Abstract
Several factors, including overexpression of lactate dehydrogenase (LDH) and monocarboxylate transporters (MCTs), promote an aerobic lactate production that allows some cancer cells to sustain higher proliferation rates in hostile environments outside the cell. To elucidate the effect of endurance training on the metabolic phenotype of solid tumours, we focused on the tumour expression of LDH-A, LDH-B, MCT1, MCT4, oestrogen-related receptor alpha (ERRα) and LDH isozymes in control (C), trained (T), control+XCT790 (CX) and trained+XCT790 (TX) mice. First, we found that the metabolically altered tumours from the trained animals exhibited lower values for lactate concentration than the control group. The decreased lactate concentration was associated with a shift in the tumour LDH isozyme profile towards LDH-1. These exercise-induced changes were also associated with decreases in the expression of the tumour MCT1, ERRα and CD147 in the trained animals. Secondly, the inhibition of ERRα by treatment of MC4-L2 human breast cancer cells with XCT790 (inverse agonist ligand of ERRα) before injection into the animals not only increased LDH-B expression in the tumour, but also decreased MCT1 expression in the CX group in comparison to the C group. The effects of ERRα inhibition were not additive to the training effects on the expressions of MCT1 and LDH-B in the solid tumours. In conclusion, our results suggest that exercise-induced suppression of ERRα expression modulates alterations in solid tumour expression of LDH-B and MCT1 and contributes towards the prevention of tumour development.
Key points
Monocarboxylate transporters (MCTs) and lactate dehydrogenase A (LDH-A) play important roles in sustaining the glycolytic phenotype seen in cancer.
Endurance training improves aerobic capacity; however, whether endurance training alters the metabolic phenotype of a solid tumour, from the perspective of lactate metabolism, is yet to be proven.
This study showed that endurance training decreases expression of the MCT1 basigin (CD147) and LDH-A, and also increases LDH-B expression in solid tumours and attenuates tumour lactate metabolism.
Similar results for MCT1 and LDH-B were found with inhibition of the oestrogen-related receptor alpha (ERRα). The training effects were not additive to the ERRα effects on MCT1 and LDH-B expression in the tumour, which indicated that exercise-induced alterations in MCT1 and LDH-B expression were modulated by ERRα.
These results suggest that endurance training could be a useful tool in cancer therapy, especially in basal-like and luminal-like breast carcinomas.
Introduction
Breast cancer is unanimously considered a highly heterogeneous disease from several distinct perspectives. Expression profiling studies classified breast carcinomas into five groups: luminal A (oestrogen receptor (ER)+); luminal B (ER+); epidermal growth factor receptor 2 (HER2) overexpressing; normal breast-like; and basal-like. Preferential conversion of glucose into lactate, even under normoxic conditions (i.e. aerobic glycolysis or the Warburg Effect), is a common feature seen in cancer cells (Warburg, 1956; Semenza, 2008; Draoui & Feron, 2011; Muñoz-Pinedo et al. 2012). Increased glycolysis in cancer cells not only provides more ATP from the substrate-level phosphorylation of ADP in glycolysis, which enables the survival of the cancer cells under hypoxic conditions, but also provides the precursors necessary for the synthesis of lipids, nucleotides and amino acids that are needed to support cell proliferation (Cairns et al. 2011). The increased glycolysis, whether under anaerobic or aerobic conditions, results in significant lactate production, which in turn results in the acidification of the tumour microenvironment as the lactate and protons are released from the cells (Cardone et al. 2005; Gatenby et al. 2006). This glycolytic phenotype of the cells may be supported by adaptations in the tumour monocarboxylate transporters (MCTs, the carriers of the single-carboxylate molecules across the biological membranes; Draoui & Feron, 2011; Halestrap, 2012) and lactate dehydrogenase (LDH) isoform expression. Indeed, solid tumours typically develop faster than the blood supply, resulting in two distinct regions, which are the well-oxygenated (aerobic) and poorly-oxygenated (hypoxic) areas within the tumour mass (Semenza, 2008; Sonveaux et al. 2008). Normally, lesser amounts of glucose are utilized by the oxygenated tumour cells; instead, these cells use more lactate, which is derived from the glycolytic metabolism of glucose by the poorly oxygenated tumour cells (Semenza, 2008; Sonveaux et al. 2008; Kennedy & Dewhirst, 2010). At the molecular level, the key players in this symbiotic relationship are MCT1, as the major transporter, ensuring lactate uptake by the oxidative tumour cells, and MCT4, as the hypoxia-induced transporter involved in the export of lactate from the glycolytic tumour cells (Draoui & Feron, 2011). The plasma membrane abundance of MCTs changes significantly in breast cancer; MCT1 is up-regulated in basal-like breast carcinoma (Pinheiro et al. 2010, 2011) and luminal-like breast cancer cell lines such as MCF-7 (Hussien & Brooks, 2010). These findings suggest that these transporters might be involved in cancer proliferation. In agreement with these findings are the observations that MCT4 up-regulation in the MDA-MB-231 cancer cell line increases tumour growth through the maturation and trafficking of basigin (CD147) to the plasma membrane (Gallagher et al. 2007). Targeting MCT1 activity would not only induce apoptosis due to cellular acidosis (Le Floch et al. 2011), it would also lead to a reduction in tumour angiogenesis, invasion and metastasis (Sonveaux et al. 2008; Végran et al. 2011).
LDH is a tetrameric enzyme, containing two major subunits (A and B), encoded by two different genes, LDH-A and LDH-B (Markert, 1975). The LDH-A and LDH-B subunits associate as tetramers to form five different isoenzymes (LDH-1 to LDH-5) which are composed of either subunits LDH-B4 (LDH-1); LDH-B3:A1 (LDH-2); LDHB2:A2 (LDH-3), LDH-B1:A3 (LDH-4) and LDH-A4 (LDH-5) subunits (Markert et al. 1975). LDH-A expression was demonstrated to be up-regulated in breast cancer cells as the result of the hypoxic microenvironment and mitochondrial gene mutation (Hussien & Brooks, 2010; Wang et al. 2012). LDH catalyses the interconversion of pyruvate and reduces nicotinamide adenine dinucleotide (NADH) generated by glycolysis to lactate and NAD+. Through regenerating the NAD+ required to drive glycolysis in cancer cells, LDH is the key enzyme involved in the Warburg effect and in sustaining the glycolytic phenotype of cancer cells. While lactate accumulation is characteristic of cancer cells, there is no consensus regarding its cause. Some researchers postulate that lactate production by tumours is due to exaggerated glycolysis, while others suggest that it is a result of the limited clearance capacity imposed by the impaired capability for oxidative phosphorylation (Moreno-Sanchez et al. 2007). Although not all researchers agree that the LDH isoform is the causative factor for the pyruvate to lactate conversion, because the LDH-catalysed reaction is reversible, it seems that overexpression of the LDH enzyme comprising ‘pure’ LDH-5 subunits may be involved, at least to some degree, in elevated tumour lactate concentration. Lactate plays an important part in extracellular matrix degradation and angiogenesis, which are highly significant in cancer metastasis (Elias & Dias, 2008). Therefore, because the MCTs and LDHs are necessary for tumour maintenance, they are potential targets for cancer therapy.
