Abstract
The gating of the KCNQ1 potassium channel is drastically regulated by auxiliary subunit KCNE proteins. KCNE1, for example, slows the activation kinetics of KCNQ1 by two orders of magnitude. Like other voltage-gated ion channels, the opening of KCNQ1 is regulated by the voltage-sensing domain (VSD; S1–S4 segments). Although it has been known that KCNE proteins interact with KCNQ1 via the pore domain, some recent reports suggest that the VSD movement may be altered by KCNE. The altered VSD movement of KCNQ1 by KCNE proteins has been examined by site-directed mutagenesis, the scanning cysteine accessibility method (SCAM), voltage clamp fluorometry (VCF) and gating charge measurements. These accumulated data support the idea that KCNE proteins interact with the VSDs of KCNQ1 and modulate the gating of the KCNQ1 channel. In this review, we will summarize recent findings and current views of the KCNQ1 modulation by KCNE via the VSD. In this context, we discuss our recent findings that KCNE1 may alter physical interactions between the S4 segment (VSD) and the S5 segment (pore domain) of KCNQ1. Based on these findings from ourselves and others, we propose a hypothetical mechanism for how KCNE1 binding alters the VSD movement and the gating of the channel.
Introduction
KCNQ1 encodes a voltage-gated potassium channel α subunit, which is widely expressed in various tissues such as heart, inner ear, lung, kidney and pancreas (Sanguinetti et al. 1996). KCNQ1, formerly known as KvLQT1, was originally identified as a causative gene for long QT syndrome, a type of cardiac arrhythmia (Wang et al. 1996). The function and biophysical properties of the KCNQ1 channel are drastically altered by co-expressing KCNE proteins. There have been five KCNE proteins identified (KCNE1–KCNE5) (Barhanin et al. 1996; Sanguinetti et al. 1996; Abbott et al. 1999; Schroeder et al. 2000; Angelo et al. 2002; Grunnet et al. 2002). KCNE1, formerly known as minK, was originally isolated as a putative potassium channel (Takumi et al. 1988), which eventually turned out to be an auxiliary subunit for KvLQT1 (KCNQ1) (Barhanin et al. 1996; Sanguinetti et al. 1996). The KCNQ1 and KCNE1 complex underlies the cardiac slow delayed rectifier potassium current (IKs) current and both genes are related to long QT syndrome; therefore, the KCNQ1/KCNE1 complex is physiologically and pathophysiologically important. On the other hand, the KCNQ1 modulation by KCNE1 is quite drastic and fascinating from a biophysical standpoint: KCNE1 increases KCNQ1 current amplitude by 10 times, slows the activation/deactivation kinetics and changes single channel conductance, pharmacological properties (Barhanin et al. 1996; Sanguinetti et al. 1996; Salata et al. 1998; Sesti & Goldstein, 1998; Yang & Sigworth, 1998; Gao et al. 2008; Mruk & Kobertz, 2009; Werry et al. 2013; Yu et al. 2013) (Fig.1A and B). In particular the activation rate, which is slowed by two orders of magnitude, has been one of the most interesting phenomena in the modulation by KCNE1. KCNE3 is also an interesting protein because it makes KCNQ1 channels constitutively active (channel is always open regardless of membrane potential) (Schroeder et al. 2000) (Fig.1C).
Figure 1.
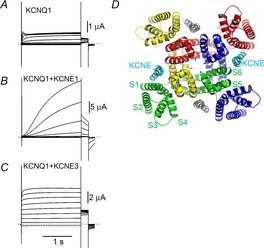
KCNE proteins modulate the gating behaviour of KCNQ1 by being located in the open space between VSDs
A–C, representative ionic current traces from KCNQ1, KCNQ1 + KCNE1 and KCNQ1 + KCNE3 expressed in Xenopus oocytes 3 days after RNA injection. The membrane potential was stepped from −100 mV to +60 mV in 20 mV steps for 2 s from the holding potential of −90 mV, and then stepped to −30 mV for tail current measurement. Dotted lines indicate zero current level. D, structural model of the open state KCNQ1 tetramer with KCNE1 (Smith et al. 2007; Kang et al. 2008). Each KCNQ1 subunit is in a different colour. The location and number of KCNE1 subunits (cyan and grey) are hypothetical. Each segment in the green subunit is labelled (S1–S6).
