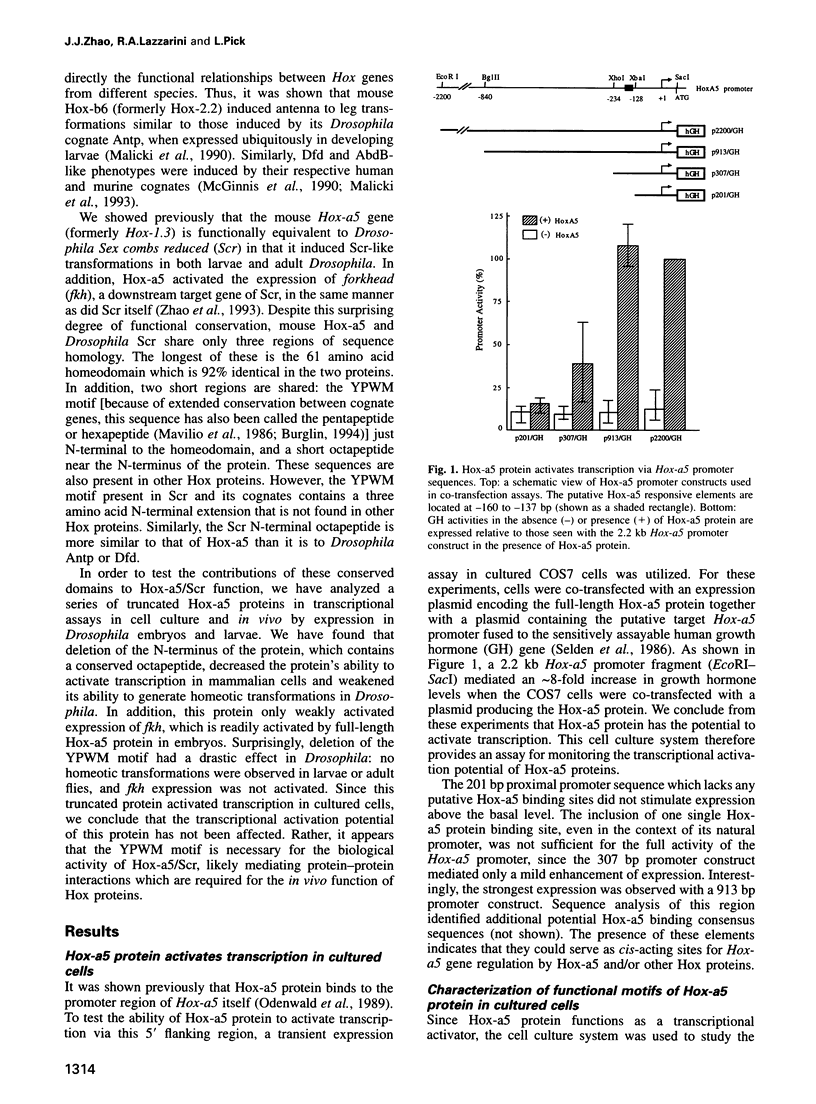
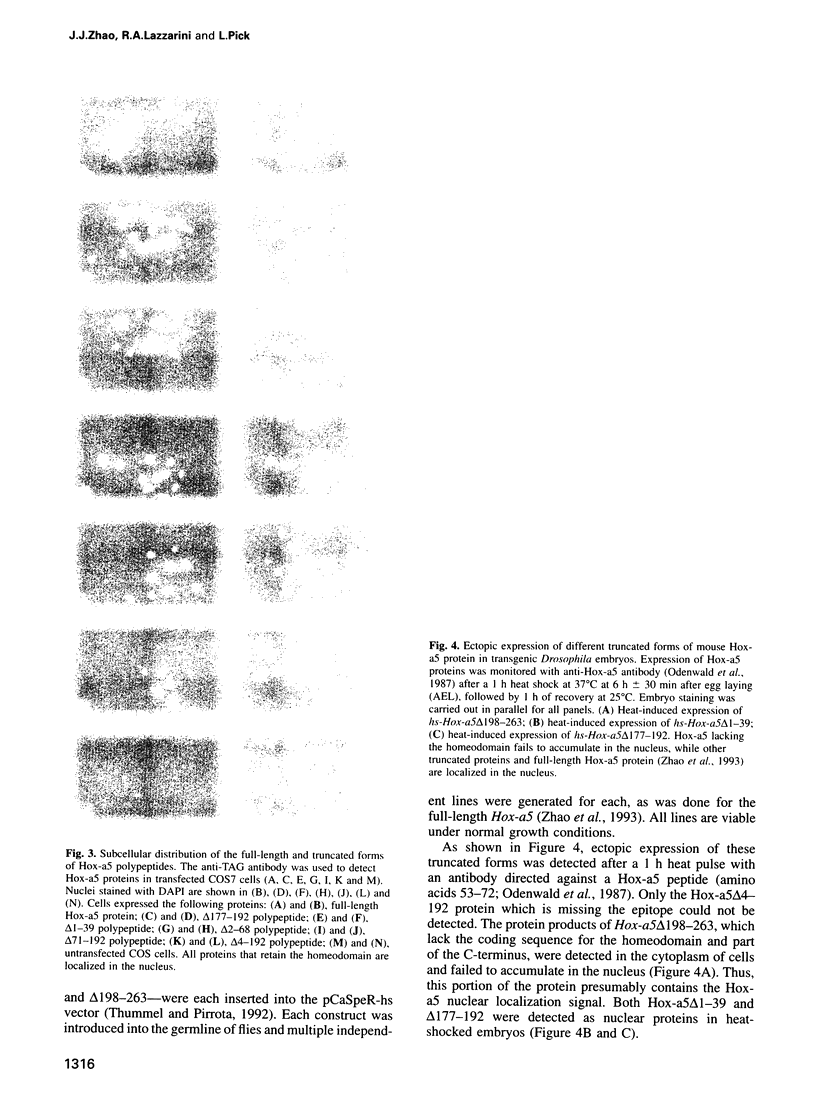
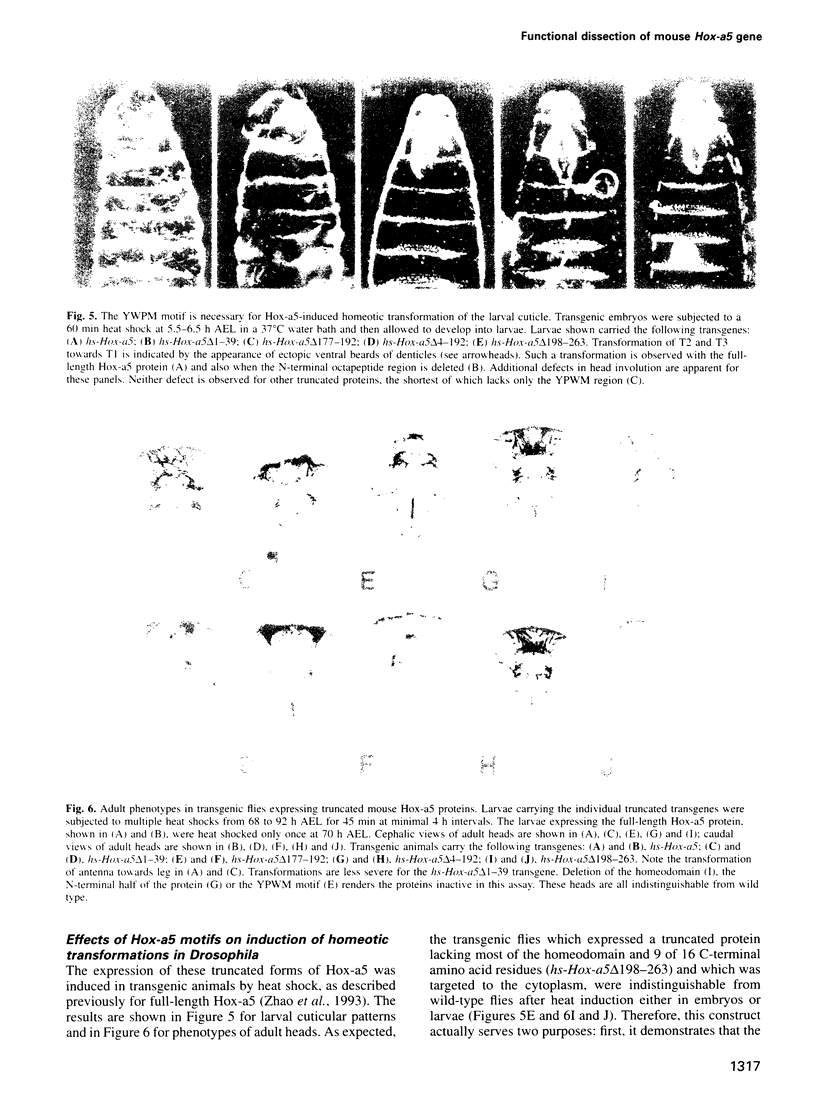
Abstract
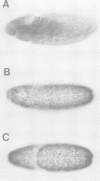
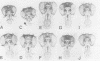
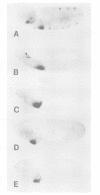
The Hox genes are clustered in evolutionarily conserved complexes and encode DNA binding proteins that determine positional identity. Ubiquitous expression of fly or mammalian Hox proteins in Drosophila embryos provides an assay for gene function, since different Hox genes induce characteristic homeotic transformations. Drosophila Sex combs reduced (Scr) and its murine cognate Hox-a5 produce identical transformations in transgenic flies. To study the contributions of domains conserved between the two proteins, truncated versions of mouse Hox-a5 were assayed for their ability to activate transcription in cultured cells and to induce homeotic transformation and activate target gene expression in transgenic embryos. The homeodomain is essential for protein function and/or nuclear targeting; the N-terminal region contributes to transcription activity and transformation potential in the embryo, but plays no role in determining functional specificity. The YPWM motif is essential for biological specificity, although it does not contribute to transcriptional activation potential. It was recently shown that the Hox-a5 YPWM motif is necessary for in vitro interactions with the co-factor Pbx1. Our results suggest that this type of protein-protein interaction may be essential for the biological activities of Hox-a5 and Scr.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. Hox and HOM: homologous gene clusters in insects and vertebrates. Cell. 1989 May 5;57(3):347–349. doi: 10.1016/0092-8674(89)90909-4. [DOI] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Chan S. K., Jaffe L., Capovilla M., Botas J., Mann R. S. The DNA binding specificity of Ultrabithorax is modulated by cooperative interactions with extradenticle, another homeoprotein. Cell. 1994 Aug 26;78(4):603–615. doi: 10.1016/0092-8674(94)90525-8. [DOI] [PubMed] [Google Scholar]