Endurance training is one of the most important stimuli for improving metabolic phenotype and is associated with a reduced risk of various types of cancers. The effect of exercise on metabolic profiles, including hormone levels (Kossman et al. 2011), inflammation (Pierce et al. 2009), and adipokine concentrations (Devries et al. 2008), are the mechanisms that link physical activity to cancer risk. There is evidence also for the effects of exercise on the immune function, oxidative stress, and possibly DNA repair capacity (Schmitz et al. 2008; Devries et al. 2008; Ulrich et al. 2012). Although the effects of exercise on MCT and LDH isoform expression have been previously reported in healthy animals and humans (Dubouchaud et al. 2000; Benton et al. 2008; Nikooie et al. 2013; Aveseh et al. 2014), no detailed research has been done on the effects of endurance training on the MCTs, LDH isoforms and their related proteins in breast cancer patients.
Furthermore, the regulation of cell metabolism by endurance training requires the expression of a great number of genes encoded by the nuclear and mitochondrial genomes. The coordination of these genes depends on the transcription factors, among which oestrogen related receptor alpha (ERRα) appears to be essential (Giguere, 2008). Recently, ERRα has been considered as a switch regulating not only mitochondrial function, but also glycolysis (Cai et al. 2013; Mirebeau-Prunier et al. 2013). Cai et al. have shown that ERRα is an important transcriptional activator of the glycolytic pathway and contributes to the Warburg effect in cancer cells (Cai et al. 2013). Mirebeau-Prunier et al. have demonstrated an inverse correlation between ERRα expression and LDH activity and suggested an important role for ERRα in the induction of a metabolic shift in thyroid cells (Mirebeau-Prunier et al. 2013). ERRα is primarily assumed to regulate energy homeostasis via interaction with the peroxisome proliferator-activated receptor γ co-activator-1α and -1β (PGC-1α and -1β; Huss et al. 2004). In several tissues, PGC-1α acts as the master regulator of MCT regulation and cell energy metabolism (Benton et al. 2008) and is a major regulator of exercise-induced phenotype adaptation (Lira et al. 2010). Therefore, it is possible that any exercise-induced change in the expression of MCTs could be mediated by changes in ERRα.
In this study, we hypothesized that endurance training can modulate MCT and LDH isoform expression in the tumour in breast cancer-bearing BALB/c mice. The primary aim of this study was to investigate and examine the long term effects of endurance training on the tumour MCT (1 and 4), CD147 and LDH isoform (A and B) expression in breast cancer bearing BALB/c mice. Second, the study investigated whether the exercise-induced changes in MCTs and LDH isoforms could be mediated by ERRα.
Methods
Ethical approval
All procedures in the present study were approved by the Ethical Committees on Animal Care at the Neuroscience Research Centre of Kerman, University of Medical Sciences, Kerman, Iran.
Animals
Fifty female BALB/c mice (5 weeks old) were purchased from the Pasteur Institute (Tehran) and maintained on a 12 h–12 h light–dark cycle in a low-stress environment (22°C, 50% humidity and low noise) and provided with food and water ad libitum. Ten days after breast cancer induction, mice with visible tumours (n = 49) were randomly separated into four groups, according to their body weight: control (C; n=12), trained (T; n=13), control + XCT790 (CX; n=12), and trained + XCT790 (TX; n=12). Over a 7 week period after breast cancer induction, deaths occurred in the C (n = 3), T (n = 1), CX (n = 2), and TX (n = 2) groups. Two animals from the T group were not able to complete the training protocol and were excluded from the final measurements. The tissues from three animals (n = 1 T, n = 1 CX, and n = 1 TX)) were lost during the tissue homogenizing protocol. Therefore, 9–10 animals from each group were used for the final measurements. Health status was monitored during the study by monitoring the body weight, physical appearance and measurable clinical signs (changes in the general appearance, hair, eyes, nose, etc.) by a specialist. No clinical signs were found in the animals throughout the experiment. Ethical approval for the experimental protocol was obtained from the Ethical Committees on Animal Care at the Neuroscience Research Centre of Kerman.
Breast cancer induction procedure
The MC4-L2 human breast cancer cell line was purchased from the Pasteur Institute (Tehran, Iran). The cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin. The cells were maintained in 5% CO2, a humidified atmosphere and at 37°C and passaged twice a week. When the number of cells reached the desired level, they were harvested using trypsin–EDTA treatment, then washed with PBS and cell counts were performed in a Neubauer chamber. The MC4-L2 cells (1.2 × 106 cells in 100 μl of PBS) were injected subcutaneously into the right dorsal mammary fat pad in both the C and T groups. Similar procedures were performed in both the CX and TX groups with the exception that MC4-L2 cells were treated with XCT790 (at a concentration of 5 μm) for 24 h prior to the injection. XCT790 is a potent and selective inverse agonist ligand of ERRα and is widely used to inhibit ERRα activity (Busch et al. 2004). XCT790 suppresses ERRα transcriptional activity by decreasing its interaction with co-activators and promoting its proteasome-dependent degradation. The final concentration of XCT790 used in the present study can significantly inhibit ERRα protein expression, and does not impact cell viability and proliferation.
Exercise protocol
Treadmill running was the exercise mode used. This mode was selected because the exercise intensity and duration could be experimentally manipulated and quantified. Endurance training was started at 9 weeks of age and was performed every day for 7 weeks. Initially, the T and TX groups were familiarized with a motor-driven treadmill running at low speeds (10 m min−1, 20min day–1) for the first 5 days. Thereafter, the speed and duration were increased progressively over the 7 week period until the animals were running at 20 m min−1 for 55 min for the last 2 weeks. The animals from the C and CX groups remained sedentary in their cages for the entire duration of the 7 week training programme.
Tumour volume assessment
Fourteen days after the subcutaneous injection of MC4-L2 cells, the tumour was measurable. The tumour volume at this time was considered as the Baseline measurement. This measurement was used for adjusting all future measurements of tumour volume. Tumour volume was measured every 3 days in two orthogonal dimensions using microdigital calipers. The greatest dimension of the tumour was recorded as tumour length, with the other dimension (at a 90 deg angle) being recorded as the width. The tumour volume was calculated as π/6 × width × length2, which is the standard formula for calculating tumour volume in mouse models of breast cancer (Jones et al. 2010). Forty-eight hours after the final exercise session, animals were anaesthetized using a mixture of 10% ketamine and 2% xylazine and then decapitated. The heart and solid tumours were excised, weighed and snap-frozen in liquid nitrogen and stored at –80°C for subsequent analysis. Solid tumours were weighed and normalized to body weight. In order to calculate the cardiac mass index, the heart was weighed and normalized to body weight minus tumour weight (Almeida et al. 2009).