To understand the mechanism(s) of how KCNE proteins modulate KCNQ1 channel properties, it is important to know how KCNE proteins interact with KCNQ1 channels. Earlier studies show that the pore domain could be the binding site for KCNE1 (and KCNE3) (Melman et al. 2001, 2002, 2004; Tapper & George, 2001; Panaghie et al. 2006). Disulfide bond formation between two exogenous cysteine residues on the extracellular ends of S1/S4/S6 and the extracellular domain of KCNE1 revealed state-dependent contacts between KCNQ1 and KCNE1 in the extracellular domains (Nakajo & Kubo, 2007; Xu et al. 2008; Chung et al. 2009). Possible interaction with the S4 segment is particularly intriguing because the S4 segment is the central part of the voltage-sensing domain (VSD). When the membrane potential is depolarized, the S4 segment moves up towards the extracellular side by sensing membrane potential with some positively charged amino acid residues on the S4 itself. This conformational change eventually leads to the channel opening (Catterall, 2010; Jensen et al. 2012). Recent determination of the open state structure of rat Kv1.2 voltage gated potassium channel and structural models of KCNQ1 based on the rat Kv1.2 structure clearly show unoccupied open space between the four VSDs (Long et al. 2005; Smith et al. 2007). These gaps allow KCNE1 to bind to the pore domain and/or the voltage-sensing domain (Kang et al. 2008; Nakajo & Kubo, 2011; Van Horn et al. 2011) (Fig.1D). The number of KCNE1 subunits bound to KCNQ1 could be variable because the gating of KCNQ1 is dependent on the expression level of KCNE1 (Cui et al. 1994; Wang et al. 1998; Nakajo et al. 2010; Wang & Kass, 2012; Yu et al. 2013). Our single subunit-counting data also support a multiple stoichiometry up to 4:4 in the KCNQ1/KCNE1 ion channel complex (Nakajo et al. 2010). However, other reports including similar subunit-counting experiments showed that the stoichiometry is fixed at 4:2 (Chen et al. 2003; Morin & Kobertz, 2008; Plant et al. 2014). Although the exact binding site and the binding number of KCNE1 subunits still remain to be determined, KCNE1 should be located between the VSDs and is thought to affect the VSD movement. Because the VSD moves from the down state to the up state during activation, closely located KCNE proteins may affect the VSD movement.
KCNE1 alters the VSD movement in KCNQ1
One of the characteristic changes in the KCNQ1 channel induced by KCNE1 is the marked positive shift of the G–V (conductance–voltage) relationship (Fig.2A; black dashed and continuous curves). The positive shift by KCNE1 is around +40 to +50 mV, suggesting that KCNE1 makes the KCNQ1 channel more resistant to activation in terms of membrane potential. It was hypothesized that the VSD movement might be altered in the presence of KCNE1, and we and others used the scanning cysteine accessibility method (SCAM) to test this hypothesis (Akabas et al. 1992; Yang & Horn, 1995; Larsson et al. 1996; Nakajo & Kubo, 2007; Rocheleau & Kobertz, 2008). Introduction of a cysteine mutation at the top of S4 allows this question to be investigated using state-dependent modification by methanethiosulfonate (MTS) reagents. If the VSD movement was hindered by the presence of KCNE1, the modification rate of the MTS reagent would be slowed. We observed that the sodium (2-sulfonatoethyl)methanethiosulphonate modification rate of the A226C KCNQ1 mutant was 13 times slower in the presence of KCNE1 and concluded that the movement of the S4 segment was stabilized in the down state (closed state) (Nakajo & Kubo, 2007). On the other hand, in the KCNQ1/KCNE3 channel, which is constitutively active regardless of membrane potential, the modification rate was not voltage dependent, suggesting the VSD was stabilized in the up state (Nakajo & Kubo, 2007). However, the slower modification rate in the KCNQ1/KCNE1 channel does not necessarily mean that the S4 segment is slowed by KCNE1. The later report by Rocheleau & Kobertz (2008) emphasizes that the KCNE proteins affect voltage sensor equilibrium and not the speed of S4 movement. Nonetheless, these results raised the possibility that the VSD movement (or equilibrium) might be altered in the presence of KCNE1.
Figure 2.

KCNE1 alters the VSD movement and the opening of KCNQ1 channels
A, G–V relationships (black curves) and F–V relationships (red curves) for KCNQ1 (dashed curves) and KCNQ1 + KCNE1 (continuous curves). The G–V relationship of KCNQ1 is shifted in the positive direction by KCNE1. The F–V relationship is split into two components (F1 and F2) by KCNE1 (Osteen et al. 2010; Barro-Soria et al. 2014). The larger F1 component is shifted well in the negative direction while the smaller F2 component is shifted in the positive direction, as is the G–V relationship. B, S4 segments (red bars with ‘+’) of the KCNQ1/KCNE1 channel sit in the down state (closed state) at negative membrane potential. Depolarization moves the S4 segments to the pre-open state (F1), in which all S4 segments are in the up state with the pore domain still closed. Higher or longer depolarization induces the channel to enter the open state (F2).