- Chan S. K., Mann R. S. The segment identity functions of Ultrabithorax are contained within its homeo domain and carboxy-terminal sequences. Genes Dev. 1993 May;7(5):796–811. doi: 10.1101/gad.7.5.796. [DOI] [PubMed] [Google Scholar]
- Chang C. P., Shen W. F., Rozenfeld S., Lawrence H. J., Largman C., Cleary M. L. Pbx proteins display hexapeptide-dependent cooperative DNA binding with a subset of Hox proteins. Genes Dev. 1995 Mar 15;9(6):663–674. doi: 10.1101/gad.9.6.663. [DOI] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988 Sep 23;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick V. D., Percival-Smith A., Ingles C. J., Krause H. M. Homeodomain-independent activity of the fushi tarazu polypeptide in Drosophila embryos. Nature. 1992 Apr 16;356(6370):610–612. doi: 10.1038/356610a0. [DOI] [PubMed] [Google Scholar]
- Furukubo-Tokunaga K., Flister S., Gehring W. J. Functional specificity of the Antennapedia homeodomain. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6360–6364. doi: 10.1073/pnas.90.13.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G., Schier A., LeMotte P., Gehring W. J. The specificities of Sex combs reduced and Antennapedia are defined by a distinct portion of each protein that includes the homeodomain. Cell. 1990 Sep 21;62(6):1087–1103. doi: 10.1016/0092-8674(90)90386-s. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Gutjahr T., Frei E., Noll M. Complex regulation of early paired expression: initial activation by gap genes and pattern modulation by pair-rule genes. Development. 1993 Feb;117(2):609–623. doi: 10.1242/dev.117.2.609. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Scott M. P. What determines the specificity of action of Drosophila homeodomain proteins? Cell. 1990 Nov 30;63(5):883–894. doi: 10.1016/0092-8674(90)90492-w. [DOI] [PubMed] [Google Scholar]