Western blotting
Approximately 100–150 mg of solid tumour was powdered using a cold mortar and pestle in liquid nitrogen. The tumour was homogenized in ice-cold RIPA buffer (50 mm Tris-HCl, 1 mm EDTA, 150 mm NaCl, NP40 1%, sodium deoxycholate 1%, SDS 1%, protease and phosphatase inhibitor 0.01 m, pH 7.4) and then centrifuged at 600 g for 10 min at 4°C to remove the nuclei and debris. One fraction of the resulting supernatant was centrifuged at 10,000 g for 30 min at 4°C to precipitate the mitochondrial fragments, and the supernatant was used for measurement of LDH-A and LDH-B (Hussien & Brooks, 2010). The pellet was washed in 1 ml of washing buffer (1 mm EDTA and 10 mm Tris, pH 7.4) and then resuspended in 100 μl of sample buffer (1.167 m KCl and 58.3 mm, Na4P2O7.10H2O, pH 7.4) and 33 μl of 16 % SDS and centrifuged at room temperature for 20 min to remove any insoluble materials. This sample was used for the measurement of cytochrome c oxidase subunit IV expression (Nikooie et al. 2013). Another fraction was centrifuged at 14,000 rpm for 15 min at 4°C. The supernatant was recovered and used to measure the MCTs and CD147. Protein concentration was determined with the Bradford protein assay using bovine serum albumin (BSA) as the standard and Western blotting was performed as previously described (Nikooie et al. 2013). In brief, 20 μg total protein from each sample was loaded and separated on 12.5% SDS–PAGE and transferred by electroblotting onto polyvinylidene difluoride membranes (Amersham). Thereafter, the PVDF membranes were stained in 0.1% Ponceau S in 1% acetic acid for 3 min with shaking. Membranes were destained by rinsing in water for 2 min. Ponceau S staining total protein loading was recorded to monitor the transfer efficiency and quantification of whole protein loading. Following washing in Tris-buffered saline–Tween 20 (TBST) for 5 min, the membranes were incubated for 1.5 h at room temperature in an orbital shaker in blocking buffer (150 mm NaCl, 5% skimmed milk, 0.1% Tween 20, and 50 mm Tris, at pH 7.5) in the primary antibody overnight at 4°C. The membranes were washed and then incubated for 90 min at room temperature with secondary antibody in TBST (Nikooie et al. 2013). The membranes were washed and protein expression was then detected by enhanced chemiluminescence according to the manufacturer’s instructions (cat number: RPN2135, Amersham, UK). The autoradiographic film was exposed to the membranes and developed. Molecular weight standards were used to identify appropriate antibody binding. Band densities were determined using the Image J densitometry software. Rabbit anti-MCT1 (LS-C2815, LSBio) and rabbit anti-MCT4 (LS-C135144, LSBio), rabbit anti-CD147 (orb127767, Biorbyt), and goat anti-rabbit IgG antibody were the primary antibodies used; further, peroxidase conjugated antibodies (AP132P, Millipore) were used as the secondary antibody. Mouse erythrocyte ghost was used as a positive control and to fix an arbitrary unit to enable a comparison between the experiments (1 equals the MCT1 signal generated by 5 μg of the mouse erythrocyte ghost).
Mouse erythrocyte ghost preparation
Fresh blood from a mouse was mixed with 7 volumes of acid citrate–dextrose buffer (75 mm sodium citrate, 38 mm citric acid, and 138 mm d-glucose) and centrifuged at 16,000 g. The supernatant and buffy coats were removed and the pellet washed 3× in 66 mm NaCl. The sedimented cell pellet was diluted in 66 mm NaCl to give a 25% cell suspension. The cell suspension was mixed with the haemolysed buffer (1 mm EDTA, 9.64 mm NaCl, 3.61 mm Na2HPO4 and 1.2 mm KH2PO4; pH 7.2) in 1:7 volume ratio and placed on ice for 20 min. The solution was centrifuged at 20,000 g for 15 min at 4°C, and the pellet diluted with ten times the volume of the buffer (containing 9.6 mm Tris-HCl, 20 mm NaCl; pH 7.2) and washed once in this buffer and again in a buffer containing 4.8 mm Tris-HCl and 10 mm NaCl. The pellet was washed once in 100 mm KCl and twice in water and diluted in the CO2-free water (Schwoch & Pasoow, 1984).
Measurement of tumour lactate concentration
The tumour lactate concentration was determined using a lactate assay kit (cat. No. K607-100, Biovision) as follows. Approximately 50 mg of the solid tumour was powdered and incubated for 10 min in 8 vol. of ice-cold 6% perchloric acid and centrifuged at 1500 g for 10 min at 4°C (Gutmann & Wahlefeld, 1974). The supernatant was removed and the lactate concentration was then measured according to the manufacturer’s instructions.
LDH separation and analysis by electrophoresis
The LDH isozymes present in the tumour homogenates were electrophoretically separated on agarose gels (1%) using a Bio-Rad SubCell system. Samples containing 15 μg of total protein and LDH marker (K770049, LDH Isotrol and Sigma) were separated by electrophoresis at 90 V for 30 min. The LDH bands were stained and visualized utilizing the LDH isoenzymes electrophoresis kit (SRE612K, Interlab) according to the manufacturer’s directions. The gels were fixed in 5% acetic acid. The different bands were scanned and quantified using the Image J software. For densitometric analysis, only those samples in which all five LDH isoform bands were distinctly visible were selected. The LDH isozyme distribution was calculated by dividing the area × mean optical density product for each isozyme by the sum of the area × mean optical density of the five isozymes. The results are expressed as a percentage of all the LDH isozymes.
Statistical analysis
Data are expressed as the means ± SD. After the normality of distribution was checked with the Shapiro-Wilk test, values between and within the groups were tested using one-way ANOVA. Tukey’s post hoc tests were used to determine where significant differences occurred. In all comparisons, the significance level was set at α = 0.05.
Results
Endurance training decreased tumour volume and weight
No significant differences in body weight gain were found in any of the groups throughout the study period (C 21.5 ± 1.6 g; T 21 ± 0.7 g; CX 22.2 ± 2. g; TX 21.1 ± 1.5). The tumour weight and volume, cardiac mass index, blood and tumour lactate concentration values in the four groups are shown in Table1. Significant differences in heart weights were found between groups C and T (P < 0.05), and CX and TX (P < 0.05) at the end of the study. Seven weeks of endurance training significantly reduced the tumour weight in the trained animals when compared with the control (P < 0.05). Endurance training significantly increased COX expression in the tumour in the T and TX groups in comparison to the C and CX mice, respectively (P < 0.01; Fig.5B).
Table 1.
Tumour weight and volume, cardiac mass index, and resting tumour and blood lactate concentrations at the end of the study
| Groups | ||||
|---|---|---|---|---|
| Variable | Control | Trained | Control + XCT790 | Trained + XCT790 |
| Heart weight/body weight (mg g–1) | 5.8 ± 0.7 | 6.5 ± 0.3a | 5.5 ± 0.4 | 6.2 ± 0.7b |
| Tumour weight/body weight (mg g–1) | 31.8 ± 1.3 | 28.5 ± 2.7a | 28 ± 2.5a | 26.3 ± 2.2 |
| Tumour volume | 7 ± 0.6 | 6.2 ± 0.6a | 6 ± 0.7a | 5.7 ± 0.54b |
| Tumour lactate (μmol g–1) | 11.2 ± 1.3 | 9.7 ± 1a | 9.4 ± 0.8a | 8.9 ± 0.8 |
| Blood lactate (mmol l–1) | 4.1 ± 0.5 | 3.4 ± 0.4a | 4.3 ± 0.4 | 3.5 ± 0.3b |
Heart and tumour weights were normalized to body weight; tumour volume was normalized to tumour volume at 14 days after injection of the MC4-L2 cells. Control n = 9, Trained n = 10, Control + XCT790 n = 10, Trained + XCT790 n = 10.