Voltage clamp fluorometry (VCF) is a more direct way to track the structural rearrangements of the S4 segment (Mannuzzu et al. 1996). In a VCF experiment, a cysteine residue is introduced in the S3–S4 linker, and that cysteine residue is pre-labelled by a fluorophore such as tetramethylrhodamine-5-maleimide (TMRM). Because fluorescence intensity is dependent on the micro-environment surrounding the fluorophore, the S4 movement can be tracked by measuring fluorescence intensity under voltage clamp. Osteen et al. first applied VCF to the KCNQ1 channel alone, and the KCNQ1/KCNE1 channel, and found that the VSD movement was altered, but in an unexpected way (Osteen et al. 2010). While the G–V relationship was shifted in the positive direction by KCNE1 as already known, the F–V (fluorescence–voltage) relationship was split into two components in the presence of KCNE1 and showed fluorescence change over a much wider range (−200 to +100 mV; red continuous curve in Fig.2A) compared to KCNQ1 alone (−100 to +60 mV; red dashed curve in Fig.2A). The main fluorescence component (F1; −200 to 0 mV), which should correspond to the main VSD movement during the transition among the non-conducting closed states, was shifted markedly in the negative direction. On the other hand, the second and smaller fluorescence component (F2; −20 to +100 mV) seemed to be related to the opening of the channel. The first report of the VCF experiment suggested that the VSD movement of KCNQ1 was not merely stabilized in the down state by KCNE1, but was probably stabilized at the intermediate (pre-open) state between the down state and the up state (Fig.2B) and that is why the F–V and the G–V were well separated in the presence of KCNE1 (Osteen et al. 2010). However, Ruscic et al. later reported that the fluorescence and current had the same kinetics in their VCF recordings, and concluded that KCNE1 slowed the opening of KCNQ1 by slowing the VSD movement (Ruscic et al. 2013). They also tried to measure gating currents from KCNQ1, and while they successfully recorded gating current from KCNQ1 alone, they could not record the KCNQ1 gating current in the presence of KCNE1. They claimed that the gating current of the KCNQ1/KCNE1 channel was kinetically too slow to be captured, and the absence of the gating current supported the slow VSD movement in the KCNQ1/KCNE1 channel. In terms of VCF experiments, the differences between Osteen et al. and Ruscic et al. are the site of cysteine introduction (G219C for Osteen et al. and V221C for Ruscic et al.) and the fluorophore (Alexa488 maleimide for Osteen et al. and TMRM for Ruscic et al.). One possible explanation at that point was that G219C with Alexa488 reported both the initial VSD movement (F1 in Fig.2) and the late VSD movement (F2) while V221C with TMRM was only sensitive to the late VSD movement (F2) (Fig.2A). To address this discrepancy, Barro-Soria et al. re-examined the VCF experiments for all combinations of K218C, G219C and V221C labelled with Alexa488 or TMRM (Barro-Soria et al. 2014). All six combinations showed two separate fluorescence components (F1 and F2). They also re-examined MTS experiments with A223C and T224C, and reported that the [2-(trimethylammonium)ethyl methanethiosulphonate modification rate for A223C showed similar voltage dependence to the F1 component of the VCF while the modification rate for T224C corresponded to the F2 component. They even successfully captured gating currents of KCNQ1 in the presence of KCNE1, and the charge-voltage (Q-V) relationship for KCNQ1/KCNE1 channels was similar to the F1 component of the VCF. These results strongly suggest that the VSD movement of KCNQ1/KCNE1 channels can be separated into two parts: the early component (F1) corresponds to the main charge movement from the down state to the pre-open state, and the late component (F2) corresponds to the channel opening (Fig.2B). In other words, KCNE1 divides the VSD movement into two steps (Barro-Soria et al. 2014).
Although there might still remain some discrepancies and controversies among these experiments (VCF, gating charge and SCAM), the VSD movement in KCNQ1 channels is unquestionably altered by the presence of KCNE1 proteins.
Molecular determinants altering the coupling between the VSD movement and the channel opening in KCNQ1/KCNE1 channels
There have been many mutagenesis studies to elucidate the mechanisms of KCNE1 modulation. Some of these mutation studies were aimed at elucidating a possible role of the S4 segment of KCNQ1 in KCNE modulations (Panaghie & Abbott, 2007; Haitin et al. 2008; Shamgar et al. 2008; Wu et al. 2010a,b2010). Although these mutation studies provided us with some good insights into the mechanisms of how KCNE1 modulates the S4 (and VSD) movements, it still remains largely unknown how KCNE1 interacts with the VSD or which amino acid residue (if any) is the most responsible for the modulation.