- Johnson F. B., Parker E., Krasnow M. A. Extradenticle protein is a selective cofactor for the Drosophila homeotics: role of the homeodomain and YPWM amino acid motif in the interaction. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):739–743. doi: 10.1073/pnas.92.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfler P. S., Kamps M. P. The pentapeptide motif of Hox proteins is required for cooperative DNA binding with Pbx1, physically contacts Pbx1, and enhances DNA binding by Pbx1. Mol Cell Biol. 1995 Oct;15(10):5811–5819. doi: 10.1128/mcb.15.10.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziora M. A., McGinnis W. A homeodomain substitution changes the regulatory specificity of the deformed protein in Drosophila embryos. Cell. 1989 Nov 3;59(3):563–571. doi: 10.1016/0092-8674(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lin L., McGinnis W. Mapping functional specificity in the Dfd and Ubx homeo domains. Genes Dev. 1992 Jun;6(6):1071–1081. doi: 10.1101/gad.6.6.1071. [DOI] [PubMed] [Google Scholar]
- Lu Q., Knoepfler P. S., Scheele J., Wright D. D., Kamps M. P. Both Pbx1 and E2A-Pbx1 bind the DNA motif ATCAATCAA cooperatively with the products of multiple murine Hox genes, some of which are themselves oncogenes. Mol Cell Biol. 1995 Jul;15(7):3786–3795. doi: 10.1128/mcb.15.7.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki J., Bogarad L. D., Martin M. M., Ruddle F. H., McGinnis W. Functional analysis of the mouse homeobox gene HoxB9 in Drosophila development. Mech Dev. 1993 Aug;42(3):139–150. doi: 10.1016/0925-4773(93)90003-g. [DOI] [PubMed] [Google Scholar]
- Malicki J., Schughart K., McGinnis W. Mouse Hox-2.2 specifies thoracic segmental identity in Drosophila embryos and larvae. Cell. 1990 Nov 30;63(5):961–967. doi: 10.1016/0092-8674(90)90499-5. [DOI] [PubMed] [Google Scholar]