Significant difference from control (P < 0.05)
significant difference from control + XCT790 (P < 0.05).
Figure 5.
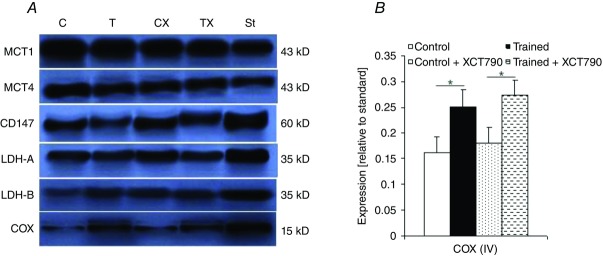
MCTs and cytochrome c oxidase expression in solid tumours
A, expression of the monocarboxylate transporters (MCTs), lactate dehydrogenase (LDH) isoforms and CD147 in the solid tumour of one mouse from each group detected by immunoblotting. The expression of MCT1 and MCT4 was normalized to an MCT1 signal generated by 5 μg of mouse erythrocyte ghost expression. CD147, LDH-A AND LDH-B expression were normalized to the expression of β-actin in solid tumours. B, cytochrome c oxidase (COX) subunit IV expression (normalized to the expression of β-actin) in solid tumours at the end of the study; Control n = 9, Trained n = 9, Control + XCT790 n = 9, Trained + XCT790 n = 9. Values are the means ± SD. *Significant difference between the groups (P < 0.01).
At the start of the study, there were no significant differences in tumour volume among the four animal groups (C 209.1 ± 12.5 mm3, T 215.1 ± 15.9 mm3, CX 188.4 ± 14.1 mm3, TX 182.9 ± 15.5 mm3). Thereafter, the mice in the control and trained groups gained ∼180 and 159 mm3 week–1, respectively, over the following 7 weeks (Fig.1). The difference between the two groups became significant at the end of the sixth week (P < 0.05; Fig.1).
Figure 1.
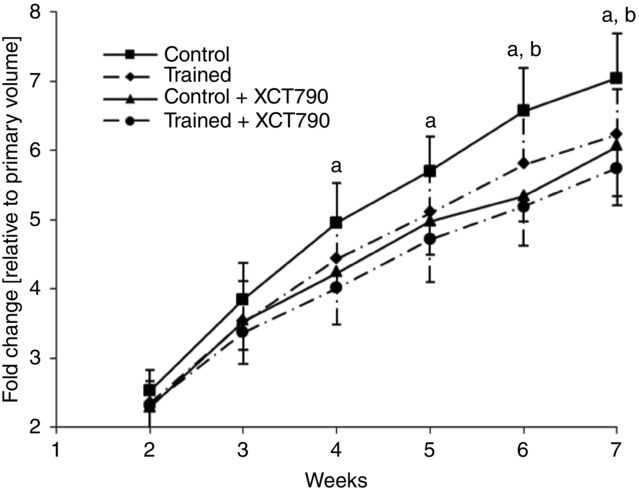
Changes over the course of time in tumour volume
Control n = 9, Trained n = 10, Control + XCT790 n = 10, Trained + XCT790 n = 10. All measurements were normalized to the initial measurement of the tumour volume in each group, which was measured 14 days after the subcutaneous injection of the MC4-L2 cells. Baseline values of tumour volume are: Control 209.1 ± 12.5, Trained 215.1 ± 15.9, Control + XCT790 188.4 ± 14.1, Trained + XCT790 182.9 ± 15.5. Values are the means ± SD. aSignificant difference between the Control and Control + XCT790 groups (P < 0.05); bsignificant difference between the Control and Trained groups (P < 0.05).
ERRα inhibition decreased tumour volume and weight
The inhibition of ERRα suppressed tumour growth in the CX group; there was a significant difference for tumour weight between the C and CX groups at the end of the study (P < 0.05). The ERRα inhibition caused no additional effects to those exposed to the exercise training; there was no significant difference for tumour weight between the TX and CX groups (Table1). The animals in the CX and TX groups gained ∼134 and 123 mm3 week–1 in tumour volume. No significant difference was found between the two groups throughout the study. However, significant differences were found regarding tumour volume between the C and CX groups at the end of the fourth week; tumour volume in the CX group remained significantly lower from the fourth week to the seventh week compared with the control (P < 0.05; Fig.1).
LDH isozymes shift to LDH-1 with endurance training
To investigate the exercise-induced change on tumour metabolic phenotype, we first measured the tumour lactate concentration. Endurance training significantly decreased the tumour lactate concentration in the T group compared with that of the C group (P< 0.05; Table1). Next, to determine whether the change in tumour lactate production was associated with LDH isozyme changes, the tumour LDH isozymes were analysed. Interestingly, the metabolically altered tumours in group T contained mostly LDH-1 and LDH-2, whereas the tumours of the group C animals exhibited a higher expression of the LDH-5 and LDH-4 isozymes (Fig.2C). To elucidate the underlying mechanism of this observation, we then examined the expression levels of LDH-A and LDH-B. A significant decrease in tumour LDH-A expression was observed in the T group compared with that of the C group (P < 0.05; Fig.3B). Concomitant increases in tumour LDH-B expression were also found in the T group compared with the C mice (P < 0.05; Fig.3B).
Figure 2.
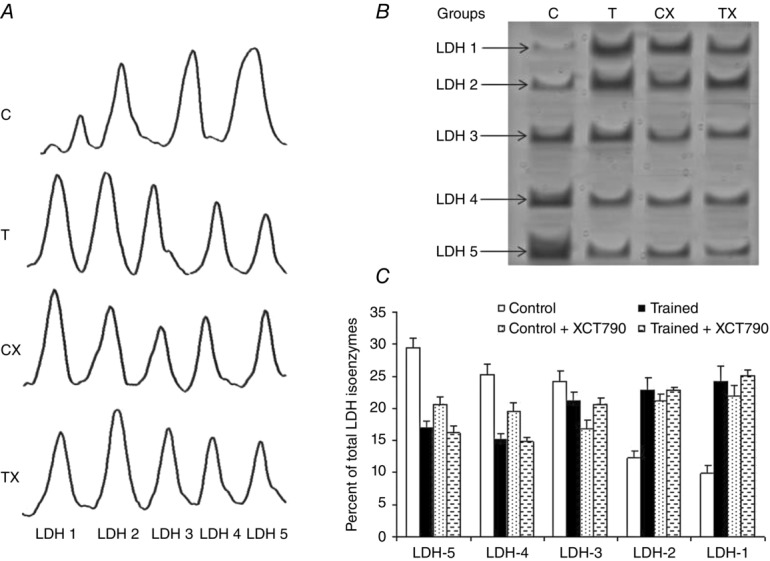
LDH isoenzymes expression in solid tumor
A, densitometryy analysis of LDH subunit expressions revealed the exercise-induced suppression of LDH-A in both the Trained (T) and Trained + XCT790 (TX) groups. B, in-gel profile of the LDH isozymes was performed as described in Methods; this is a typical example of a mouse tumour in which the shift towards aerobic metabolism was associated with the LDH isozyme shift via repression of LDH-A. C, distribution of the LDH isozymes in the solid tumours of the groups of the study. Values are the means ± SD; Control n = 7, Trained n = 7, Control + XCT790 n = 6, Trained + XCT790 n = 6.