We recently demonstrated that Phe232 in KCNQ1 plays an important role in the KCNE1-mediated G–V shift (Nakajo & Kubo, 2014). The F232A mutant showed almost no positive G–V shift in the presence of KCNE1. Phe232 is located right next to the 2nd positively-charged arginine residue (Arg231). According to the structural model of KCNQ1 (Smith et al. 2007), Phe232 faces the S5 segment and possibly interacts with Phe279 on the S5. We mutated Phe279 to alanine and F279A again showed no positive G–V shift in the presence of KCNE1. More interestingly, the degree of G–V shift was dependent on the side chain volume at 232 or 279, suggesting that the bulky phenylalanine residues might hinder each other during the VSD movement upon depolarization. We next applied VCF experiments to reveal whether the VSD movement is actually hindered by the bulky amino acid residues on the S4 and the S5 segments, and found that the relationship between the VSD movement and the channel opening was altered in the F232A and F279A mutants. While wild-type KCNQ1/KCNE1 channels showed delayed ionic current after the VSD movement, the VSD movements and the ionic currents were almost synchronized in the F232A and F279A mutants with KCNE1 (Fig.3A). In wild-type KCNQ1/KCNE1 channels, the interaction of the two bulky amino acid residues Phe232 and Phe279 may hinder the final opening step, and that could be one of the mechanisms why KCNQ1/KCNE1 channels require a higher membrane potential to be activated (Fig.3B). Very importantly, this steric hindrance does not occur in the absence of KCNE1. Phe232 and Phe279 of KCNQ1 are definitely important amino acid residues in the gating modulation by KCNE1; however, the reason why they interact with each other only in the presence of KCNE1 is still an enigma. This could be a key question for understanding the gating modulation by KCNE1 and we will propose our hypothesis in the later section.
Figure 3.
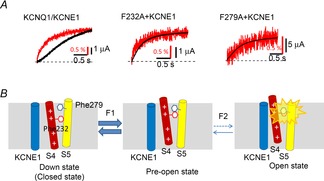
Two bulky phenylalanine residues hamper the opening of the KCNQ1/KCNE1 channel
A, simultaneous recording of VCF (VSD movement; red traces) and ionic current (black traces) from the wild-type KCNQ1/KCNE1 channel, F232A with KCNE1 and F279A with KCNE1. The wild-type KCNQ1/KCNE1 channel shows a delay of ionic current after the VSD movement; on the other hand, VSD movement and ionic currents from the two F–A mutants are almost synchronized (data from Nakajo & Kubo, Nature Communications, 2014). Red bars indicate 0.5% increase from the basal fluorescence of Alexa488 maleimide attached to G219C on the S3–S4 linker. B, schematic diagrams showing how Phe232 and Phe279 interact with each other during activation. Phe232 on the S4 segment moves by depolarization, up to the pre-open state (F1). Further depolarization rearranges the S4 a little more; however, physical interaction between Phe232 and Phy279 prevents the channel from entering the open state (F2). This would hamper the opening after the VSD movement in the wild-type KCNQ1/KCNE1 channels (A) (Nakajo & Kubo, 2014).
We do not know if our hypothesis, the interaction between the S4 and the S5 via bulky amino acid residues, can be applied to other voltage-gated ion channels at present. Phe232 is conserved in the other KCNQ family members (KCNQ2–KCNQ5), but Phe279 is not. In addition, even KCNQ1 requires KCNE1 to facilitate the interaction between the two phenylalanine residues. Therefore, this mechanism may be specific to the KCNQ1/KCNE1 channel. On the other hand, phenylalanine is commonly found in the hydrophobic transmembrane region. There might exist other ion channels which utilize bulky phenylalanine residues for altering the coupling between the VSD and the pore domain.
Phe232 and Phe279 are not the only important sites for coupling between the VSD and the pore domain in KCNQ1. Phosphatidylinositol 4,5-bisphosphate (PIP2) is required for the coupling of voltage sensing to pore opening (Zaydman et al. 2013; Zaydman & Cui, 2014). They applied VCF and showed that PIP2 depletion suppresses pore opening while the VSD movement is intact, suggesting that PIP2 depletion decouples the VSD and the pore domain. Putative PIP2 binding sites are located in the intracellular VSD–pore domain interface including the S2–S3 linker, the S4–S5 linker and the proximal C-terminus. Interestingly, KCNE1 enhances the PIP2 sensitivity of KCNQ1 (Li et al. 2011). This suggests that PIP2 may contribute to the KCNE1-induced gating modulation by changing the coupling between the VSD and the pore domain (Zaydman et al. 2014).
Possible roles of non-S4 segments in KCNE modulation
While the S4 segment bears the positively charged amino acid residues for voltage sensing, the rest of the segments S1–S3 have been regarded as less important segments in voltage sensing. Nevertheless, the recent disulfide cross-linking studies of KCNQ1/KCNE1 channels suggest that the S1 segment is probably one of the closest segments to KCNE1 (Xu et al. 2008; Chung et al. 2009). In addition, we identified Phe127 and Phe130 on the S1 segment as possible interaction sites for KCNE3 (Fig.4; green amino acid residues) (Nakajo et al. 2011). If KCNE proteins share the binding site on the KCNQ1 channel, amino acid residues in S1 may also be binding sites for KCNE1 (although Phe127 and Phe130 were not critical for the KCNE1 modulation in our experiments). According to the structural models shown in Fig.4, it appears possible that KCNE1 interacts with the pore domain (S5–S6) and the S1 segment simultaneously both in the closed state and the open state.
