 3.
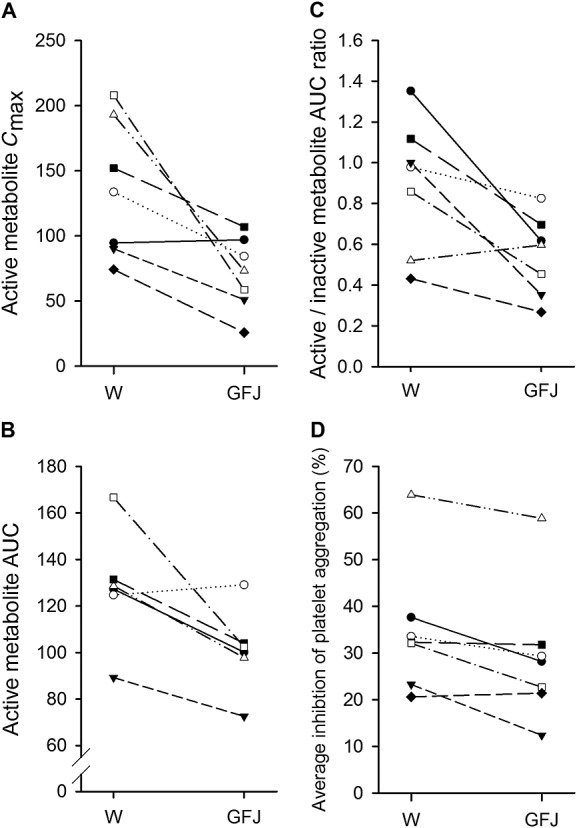
The peak plasma concentrations (Cmax, ng ml−1; A) and area under the plasma concentration–time curve (AUC0–∞, ng h ml−1; B) of prasugrel active metabolite R-138727, the active/inactive R-95913 metabolite AUC ratio (C) and average inhibition of platelet aggregation at 0–24 h in individual subjects following a single 10 mg dose of prasugrel ingested in the water (control; W) and the grapefruit juice (GFJ) phases
Platelet inhibition
Grapefruit juice had only a minor effect on the platelet inhibitory activity of prasugrel, as assessed with the VerifyNow® P2Y12 test. The average inhibition of platelet aggregation at 0–24 h was 29% in the grapefruit juice phase and 35% in the control phase (95% CI for difference −10 to −1 percentage points, P = 0.034; Figure 4, Table 1). The maximal inhibition and average inhibition at 0–48 h were not significantly different between the grapefruit juice and control phases.
Figure 4.
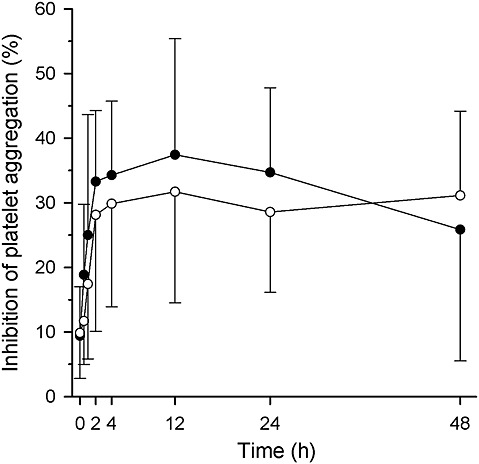
Platelet function after a single 10 mg dose of prasugrel in seven healthy volunteers, as measured with optical aggregometry VerifyNow® P2Y12 test. The results are given as arithmetic means (SD). The volunteers ingested 200 ml of grapefruit juice or water three times a day for 4 days, and a single 10 mg dose of prasugrel on day 3 with an additional 200 ml of grapefruit juice or water. Some error bars have been omitted for clarity.  , water phase;
, water phase;  , grapefruit juice phase
, grapefruit juice phase
Discussion
The present study shows that regular use of grapefruit juice reduces the bioactivation of prasugrel in humans. The active metabolite Cmax was halved and its AUC decreased by one-quarter by grapefruit juice. This pharmacokinetic interaction, however, did not markedly impair the antiplatelet effect of prasugrel after a single 10 mg dose.