Figure 3.
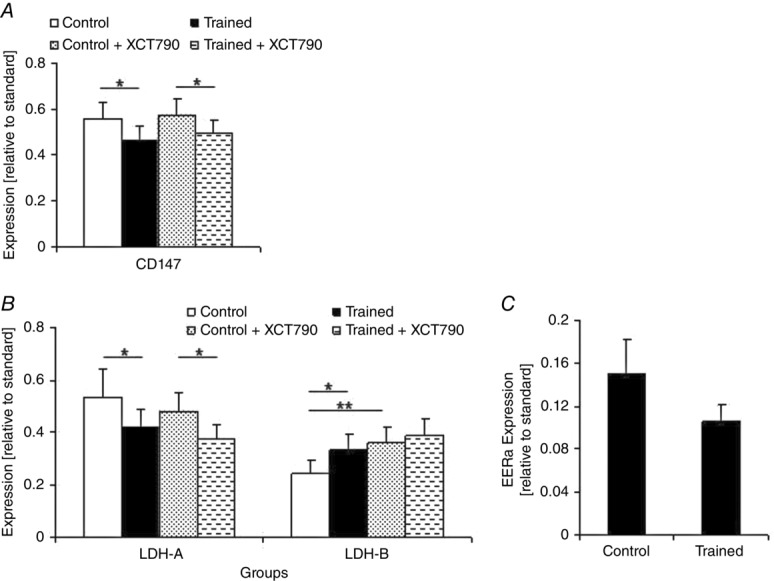
LDH-A, LDH-B, CD147 and ERRα expression in the tumour at the end of the study
A, CD147; B, LDH-A and LDH-B; C, ERRα. In both the Control + XCT790 and Trained + XCT790 groups the ERRα was inhibited by incubation of the MC4-L2 cells in XCT790 solution before the injection. Refer to Methods for details. Values are the means ± SD; Control n = 9, Trained n = 9, Control + XCT790 n = 9, Trained + XCT790 n = 9. Asterisks show significant difference between the groups: *P < 0.05, **P < 0.01.
Endurance training reduced tumour MCT1 and CD147 expression
Next, analysis was done to determine exercise-induced changes in the MCTs and their chaperone, MCT1, MCT4 and CD147 expression in the tumour. After 7 weeks of endurance training, the expression of MCT1 (P < 0.01; Fig.4) and CD147 (P < 0.05) in the tumour were significantly lower in the T group compared with the C group (Fig.3A). There was no significant difference regarding the tumour MCT4 expression among the groups at the end of the study period (Fig.4).
Figure 4.
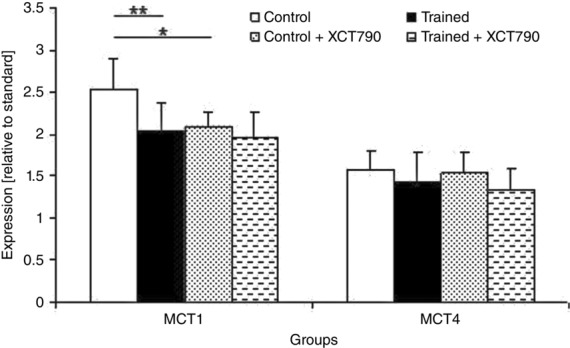
MCT1 and MCT2 expression of the tumour at the end of the study
MCT1 and MCT2 expression shown relative to the standard (MCT1 signal generated by 5 μg of rat erythrocyte ghost). In both Control + XCT790 and Trained + XCT790 groups ERRα was inhibited by the incubation of the MC4-L2 cells in XCT790 solution before the injection. Refer to Methods for details. Values are the means ± SD; Control n = 9, Trained n = 9, Control + XCT790 n = 9, Trained + XCT790 n = 9. Asterisks show significant difference between the groups: *P < 0.05, **P < 0.01.
ERRα inhibition suppressed tumour MCT1 expression and increased LDH-B expression
Next, by inhibiting ERRα by treating the MC4-L2 cells with XCT790 before injection into the TX animals, we elucidated the role played by ERRα in tumour expression of LDH and the MCT1 expression and LDH isozymes. As a result of ERRα inhibition, the solid tumours in the CX group contained all five LDH isozymes with a slight trend towards LDH-1. This observation was associated with a significant increase in the expression of LDH-B in the tumours of the CX mice compared with the C group (P < 0.01, Fig.3B). LDH-A expression in the tumour was not different between the two groups. The treatment of the MC4-L2 cells with XCT790 before the injection significantly decreased MCT1 expression in the tumour of the CX mice compared with the C group (P < 0.05) (Fig.4). However, it had no effect on MCT4 and CD147 expression in the tumour, and there was no significant difference regarding these variables between the C and CX groups at the end of the study (Fig.4). Taken together, these results suggest that ERRα may play a critical role in the metabolic shift, including the enhanced expression of MCT1 and LDH-B suppression, during tumour development.
ERRα inhibition effects were not additive to the training effects on MCT1 and LDH-B expression in the tumour
We next checked to see whether the exercise-induced alterations in the LDH and MCT isoforms were related to the changes in ERRα expression. We compared the TX and CX groups to clarify the effects of ERRα in the exercise-induced alterations on the expression of the isoforms of LDH and MCTs. ERRα inhibition effects were not additive to the training effects on MCT1 and LDH-B expression in the solid tumours. No significant difference in tumour MCT1 and LDH-B expression was found between the CX and TX groups (Fig.3B). Exercise-induced alterations in LDH-A and CD147 expression in the tumour were independent of ERRα; significant differences were observed for LDH-A (P < 0.05) and CD147 expression (P < 0.05; Fig.3A and B) between tumours in the XT and CX groups. Considering the significant decrease in ERRα expression in tumours in the T group compared with those of the C mice (P < 0.01; Fig.3C), these results suggest that exercise-induced alterations in MCT1 and LDH-B expression are modulated by ERRα.
Discussion
This study examined the effects of endurance training on the monocarboxylate transporters (MCT1 and MCT4) and lactate dehydrogenase isoform (LDH-A and LDH-B) expression in breast cancer tumour-bearing BALB/c mice. Our results generated three novel conclusions that are discussed in more detail below: (1) endurance training markedly decreased tumour expression of MCT1, CD147 and LDH-A and increased tumour LDH-B expression in breast cancer-bearing BALB/c mice; (2) ERRα plays an important role in MCT1 and LDH-B expression in tumours of breast cancer-bearing BALB/c mice; (3) exercise-induced changes in tumour MCT1 and LDH-B expression appear to be mediated by ERRα in breast cancer-bearing BALB/c mice.