Figure 4.
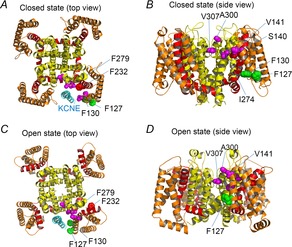
The putative interaction sites between KCNE protein and the S1/S5 segments
A–D, structural models of closed (A and B) and open (C and D) states (Smith et al. 2007). A and C are top views from the extracellular side. B and D are side views. The transmembrane region of KCNE1 (cyan) (Kang et al. 2008) is also depicted in A and C. S1–S3, S4 & S4–S5 linker and S5–S6 are coloured in orange, red and yellow, respectively. The side chain of amino acid residues whose mutation causes short QT syndrome or gain-of-function (S140, V141, I274, A300 and V307) are coloured in magenta (Smith et al. 2007). The amino acid residues responsible for the constitutive activity induced by KCNE3 (F127 and F130) are coloured in green (Nakajo et al. 2011). The side chain of F232 (red) and F279 (yellow) are also depicted. Only side chains around KCNE protein (cyan) are depicted.
KCNQ1 is well known as a causative gene for long QT syndrome, in which the depolarization of ventricular myocytes is prolonged due to reduced potassium conductance during repolarization. KCNQ1 is also a causative gene for a rare cardiac arrhythmia, short QT syndrome, in which the depolarization of ventricular myocytes is shortened by enhanced potassium conductance. Interestingly, those short QT or gain-of-function mutation sites are clustered in the upper part of the S1 and S5 segments and the pore helix (Fig.4; magenta amino acid residues) (Smith et al. 2007). These amino acid residues might have an interaction with other amino acid residues to stabilize the closed state. Therefore, mutations on these amino acid residues destabilize the closed state. An alternative explanation is that the mutations somehow stabilize the open state (or pre-open state). In either case, the KCNQ1 channel becomes easier to be activated and thus shorten the depolarization. Interestingly, some of the mutations are effective only in the presence of KCNE1 (Restier et al. 2008; Chan et al. 2012).
If the S1 segment is the interaction site for KCNE1, how does KCNE1 regulate the VSD movement? One possible role for KCNE1 is packing the pore domain and the VSD. As described in the previous section, Phe232 of the S4 and Phe279 of the S5 only interact with each other in the presence of KCNE1 (Nakajo & Kubo, 2014). Although they are located close to each other in the KCNQ1 structural models (Smith et al. 2007), they might not be tightly coupled especially in the absence of KCNE1 (Fig.5A). A recent molecular dynamics (MD) simulation of a Kv1.2/2.1 chimera presented the VSD motion during depolarization/hyperpolarization (Jensen et al. 2012). In that simulation, the VSDs loosely move around the pore domain especially in the absence of the N-terminal T1 domain. KCNE1 may play a similar role to the T1 domain in Kv1.2, to pack the VSD to the pore domain (Fig.5B). The packing of these domains would enhance the interaction between the S4 (Phe232) and the S5 (Phe279), and affect the gating behaviour as a result. This is our current hypothesis for the molecular mechanisms of the KCNE modulations. Future experiments including MD simulation would be required to test this hypothesis.
Figure 5.
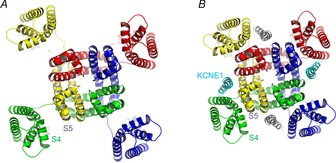
Hypothetical role of KCNE1 in packing the VSDs and the pore domain
A, VSDs move laterally around the pore domain. In this situation the distance between the S4 and S5 segments would fluctuate depending on the location of the VSD. B, KCNE1 proteins (cyan and grey) pack the VSDs and the pore domain. In tightly packed channels with KCNE1, the interaction between the S4 and the S5 segments may be facilitated and that may affect the gating of KCNQ1 channels.
Concluding remarks
Interactions between KCNQ1 and KCNE proteins occur not only in the transmembrane region but also on the extracellular and intracellular sides. However, the modulation of the VSD movement is definitely a very important component of the modulations by KCNE, if not the most important one. Especially in the past several years, new findings from VCF and other experiments have boosted our understanding of the molecular mechanisms. On the other hand, simple mutagenesis studies might no longer be enough to elucidate the mechanism of interaction and modulation. Future studies including structural determination and MD simulation would be required to fully understand this biophysically fascinating and physiologically important ion channel complex.