Grapefruit juice is known to be a strong inhibitor of intestinal CYP3A4 and by this mechanism it can affect the pharmacokinetics of several drugs 12–15,20. In addition to its inhibition of intestinal CYP3A4, repeated doses of grapefruit juice can inhibit hepatic CYP3A4 in an exposure-dependent fashion and prolong the elimination half-lives of drugs such as buspirone, triazolam and midazolam 21–23. Furthermore, grapefruit juice components bergamottin and nootkatone have been shown to inhibit other CYPs, including CYP2C19, which is also expressed in the intestinal wall 24,25. Parent prasugrel is metabolized by carboxylesterase (CES) 2 during first pass in the intestine to inactive thiolactone R-95913 (Figure 1). CYP3A4 and CYP2B6 are the major enzymes that catalyse the formation of the subsequent active metabolite R-138727, with a minor contribution of CYP2C9 and CYP2C19 in vitro 7. We have previously shown that grapefruit juice severely impairs the formation of the active metabolite of clopidogrel, suggesting a major role of intestinal first-pass metabolism in clopidogrel bioactivation 17. Similar to prasugrel, clopidogrel requires metabolic activation for its antiplatelet efficacy. However, the major metabolic pathway of clopidogrel is inactivation by CES1, and only a small portion of clopidogrel is activated in two steps by CYP enzymes 26,27. CYP2C19 is considered the most important enzyme for clopidogrel bioactivation, with other CYPs, such as CYP3A4, contributing to a lesser extent 28.
The results of our study suggest that prasugrel bioactivation is much less susceptible to the inhibition of intestinal CYP activity by grapefruit juice than clopidogrel bioactivation. This finding indicates that mechanisms other than intestinal CYP3A metabolism also contribute to the formation of the active metabolite of prasugrel. It should also be noted that only the second step of prasugrel activation is mediated by CYP enzymes, which is in line with the observed decrease in active/inactive (secondary/primary) metabolite AUC ratio (Table 1). While there was a clear decrease in the Cmax of the active metabolite of prasugrel by grapefruit juice, its total exposure (AUC) was much less affected, most probably reflecting that the hepatic metabolism of prasugrel and its metabolites was not significantly affected by grapefruit juice. In accordance with this, the differences in prasugrel metabolite pharmacokinetics between grapefruit juice and control phases were most prominent during the absorption phase (Figure 2).
Ritonavir, which is a strong CYP3A4 inhibitor, inhibited the formation of the active metabolite of prasugrel in a recent study, with only a slightly more pronounced effect on active metabolite exposure than observed here with grapefruit juice (45% decrease in Cmax and 38% decrease in AUC by ritonavir) 10. It should be noted that, in the same study, midazolam AUC was increased to 26 times the control value by ritonavir, underlining the strong CYP3A4-inhibiting effect. However, ketoconazole, which also is known for its strong CYP3A4 inhibitory effect, did not affect prasugrel active metabolite AUC or antiplatelet activity even though it decreased the Cmax in a similar manner to ritonavir and grapefruit juice (46% decrease by ketoconazole) 9.
Grapefruit juice has clinically relevant interactions with the other P2Y12 receptor antagonists, clopidogrel and ticagrelor. The bioactivation of clopidogrel was greatly impaired by grapefruit juice, resulting in a decreased platelet inhibitory effect in healthy volunteers 17. In contrast, grapefruit juice increased ticagrelor exposure by more than twofold, leading to an enhanced and prolonged antiplatelet effect 16. In the present study, a similar exposure to grapefruit juice as in our previous interaction studies with clopidogrel and ticagrelor decreased the AUC of prasugrel active metabolite by 26%. This suggests that prasugrel pharmacokinetics is less susceptible than clopidogrel or ticagrelor pharmacokinetics to the inhibition of intestinal CYP3A4. The small sample size decreased the statistical power of this study, especially in the analysis of the pharmacodynamic data. However, we were able to show a statistically significant interaction with prasugrel active metabolite Cmax, AUC and platelet inhibition activity during the first 24 h after prasugrel administration. Although the antiplatelet effect of prasugrel was only decreased slightly in this homogeneous group of healthy volunteers, the reduced formation of the active metabolite suggests that regular consumption of grapefruit juice could also have a significant interaction with prasugrel in some patients.