The MC4-L2 mouse mammary carcinoma line was chosen for this study. This cell line is derived from murine mammary ductal carcinomas (Lanari et al. 2001). This cell line has a positive status for ERα and the ability to rapidly grow in vitro, making it a suitable model for breast cancer. These multinucleated cells, when injected into syngeneic mice, develop tumours disclosing a biphasic growth pattern, with sarcomatoid areas showing no morphological signs of epithelial differentiation and well-vascularized areas that show a definite carcinomatous growth pattern (Lanari et al. 2001). Compared with sedentary animals, the heart weight/body weight ratio and COX expression in the tumour were significantly higher in the T and TX groups at the end of the study, providing evidence for an effect of the training. Endurance training markedly reduced the weight and volume of the solid tumour in the T group. These results concur with those of prior studies (Almeida et al. 2009; Murphy et al. 2011; Goh et al. 2013; Steiner et al. 2013). However, some studies failed to demonstrate any beneficial effects of physical training on tumour development and progression (Jones et al. 2010, Steiner et al. 2013). The reasons for the contrasting findings are not clear, but are probably explained by the differences in breast cancer models, the species evaluated, and, more likely, may be dependent on the exercise protocol (forced vs. voluntary exercise). In the present investigation, the treadmill running protocol forced animals to maintain a constant running volume and intensity throughout the study. By contrast, voluntary exercise reveals each individual animal’s voluntary capacity for running, and it is possible that animals decline in their running ability at a critical point of time when exercise could maximize the benefits of physical activity on tumour growth. The mechanisms for the beneficial effect of exercise on breast cancer progression are potentially very complex and multifaceted (Eickmeyer et al. 2012). Our results showing an exercise-induced reduction in MCT1 and lactate accumulation in tumour tissue suggest another molecular mechanism. MCT1 and lactate together play several roles in tumour progression. In tumours, the lactate produced by the poorly-oxygenated regions is taken up by the well-oxygenated regions where it is used as their principal substrate for oxidative phosphorylation (Semenza, 2008; Sonveaux et al. 2008; Feron, 2009). There is evidence that the lactate uptake and utilization by the oxygenated (tumour) cells increases the availability of glucose for the hypoxic (tumour) cells and enables them to survive and proliferate (Sonveaux et al. 2008; Feron, 2009). In this process, MCT1, as the key monocarboxylate transporter responsible for lactate uptake by oxygenated (tumour) cells, plays an important role. Moreover, MCT1 plays a critical role in tumours with elevated glycolysis by not only preventing intracellular acidification by exporting lactic acid, but also by sustaining growth by importing the lactate (Semenza, 2008; Sonveaux et al. 2008). Support for this theory comes from the observation that inhibiting MCT1 with α-cyano-4-hydroxycinnamate (CHC) selectively kills the hypoxic tumour cells in mice (Sonveaux et al. 2008). Therefore, it could be speculated that exercise-induced reductions in MCT1 expression in tumours may result in the disruption of the metabolic symbiosis between the oxygenated and hypoxic (tumour) cells. Disruption of this metabolic symbiosis can force the oxygenated (tumour) cells to take up the glucose rather than the lactate, causing glucose depletion and the ultimately the death of the hypoxic (tumour) cells. This decrease in tumour MCT1 expression after endurance training can be partially explained by the exercise-induced reduction in CD147 expression in the tumour. CD147 is an ancillary protein required for the expression of MCTs and interaction between CD147 and MCT1 or MCT4 may be required for their translocation and correct localization to the plasma membrane (Gallagher et al. 2007; Schneiderhan et al. 2009; Le Floch et al. 2011). The significant correlation found in the present study between CD147 and MCT1 expression in the tumour also confirms this hypothesis (authors’unreported data).
The data collected in this study are the first to describe the extensive changes occurring in LDH isoform expression in tumours in breast cancer-bearing animals exposed to endurance training. The elevated LDH levels are the hallmark of many tumours, the majority of which are highly glycolytic, which plays a crucial part in tumour progression. In one study (Le et al. 2010), the inhibition of LDH reduced ATP levels and induced significant oxidative stress and cell death and inhibited tumour progression in pancreatic cancer. Similar results have also been reported in breast cancer and breast cancer tumorigenicity has been suppressed by LDH silencing (Wang et al. 2012). The results of these studies indicate that the up-regulation of LDH by cancer cells may facilitate glycolytic metabolism and reduce tumour dependence on the presence of oxygen. Our results demonstrated that an exercise-induced shift towards increased LDH-1 and 2 was concurrent with a decrease in LDH-A expression in the tumours; therefore, some of the LDH isozyme shift observed can be explained by the decreased LDH-A expression. However, it is clear from our results that LDH-A down-regulation does not fully explain the shift towards LDH-1 in the tumours of those animals that underwent endurance training. The changes observed in tumour LDH-B expression suggest that the enhanced expression of LDH-B can also induce the observed LDH isozyme shift. Previous studies demonstrated an exercise-induced increase in LDH-B expression in human skeletal muscle (Dubouchaud et al. 2000; Hittel et al. 2005); however, there is no evidence regarding the effect of endurance training on LDH-B expression in solid tumours. The increased expression of LDH-B in tumours after endurance training found in the current study is reported for the first time, and may have a physiologically important role in tumour invasion. Down-regulation of LDH-B has a greater effect on lactate production than the induction of LDH-A (Gatenby & Gillies, 2004; Kim et al. 2011). LDH-B suppression can initiate and maintain a metabolic shift towards aerobic glycolysis, not only by activating glycolysis, but also by reducing mitochondrial respiration, and can potentiate cancer cell invasion (Kim et al. 2011). Therefore, it is possible that the major causative factor for tumour development is a decrease in mitochondrial respiration. However, it appears that the reduction in tumour LDH-A and the concomitant increase in LDH-B expression induced by endurance exercise may provide other mechanisms for a beneficial effect of the exercise in case of breast cancer. The LDH isozyme shifts observed in the current study were concomitant with decreased tumour lactate concentrations. Therefore, it could be speculated that these effective LDH isozyme shifts may attenuate lactate production and subsequent extracellular lactate release, thereby preventing the acidification-facilitated tumour invasion. However, this interpretation should be made with caution, because the tumour lactate concentrations may be affected by lactate utilization, anaerobic production of lactate or both. For instance, the mitochondria play an important role in lactate oxidation and its altered activity in the cancer cells may be crucial in tumour development. Activities of the respiratory chain complexes, cellular oxygen consumption, and ATP synthesis rates are much lower in breast cancer cells (Kim et al. 2011; Wang et al. 2012). Endurance training appears not only to increase the number of mitochondria, but also to improve the function of the mitochondrial pool in tissues including cardiac and skeletal muscle. Therefore, there is a possibility that endurance training induces changes in mitochondrial volume/activity in tumour cells. Unfortunately, mitochondrial volume/activity in the solid tumour was not measured in the current study and it is possible that some of the changes observed in tumour lactate concentration could be attributed to the exercise-induced improvement in mitochondrial function. The mechanisms by which endurance exercise modulated the LDH isozyme shift and consequent tumour growth were not investigated here. However, a recent genetic study suggested that the loss of expression of the LDH-B subunit may occur in human breast cancer, with an underlying mechanism probably involving the methylation of the LDH-B promoter (Leiblich et al. 2013). It is clear that physical activity is usually associated with higher levels of global genomic DNA methylation and, thus, it could restore, at least to some extent, the hypomethylated genome in cancer (Ntanasis-Stathopoulos et al. 2013). However, aberrant DNA methylation may result in silencing some tumour suppressor genes such as CACNA2D3 and L3MBTL1 (Ntanasis-Stathopoulos et al. 2013). Previous studies have revealed that endurance training may decrease the methylation status of these particular genes and can have a positive effect against tumorigenesis (Yuasa et al. 2009; Zeng et al. 2012). A similar mechanism could also explain the exercise-induced increase observed in LDH-B expression; however, this hypothesis has not yet been investigated.