Acknowledgments
We thank Dr A. Collins (Queen’s University, Belfast) for text editing and correction.
Glossary
- MTS
methanethiosulfonate
- SCAM
scanning cysteine accessibility method
- TMRM
tetramethylrhodamine-5-maleimide
- VCF
voltage clamp fluorometry
- VSD
voltage-sensing domain
Biographies
 Koichi Nakajo (left) received his PhD from the University of Tokyo working with Dr Yasushi Okamura.He thenmoved toTokyoMedical andDentalUniversity to performpostdoctoral work with Professor Yoshihiro Kubo where he started his KCNQ channel studies. He is currently an assistant professor at the National Institute for Physiological Sciences.
Koichi Nakajo (left) received his PhD from the University of Tokyo working with Dr Yasushi Okamura.He thenmoved toTokyoMedical andDentalUniversity to performpostdoctoral work with Professor Yoshihiro Kubo where he started his KCNQ channel studies. He is currently an assistant professor at the National Institute for Physiological Sciences.
 Yoshihiro Kubo (right) received his PhD from The University of Tokyo (supervisor: the late Professor Kunitaro Takahashi) and performed post doctoral work at the University of California San Francisco in lab of Professor Lily Jan. He engaged in structure–function studies of membrane proteins at Tokyo Metropolitan Institute for Neuro sciences and then at Tokyo Medical and Dental University. He has been a professor at the National Institute for Physiological Sciences since 2003.
Yoshihiro Kubo (right) received his PhD from The University of Tokyo (supervisor: the late Professor Kunitaro Takahashi) and performed post doctoral work at the University of California San Francisco in lab of Professor Lily Jan. He engaged in structure–function studies of membrane proteins at Tokyo Metropolitan Institute for Neuro sciences and then at Tokyo Medical and Dental University. He has been a professor at the National Institute for Physiological Sciences since 2003.
Additional information
Competing interests
The authors have no conflicts of interest to disclose.
Funding
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant nos 24590292 and 25136724) to K.N.
References
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT. Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- Akabas MH, Stauffer DA, Xu M. Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Angelo K, Jespersen T, Grunnet M, Nielsen MS, Klaerke DA. Olesen SP. KCNE5 induces time- and voltage-dependent modulation of the KCNQ1 current. Biophys J. 2002;83:1997–2006. doi: 10.1016/S0006-3495(02)73961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M. Romey G. KVLQT1 and lsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Barro-Soria R, Rebolledo S, Liin SI, Perez ME, Sampson KJ, Kass RS. Larsson HP. KCNE1 divides the voltage sensor movement in KCNQ1/KCNE1 channels into two steps. Nat Commun. 2014;5:3750. doi: 10.1038/ncomms4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67:915–928. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PJ, Osteen JD, Xiong D, Bohnen MS, Doshi D, Sampson KJ, Marx SO, Karlin A. Kass RS. Characterization of KCNQ1 atrial fibrillation mutations reveals distinct dependence on KCNE1. J Gen Physiol. 2012;139:135–144. doi: 10.1085/jgp.201110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kim LA, Rajan S, Xu S. Goldstein SA. Charybdotoxin binding in the IKs pore demonstrates two MinK subunits in each channel complex. Neuron. 2003;40:15–23. doi: 10.1016/s0896-6273(03)00570-1. [DOI] [PubMed] [Google Scholar]
- Chung DY, Chan PJ, Bankston JR, Yang L, Liu G, Marx SO, Karlin A. Kass RS. Location of KCNE1 relative to KCNQ1 in the IKS potassium channel by disulfide cross-linking of substituted cysteines. Proc Natl Acad Sci U S A. 2009;106:743–748. doi: 10.1073/pnas.0811897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Kline RP, Pennefather P. Cohen IS. Gating of IsK expressed in Xenopus oocytes depends on the amount of mRNA injected. J Gen Physiol. 1994;104:87–105. doi: 10.1085/jgp.104.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Xiong Q, Sun H. Li M. Desensitization of chemical activation by auxiliary subunits: convergence of molecular determinants critical for augmenting KCNQ1 potassium channels. J Biol Chem. 2008;283:22649–22658. doi: 10.1074/jbc.M802426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Rasmussen HB, Ljungstrøm T, Jorgensen NK, Olesen SP. Klaerke DA. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol. 2002;542:119–130. doi: 10.1113/jphysiol.2002.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haitin Y, Yisharel I, Malka E, Shamgar L, Schottelndreier H, Peretz A, Paas Y. Attali B. S1 constrains S4 in the voltage sensor domain of Kv7.1 K+ channels. PloS One. 2008;3:e1935. doi: 10.1371/journal.pone.0001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MØ, Jogini V, Borhani DW, Leffler AE, Dror RO. Shaw DE. Mechanism of voltage gating in potassium channels. Science. 2012;336:229–233. doi: 10.1126/science.1216533. [DOI] [PubMed] [Google Scholar]