- Mavilio F., Simeone A., Giampaolo A., Faiella A., Zappavigna V., Acampora D., Poiana G., Russo G., Peschle C., Boncinelli E. Differential and stage-related expression in embryonic tissues of a new human homoeobox gene. Nature. 1986 Dec 18;324(6098):664–668. doi: 10.1038/324664a0. [DOI] [PubMed] [Google Scholar]
- McGinnis N., Kuziora M. A., McGinnis W. Human Hox-4.2 and Drosophila deformed encode similar regulatory specificities in Drosophila embryos and larvae. Cell. 1990 Nov 30;63(5):969–976. doi: 10.1016/0092-8674(90)90500-e. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Krumlauf R. Homeobox genes and axial patterning. Cell. 1992 Jan 24;68(2):283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Kuziora M. The molecular architects of body design. Sci Am. 1994 Feb;270(2):58-61, 64-6. doi: 10.1038/scientificamerican0294-58. [DOI] [PubMed] [Google Scholar]
- Odenwald W. F., Garbern J., Arnheiter H., Tournier-Lasserve E., Lazzarini R. A. The Hox-1.3 homeo box protein is a sequence-specific DNA-binding phosphoprotein. Genes Dev. 1989 Feb;3(2):158–172. doi: 10.1101/gad.3.2.158. [DOI] [PubMed] [Google Scholar]
- Odenwald W. F., Taylor C. F., Palmer-Hill F. J., Friedrich V., Jr, Tani M., Lazzarini R. A. Expression of a homeo domain protein in noncontact-inhibited cultured cells and postmitotic neurons. Genes Dev. 1987 Jul;1(5):482–496. doi: 10.1101/gad.1.5.482. [DOI] [PubMed] [Google Scholar]
- Rauskolb C., Peifer M., Wieschaus E. extradenticle, a regulator of homeotic gene activity, is a homolog of the homeobox-containing human proto-oncogene pbx1. Cell. 1993 Sep 24;74(6):1101–1112. doi: 10.1016/0092-8674(93)90731-5. [DOI] [PubMed] [Google Scholar]
- Schneuwly S., Klemenz R., Gehring W. J. Redesigning the body plan of Drosophila by ectopic expression of the homoeotic gene Antennapedia. 1987 Feb 26-Mar 4Nature. 325(6107):816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- Schughart K., Utset M. F., Awgulewitsch A., Ruddle F. H. Structure and expression of Hox-2.2, a murine homeobox-containing gene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5582–5586. doi: 10.1073/pnas.85.15.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P. Vertebrate homeobox gene nomenclature. Cell. 1992 Nov 13;71(4):551–553. doi: 10.1016/0092-8674(92)90588-4. [DOI] [PubMed] [Google Scholar]