ERRα is known to co-regulate and coordinate the gene encoding enzymes of the biochemical pathways involved in not only mitochondrial function, but also in glycolysis (Cai et al. 2013; Mirebeau-Prunier et al. 2013). In breast cancer, the activation of aerobic glycolysis by the ERRα–PGC1β complex plays an important role in sustaining energy needs during tumorgenesis (Eichner et al. 2010). We have shown for the first time that ERRα promotes aerobic glycolysis in tumours in breast cancer-bearing BALB/c mice, perhaps partly by suppressing LDH-B and up-regulating MCT1 expression. In fact, in our study, the solid tumours of the C group had a higher LDH-A/LDH-B ratio than those of the CX group. As their solid tumours expressed more ERRα, we hypothesized that this aerobic glycolysis could have been orchestrated by ERRα. Our study of ERRα inhibition in the CX group provided additional support for the role of ERRα in the metabolic switch towards anaerobic glycolysis; these mice had a lower LDHA/LDHB ratio than the C group mice. Hypothesizing that the effects of exercise on the tumour metabolic phenotype are mediated by ERRα, we investigated the effect of ERRα inhibition on the exercise-induced changes to the isoform expression of MCTs and LDH. The fact that the effects of the exercise training on MCT1 and LDH-B expression were not enhanced by ERRα inhibition suggests that their regulation by endurance exercise is mediated by ERRα. Recruitment of ERRα to the LDH-B promoter has been reported earlier in mouse liver cells (Charest-Marcotte et al. 2010), mouse skeletal muscle (Summermatter et al. 2013), and human thyroid tumours (Mirebeau-Prunier et al. 2013). The three regions identified for the LDH-B promoter have been introduced as the potential ERRα binding sites (Mirebeau-Prunier et al. 2013). LDH-B suppression is the functional consequence of ERRα binding to the LDH-B promoter ((Mirebeau-Prunier et al. 2013). Therefore, our results related to tumour ERRα expression suggest that endurance training can increase LDH-B expression by suppressing ERRα expression in the tumour, but the exact mechanisms through which ERRα regulates MCT1 expression were not investigated here. However, earlier studies have shown that the ERRα effects on metabolic changes are usually mediated by the PGC-1 family and the PGC-1-related co-activator (PRC) (Giguere, 2008; Mirebeau-Prunier et al. 2013). PGC-1α is a major regulator of exercise-induced phenotype adaptation (Lira et al. 2010) and mediates exercise-induced changes in MCT1 regulation with no effect on MCT4 expression (Benton et al. 2008). Given that ERRα inhibition reduced the effects of exercise on MCT1 expression and had no effect on MCT4 expression, it can be postulated that the PGC-1α-induced regulation of MCT1 expression is mediated by ERRα. Unfortunately, we did not measure the tumour PGC-1α expression and thus have no evidence to confirm this hypothesis. Further research is warranted to clarify this hypothesis. Exactly which exercise signals (myokines) might have triggered the tumour changes observed is also not very clear. Prior studies have demonstrated that interleukin-6 (IL-6) promotes breast cancer cell growth. ERα-negative breast cancer cell lines produce autocrine signalling (IL-6) whereas the ERα-positive breast cancer cell lines do not. Therefore, ERα-positive breast cancer cells could only utilize paracrine IL-6 signalling. Reduction in systemic IL-6 has been reported after endurance training and could be considered as a potential modulator.
In conclusion, the results of this study illustrated that endurance training initiated a shift in the tumour LDH isozyme profile towards LDH-1 along with a reduction in LDH-A expression and an increase in LDH-B expression. There was a concomitant decrease in the tumour lactate concentration. Other possibilities such as changes in the tumour mitochondrial volume/activity were not addressed in the current study. ERRα is important in the induction of a metabolic shift in solid tumours of BALB/c mice, and exercise-induced suppression in ERRα expression modulates the alterations in the solid tumour expression of LDH-B and MCT1 and contributes towards the prevention of tumour development. Breast cancer is a heterogeneous disease in terms of therapeutic response and patient outcomes. The results of the current study provide evidence for the therapeutic benefits of endurance training in breast cancer. Therefore, regular endurance training could be a useful tool in cancer treatment or prevention, especially in basal-like and luminal-like breast carcinomas.
Acknowledgments
We thank Professor Vahid Sheibani and Dr Saeed Esmaeili-Mahani for their contribution of technical assistance with some of the experiments. We also gratefully acknowledge the support of all our collaborators.
Glossary
- CD147
cluster of differentiation 147
- CHC
α-cyano-4-hydroxycinnamate
- ER
oestrogen receptor
- ERRα
oestrogen-related receptor alpha
- FBS
fetal bovine serum
- HER2
epidermal growth factor receptor 2
- LDH
lactate dehydrogenase
- IL-6
interleukin 6
- MCT1
monocarboxylate transporter 1
- MCT4
monocarboxylate transporter 4
- XCT790
(2E)-3-(4-{[2,4-bis(trifluoromethyl)benzyl] oxy}-3-methoxyphenyl)-2-cyano-N-[5-(trifluoromethyl)-1,3,4-thiadiazol-2-yl] acrylamide
- PGC-1α
proliferator-activated receptor γ co-activator-1α
- PRC
PGC-1-related coactivator
Additional information
Competing interests
The authors declare no competing interests.
Author contributions
M.A. performed the experiments and wrote the manuscript; R.N. designed the experiments and revised the manuscript; A.M. analysed the data. All authors read and approved the final version of the manuscript.