In conclusion, grapefruit juice moderately decreases the concentrations of the active metabolite of prasugrel, but does not markedly impair its antiplatelet effect in healthy volunteers. Intestinal CYP3A4 activity contributes to, but does not seem crucial for prasugrel bioactivation.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This study was supported by the Helsinki University Central Hospital Research Fund and the Sigrid Jusélius Foundation (Helsinki, Finland). We thank Jouko Laitila, Eija Mäkinen-Pulli and Lisbet Partanen for skilful technical assistance.
Contributors
MTH, AT, HH, MNe., PJN, JTB and MNi. designed and performed the research, analysed the data and wrote the manuscript.
References
- 1.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 2.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Meneveau N, Steg PG, Ferrieres J, Danchin N, Becquemont L. French Registry of Acute ST-Elevation and Non-ST- Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–75. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 3.Weerakkody GJ, Jakubowski JA, Brandt JT, Payne CD, Naganuma H, Winters KJ. Greater inhibition of platelet aggregation and reduced response variability with prasugrel versus clopidogrel: an integrated analysis. J Cardiovasc Pharmacol Ther. 2007;12:205–12. doi: 10.1177/1074248407304731. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulos D, Dimitropoulos G, Davlouros P, Xanthopoulou I, Kassimis G, Stavrou EF, Hahalis G, Athanassiadou A. Prasugrel overcomes high on-clopidogrel platelet reactivity post- stenting more effectively than high-dose (150-mg) clopidogrel: the importance of CYP2C19*2 genotyping. JACC Cardiovasc Interv. 2011;4:403–10. doi: 10.1016/j.jcin.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Sugidachi A, Asai F, Yoneda K, Iwamura R, Ogawa T, Otsuguro K, Koike H. Antiplatelet action of R-99224, an active metabolite of a novel thienopyridine-type Gi-linked P2T antagonist, CS-747. Br J Pharmacol. 2001;132:47–54. doi: 10.1038/sj.bjp.0703761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asai F, Jakubowski JA, Naganuma H, Brandt JT, Matsushima N, Hirota T, Freestone S, Winters KJ. Platelet inhibitory activity and pharmacokinetics of prasugrel (CS-747) a novel thienopyridine P2Y12 inhibitor: a single ascending dose study in healthy humans. Platelets. 2006;17:209–17. doi: 10.1080/09537100600565551. [DOI] [PubMed] [Google Scholar]
- 7.Rehmel JL, Eckstein JA, Farid NA, Heim JB, Kasper SC, Kurihara A, Wrighton SA, Ring BJ. Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab Dispos. 2006;34:600–7. doi: 10.1124/dmd.105.007989. [DOI] [PubMed] [Google Scholar]
- 8.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias WL, Braunwald E, Sabatine MS. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–60. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 9.Farid NA, Payne CD, Small DS, Winters KJ, Ernest CS, II, Brandt JT, Darstein C, Jakubowski JA, Salazar DE. Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 2007;81:735–41. doi: 10.1038/sj.clpt.6100139. [DOI] [PubMed] [Google Scholar]
- 10.Ancrenaz V, Deglon J, Samer C, Staub C, Dayer P, Daali Y, Desmeules J. Pharmacokinetic interaction between prasugrel and ritonavir in healthy volunteers. Basic Clin Pharmacol Toxicol. 2013;112:132–7. doi: 10.1111/j.1742-7843.2012.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farid NA, Jakubowski JA, Payne CD, Li YG, Jin Y, Ernest CS, II, Winters KJ, Brandt JT, Salazar DE, Small DS. Effect of rifampin on the pharmacokinetics and pharmacodynamics of prasugrel in healthy male subjects. Curr Med Res Opin. 2009;25:1821–9. doi: 10.1185/03007990903018360. [DOI] [PubMed] [Google Scholar]
- 12.Bailey DG, Spence JD, Munoz C, Arnold JM. Interaction of citrus juices with felodipine and nifedipine. Lancet. 1991;337:268–9. doi: 10.1016/0140-6736(91)90872-m. [DOI] [PubMed] [Google Scholar]