- Selden R. F., Howie K. B., Rowe M. E., Goodman H. M., Moore D. D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986 Sep;6(9):3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y., Inoko H., Ando A., Iwasaki K., Fujii A., Suemizu H., Yoshimura S., Moriuchi T. Structural analysis of the human HOX4A homeobox gene. Nucleic Acids Symp Ser. 1991;(25):31–32. [PubMed] [Google Scholar]
- Van Dijk M. A., Voorhoeve P. M., Murre C. Pbx1 is converted into a transcriptional activator upon acquiring the N-terminal region of E2A in pre-B-cell acute lymphoblastoid leukemia. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6061–6065. doi: 10.1073/pnas.90.13.6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappavigna V., Sartori D., Mavilio F. Specificity of HOX protein function depends on DNA-protein and protein-protein interactions, both mediated by the homeo domain. Genes Dev. 1994 Mar 15;8(6):732–744. doi: 10.1101/gad.8.6.732. [DOI] [PubMed] [Google Scholar]
- Zeng W., Andrew D. J., Mathies L. D., Horner M. A., Scott M. P. Ectopic expression and function of the Antp and Scr homeotic genes: the N terminus of the homeodomain is critical to functional specificity. Development. 1993 Jun;118(2):339–352. doi: 10.1242/dev.118.2.339. [DOI] [PubMed] [Google Scholar]
- Zhao J. J., Lazzarini R. A., Pick L. The mouse Hox-1.3 gene is functionally equivalent to the Drosophila Sex combs reduced gene. Genes Dev. 1993 Mar;7(3):343–354. doi: 10.1101/gad.7.3.343. [DOI] [PubMed] [Google Scholar]
- van Dijk M. A., Murre C. extradenticle raises the DNA binding specificity of homeotic selector gene products. Cell. 1994 Aug 26;78(4):617–624. doi: 10.1016/0092-8674(94)90526-6. [DOI] [PubMed] [Google Scholar]