- Kang C, Tian C, Sonnichsen FD, Smith JA, Meiler J, George AL, Jr, Vanoye CG, Kim HJ. Sanders CR. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry. 2008;47:7999–8006. doi: 10.1021/bi800875q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HP, Baker OS, Dhillon DS. Isacoff EY. Transmembrane movement of the Shaker K+ channel S4. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- Li Y, Zaydman MA, Wu D, Shi J, Guan M, Virgin-Downey B. Cui J. KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity. Proc Natl Acad Sci U S A. 2011;108:9095–9100. doi: 10.1073/pnas.1100872108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Campbell EB. Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- Mannuzzu LM, Moronne MM. Isacoff EY. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- Melman YF, Domenech A, de la Luna S. McDonald TV. Structural determinants of KvLQT1 control by the KCNE family of proteins. J Biol Chem. 2001;276:6439–6444. doi: 10.1074/jbc.M010713200. [DOI] [PubMed] [Google Scholar]
- Melman YF, Krumerman A. McDonald TV. A single transmembrane site in the KCNE-encoded proteins controls the specificity of KvLQT1 channel gating. J Biol Chem. 2002;277:25187–25194. doi: 10.1074/jbc.M200564200. [DOI] [PubMed] [Google Scholar]
- Melman YF, Um SY, Krumerman A, Kagan A. McDonald TV. KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron. 2004;42:927–937. doi: 10.1016/j.neuron.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Morin TJ. Kobertz WR. Counting membrane-embedded KCNE β-subunits in functioning K+ channel complexes. Proc Natl Acad Sci U S A. 2008;105:1478–1482. doi: 10.1073/pnas.0710366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk K. Kobertz WR. Discovery of a novel activator of KCNQ1-KCNE1 K channel complexes. PloS One. 2009;4:e4236. doi: 10.1371/journal.pone.0004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo K. Kubo Y. KCNE1 and KCNE3 stabilize and/or slow voltage sensing S4 segment of KCNQ1 channel. J Gen Physiol. 2007;130:269–281. doi: 10.1085/jgp.200709805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo K. Kubo Y. Nano-environmental changes by KCNE proteins modify KCNQ channel function. Channels. 2011;5:397–401. doi: 10.4161/chan.5.5.16468. [DOI] [PubMed] [Google Scholar]
- Nakajo K. Kubo Y. Steric hindrance between S4 and S5 of the KCNQ1/KCNE1 channel hampers pore opening. Nat Commun. 2014;5:4100. doi: 10.1038/ncomms5100. [DOI] [PubMed] [Google Scholar]
- Nakajo K, Nishino A, Okamura Y. Kubo Y. KCNQ1 subdomains involved in KCNE modulation revealed by an invertebrate KCNQ1 orthologue. J Gen Physiol. 2011;138:521–535. doi: 10.1085/jgp.201110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajo K, Ulbrich MH, Kubo Y. Isacoff EY. Stoichiometry of the KCNQ1-KCNE1 ion channel complex. Proc Natl Acad Sci U S A. 2010;107:18862–18867. doi: 10.1073/pnas.1010354107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteen JD, Gonzalez C, Sampson KJ, Iyer V, Rebolledo S, Larsson HP. Kass RS. KCNE1 alters the voltage sensor movements necessary to open the KCNQ1 channel gate. Proc Natl Acad Sci U S A. 2010;107:22710–22715. doi: 10.1073/pnas.1016300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaghie G. Abbott GW. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J Gen Physiol. 2007;129:121–133. doi: 10.1085/jgp.200609612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaghie G, Tai KK. Abbott GW. Interaction of KCNE subunits with the KCNQ1 K+ channel pore. J Physiol. 2006;570:455–467. doi: 10.1113/jphysiol.2005.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant LD, Xiong D, Dai H. Goldstein SA. Individual IKs channels at the surface of mammalian cells contain two KCNE1 accessory subunits. Proc Natl Acad Sci U S A. 2014;111:E1438–1446. doi: 10.1073/pnas.1323548111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restier L, Cheng L. Sanguinetti MC. Mechanisms by which atrial fibrillation-associated mutations in the S1 domain of KCNQ1 slow deactivation of IKs channels. J Physiol. 2008;586:4179–4191. doi: 10.1113/jphysiol.2008.157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocheleau JM. Kobertz WR. KCNE peptides differently affect voltage sensor equilibrium and equilibration rates in KCNQ1 K+ channels. J Gen Physiol. 