- 13.Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, Brown MB, Guo W, Watkins PB. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–53. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey DG, Dresser GK, Kreeft JH, Munoz C, Freeman DJ, Bend JR. Grapefruit-felodipine interaction: effect of unprocessed fruit and probable active ingredients. Clin Pharmacol Ther. 2000;68:468–77. doi: 10.1067/mcp.2000.110774. [DOI] [PubMed] [Google Scholar]
- 15.Bailey DG, Dresser G, Arnold JMO. Grapefruit–medication interactions: forbidden fruit or avoidable consequences? Can Med Assoc J. 2013;185:309–16. doi: 10.1503/cmaj.120951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmberg MT, Tornio A, Joutsi-Korhonen L, Neuvonen M, Neuvonen PJ, Lassila R, Niemi M, Backman JT. Grapefruit juice markedly increases the plasma concentrations and antiplatelet effects of ticagrelor in healthy subjects. Br J Clin Pharmacol. 2013;75:1488–96. doi: 10.1111/bcp.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmberg M, Tornio A, Neuvonen M, Neuvonen PJ, Backman JT, Niemi M. Grapefruit juice inhibits the metabolic activation of clopidogrel. Clin Pharmacol Ther. 2014;95:307–13. doi: 10.1038/clpt.2013.192. [DOI] [PubMed] [Google Scholar]
- 18.Farid NA, McIntosh M, Garofolo F, Wong E, Shwajch A, Kennedy M, Young M, Sarkar P, Kawabata K, Takahashi M, Pang H. Determination of the active and inactive metabolites of prasugrel in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:169–79. doi: 10.1002/rcm.2813. [DOI] [PubMed] [Google Scholar]
- 19.Varenhorst C, James S, Erlinge D, Braun OO, Brandt JT, Winters KJ, Jakubowski JA, Olofsson S, Wallentin L, Siegbahn A. Assessment of P2Y12 inhibition with the point-of- care device VerifyNow P2Y12 in patients treated with prasugrel or clopidogrel coadministered with aspirin. Am Heart J. 2009;157:562.e1–e9. doi: 10.1016/j.ahj.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Hanley MJ, Cancalon P, Widmer WW, Greenblatt DJ. The effect of grapefruit juice on drug disposition. Expert Opin Drug Metab Toxicol. 2011;7:267–86. doi: 10.1517/17425255.2011.553189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lilja JJ, Kivistö KT, Backman JT, Lamberg TS, Neuvonen PJ. Grapefruit juice substantially increases plasma concentrations of buspirone. Clin Pharmacol Ther. 1998;64:655–60. doi: 10.1016/S0009-9236(98)90056-X. [DOI] [PubMed] [Google Scholar]
- 22.Lilja JJ, Kivistö KT, Backman JT, Neuvonen PJ. Effect of grapefruit juice dose on grapefruit juice-triazolam interaction: repeated consumption prolongs triazolam half-life. Eur J Clin Pharmacol. 2000;56:411–5. doi: 10.1007/s002280000156. [DOI] [PubMed] [Google Scholar]
- 23.Veronese ML, Gillen LP, Burke JP, Dorval EP, Hauck WW, Pequiqnot E, Waldman SA, Greenberg HE. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J Clin Pharmacol. 2003;43:831–9. doi: 10.1177/0091270003256059. [DOI] [PubMed] [Google Scholar]
- 24.Tassaneeyakul W, Guo LQ, Fukuda K, Ohta T, Yamazoe Y. Inhibition selectivity of grapefruit juice components on human cytochromes P450. Arch Biochem Biophys. 2000;378:356–63. doi: 10.1006/abbi.2000.1835. [DOI] [PubMed] [Google Scholar]
- 25.Läpple F, von Richter O, Fromm MF, Richter T, Thon KP, Wisser H, Griese EU, Eichelbaum M, Kivistö KT. Differential expression and function of CYP2C isoforms in human intestine and liver. Pharmacogenetics. 2003;13:565–75. doi: 10.1097/00008571-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Tang M, Mukundan M, Yang J, Charpentier N, LeCluyse EL, Black C, Yang D, Shi D, Yan B. Antiplatelet agents aspirin and clopidogrel are hydrolyzed by distinct carboxylesterases, and clopidogrel is transesterificated in the presence of ethyl alcohol. J Pharmacol Exp Ther. 2006;319:1467–76. doi: 10.1124/jpet.106.110577. [DOI] [PubMed] [Google Scholar]
- 27.Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126–42. doi: 10.1177/0091270009343005. [DOI] [PubMed] [Google Scholar]
- 28.Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos. 2010;38:92–9. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]