2008;131:59–68. doi: 10.1085/jgp.200709816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscic KJ, Miceli F, Villalba-Galea CA, Dai H, Mishina Y, Bezanilla F. Goldstein SA. IKs channels open slowly because KCNE1 accessory subunits slow the movement of S4 voltage sensors in KCNQ1 pore-forming subunits. Proc Natl Acad Sci U S A. 2013;110:E559–566. doi: 10.1073/pnas.1222616110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salata JJ, Jurkiewicz NK, Wang J, Evans BE, Orme HT. Sanguinetti MC. A novel benzodiazepine that activates cardiac slow delayed rectifier K+ currents. Mol Pharmacol. 1998;54:220–230. doi: 10.1124/mol.54.1.220. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL. Keating MT. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R. Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- Sesti F. Goldstein SA. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J Gen Physiol. 1998;112:651–663. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamgar L, Haitin Y, Yisharel I, Malka E, Schottelndreier H, Peretz A, Paas Y. Attali B. KCNE1 constrains the voltage sensor of Kv7.1 K+ channels. PloS One. 2008;3:e1943. doi: 10.1371/journal.pone.0001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Vanoye CG, George AL, Jr, Meiler J. Sanders CR. Structural models for the KCNQ1 voltage-gated potassium channel. Biochemistry. 2007;46:14141–14152. doi: 10.1021/bi701597s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T, Ohkubo H. Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Tapper AR. George AL., Jr Location and orientation of minK within the IKs potassium channel complex. J Biol Chem. 2001;276:38249–38254. doi: 10.1074/jbc.M103956200. [DOI] [PubMed] [Google Scholar]
- Van Horn WD, Vanoye CG. Sanders CR. Working model for the structural basis for KCNE1 modulation of the KCNQ1 potassium channel. Curr Opin Struct Biol. 2011;21:283–291. doi: 10.1016/j.sbi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD. Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Wang M. Kass RS. Stoichiometry of the slow Iks potassium channel in human embryonic stem cell-derived myocytes. Pediatr Cardiol. 2012;33:938–942. doi: 10.1007/s00246-012-0255-2. [DOI] [PubMed] [Google Scholar]
- Wang W, Xia J. Kass RS. MinK-KvLQT1 fusion proteins, evidence for multiple stoichiometries of the assembled IsK channel. J Biol Chem. 1998;273:34069–34074. doi: 10.1074/jbc.273.51.34069. [DOI] [PubMed] [Google Scholar]
- Werry D, Eldstrom J, Wang Z. Fedida D. Single-channel basis for the slow activation of the repolarizing cardiac potassium current, IKs. Proc Natl Acad Sci U S A. 2013;110:E996–1005. doi: 10.1073/pnas.1214875110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Delaloye K, Zaydman MA, Nekouzadeh A, Rudy Y. Cui J. a State-dependent electrostatic interactions of S4 arginines with E1 in S2 during Kv7.1 activation. J Gen Physiol. 2010;135:595–606. doi: 10.1085/jgp.201010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Pan H, Delaloye K. Cui J. b KCNE1 remodels the voltage sensor of Kv7.1 to modulate channel function. Biophys J. 2010;99:3599–3608. doi: 10.1016/j.bpj.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Jiang M, Hsu KL, Zhang M. Tseng GN. KCNQ1 and KCNE1 in the IKs channel complex make state-dependent contacts in their extracellular domains. J Gen Physiol. 2008;131:589–603. doi: 10.1085/jgp.200809976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N. Horn R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron. 1995;15:213–218. doi: 10.1016/0896-6273(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Yang Y. Sigworth FJ. Single-channel properties of IKs potassium channels. J Gen Physiol. 1998;112:665–678. doi: 10.1085/jgp.112.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Lin Z, Mattmann ME, Zou B, Terrenoire C, Zhang H, Wu M, McManus OB, Kass RS, Lindsley CW, Hopkins CR. Li M. Dynamic subunit stoichiometry confers a progressive continuum of pharmacological sensitivity by KCNQ potassium channels. Proc Natl Acad Sci U S A. 2013;110:8732–8737. doi: 10.1073/pnas.1300684110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaydman MA. Cui J. PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front Physiol. 2014;5:195. doi: 10.3389/fphys.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaydman MA, Kasimova MA, McFarland K, Beller Z, Hou P, Kinser HE, Liang H, Zhang G, Shi J, Tarek M. Cui J. Domain-domain interactions determine the gating, permeation, pharmacology, and subunit modulation of the IKs ion channel. Elife. 2014;3:e03606. doi: 10.7554/eLife.03606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaydman MA, Silva JR, Delaloye K, Li Y, Liang H, Larsson HP, Shi J. Cui J. Kv7.1 ion channels require a lipid to couple voltage sensing to pore opening. Proc Natl Acad Sci U S A. 2013;110:13180–13185. doi: 10.1073/pnas.1305167110. [DOI] [PMC free article] [PubMed] [Google Scholar]