References
- Almeida PW, Gomes-Filho A, Ferreira AJ, Rodrigues CE, Dias-Peixoto MF, Russo RC, Teixeira MM, Cassali GD, Ferreira E, Santos IC, Garcia AM, et al. Swim training suppresses tumor growth in mice. J Appl Physiol. 2009;107:261–265. doi: 10.1152/japplphysiol.00249.2009. [DOI] [PubMed] [Google Scholar]
- Aveseh M, Nikooie R, Sheibani V, Esmaeili-Mahani S. Endurance training increases brain lactate uptake during hypoglycemia by upregulation of brain lactate transporters. Mol Cell Endocrinol. 2014;394:29–36. doi: 10.1016/j.mce.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Benton CR, Yoshida Y, Lally J, Han XX, Hatta H. Bonen A. PGC-1alpha increases skeletal muscle lactate uptake by increasing the expression of MCT1 but not MCT2 or MCT4. Physiol Genomics. 2008;35:45–54. doi: 10.1152/physiolgenomics.90217.2008. [DOI] [PubMed] [Google Scholar]
- Busch BB, Stevens WC, Jr, Martin R, Ordentlich P, Zhou S, Sapp DW, Horlick RA. Mohan R. Identification of a selective inverse agonist for the orphan nuclear receptor estrogen-related receptor alpha. J Med Chem. 2004;47:5593–5596. doi: 10.1021/jm049334f. [DOI] [PubMed] [Google Scholar]
- Cai Q, Lin T, Kamarajugadda S. Lu J. Regulation of glycolysis and the Warburg effect by estrogen-related receptors. Oncogene. 2013;32:2079–2086. doi: 10.1038/onc.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Harris IS. Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Cardone RA, Casavola V. Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5:786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- Charest-Marcotte A, Dufour CR, Wilson BJ, Tremblay AM, Eichner LJ, Arlow DH, Mootha VK. Giguère V. The homeobox protein Prox1 is a negative modulator of ERRα/PGC-1α bioenergetic functions. Genes Dev. 2010;24:537–542. doi: 10.1101/gad.1871610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries MC, Hamadeh MJ, Glover AW, Raha S, Samjoo IA. Tarnopolsky MA. Endurance training without weight loss lowers systemic, but not muscle, oxidative stress with no effect on inflammation in lean and obese women. Free Radic Biol Med. 2008;45:503–511. doi: 10.1016/j.freeradbiomed.2008.04.039. [DOI] [PubMed] [Google Scholar]
- Draoui N. Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4:727–732. doi: 10.1242/dmm.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC. Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Eichner LJ, Perry MC, Dufour CR, Bertos N, Park M, St-Pierre J. Giguère V. miR-378 mediates metabolic shift in breast cancer cells via the PGC-1beta/ERRgamma transcriptional pathway. Cell Metab. 2010;12:352–361. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Eickmeyer SM, Gamble GL, Shahpar S. Do KD. The role and efficacy of exercise in persons with cancer. PM R. 2012;4:874–81. doi: 10.1016/j.pmrj.2012.09.588. [DOI] [PubMed] [Google Scholar]
- Elias AP. Dias S. Microenvironment changes (in pH) affect VEGF alternative splicing. Cancer Microenviron. 2008;1:131–139. doi: 10.1007/s12307-008-0013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feron O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother Oncol. 2009;92:329–333. doi: 10.1016/j.radonc.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Castorino JJ, Wang D. Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007;67:4182–4189. doi: 10.1158/0008-5472.CAN-06-3184. [DOI] [PubMed] [Google Scholar]
- Gatenby RA, Gawlinski ET, Gmitro AF, Kaylor B. Gillies RJ. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 2006;66:5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- Gatenby RA. Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Giguere V. Transcriptional control of energy homeostasis by the estrogen related receptors. Endocr Rev. 2008;29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- Goh J, Tsai J, Bammler TK, Farin FM, Endicott E. Ladiges WC. Exercise training in transgenic mice is associated with attenuation of early breast cancer growth in a dose-dependent manner. PLoS One. 2013;11:e80123. doi: 10.1371/journal.pone.0080123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granchi C, Bertini S, Macchia M. Minutolo F. Inhibitors of lactate dehydrogenase isoforms and their therapeutic potentials. Curr Med Chem. 2010;17:672–697. doi: 10.2174/092986710790416263. [DOI] [PubMed] [Google Scholar]
- Gutmann L. Wahlefeld AW. L-lactate determination with lactate dehydrogenase and NAD. Meth Enzym Anal. 1974:1464–1472. [Google Scholar]
- Halestrap AP. The monocarboxylate transporter family – structure and functional characterization. IUBMB Life. 2012;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- Hittel DS, Kraus WE, Tanner CJ, Houmard JA. Hoffman EP. Exercise training increases electron and substrate shuttling proteins in muscle of overweight men and women with the metabolic syndrome. J Appl Physiol. 2005;98:168–179. doi: 10.1152/japplphysiol.00331.2004. [DOI] [PubMed] [Google Scholar]
- Huss JM, Torra IP, Staels B, Giguère V. Kelly DP. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussien R. Brooks GA. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics. 2010;43:255–264. doi: 10.1152/physiolgenomics.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, Potter MQ, Moon EJ, Schroeder T, Herndon JE. Dewhirst MW. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol. 2010;108:343–348. doi: 10.1152/japplphysiol.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM. Dewhirst MW. Tumor metabolism of lactate: the influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010;6:127–48. doi: 10.2217/fon.09.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH1, Kim EL, Lee YK, Park CB, Kim BW, Wang HJ, Yoon CH, Lee SJ. Yoon G. Decreased lactate dehydrogenase B expression enhances claudin 1-mediated hepatoma cell invasiveness via mitochondrial defects. Exp Cell Res. 2011;317:1108–1118. doi: 10.1016/j.yexcr.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Kossman DA, Williams NI, Domchek SM, Kurzer MS, Stopfer JE. Schmitz KH. Exercise lowers estrogen and progesterone levels in premenopausal women at high risk of breast cancer. J Appl Physiol. 2011;111:1687–1693. doi: 10.1152/japplphysiol.00319.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanari C, Lüthy I, Lamb CA, Fabris V, Pagano E, Helguero LA, Sanjuan N, Merani S. Molinolo AA. Five novel hormone-responsive cell lines derived from murine mammary ductal carcinomas: in vivo and in vitro effects of estrogens and progestins. Cancer Res. 2001;61:293–302. [PubMed] [Google Scholar]
- Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL. Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Floch R, Chiche J, Marchiq I, Naiken T, Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP. Pouysségur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci USA. 2011;108:16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiblich A, Cross SS, Catto JW, Phillips JT, Leung HY, Hamdy FC. Rehman I. Lactate dehydrogenase-B is silenced by promoter hypermethylation in human prostate cancer. Oncogene. 2006;25:2953–2960. doi: 10.1038/sj.onc.1209262. [DOI] [PubMed] [Google Scholar]
- Leiblich A, Cross SS, Catto JWF, Phillips JT, Leung HY, Hamdy FC. Rehman I. Lactate dehydrogenase-B is silenced by promoter methylation in a high frequency of human breast cancers. PLoS One. 2013;2:e57697. doi: 10.1371/journal.pone.0057697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira VA, Benton CR, Yan Z. Bonen A. PGC-1α regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299:E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markert CL, Shaklee JB. Whitt GS. Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science. 1975;189:102–114. doi: 10.1126/science.1138367. [DOI] [PubMed] [Google Scholar]
- Mirebeau-Prunier D, Le Pennec S, Jacques C, Fontaine JF, Gueguen N, Boutet-Bouzamondo N, Donnart A, Malthièry Y. Savagner F. Estrogen-related receptor alpha modulates lactate dehydrogenase activity in thyroid tumors. PLoS One. 2013;8:e58683. doi: 10.1371/journal.pone.0058683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A. Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- Muñoz-Pinedo C, Mjiyad NEl. Ricci J-E. Cancer metabolism: current perspectives and future directions. Cell Death Dis. 2012;3:e248. doi: 10.1038/cddis.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EA, Davis JM, Barrilleaux TL, McClellan JL, Steiner JL, Carmichael MD, Pena MM, Hebert JR. Green JE. Benefits of exercise training on breast cancer progression and inflammation in C3(1)SV40Tag mice. Cytokine. 2011;55:274–279. doi: 10.1016/j.cyto.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikooie R, Rajabi H, Gharakhanlu R, Atabi F, Omidfar K, Aveseh M. Larijani B. Exercise-induced changes of MCT1 in cardiac and skeletal muscles of diabetic rats induced by high-fat diet and STZ. J Physiol Biochem. 2013;69:865–877. doi: 10.1007/s13105-013-0263-6. [DOI] [PubMed] [Google Scholar]
- Ntanasis-Stathopoulos J, Tzanninis JG, Philippou A. Koutsilieris M. Epigenetic regulation on gene expression induced by physical exercise. J Musculoskelet Neuronal Interact. 2013;2:133–146. [PubMed] [Google Scholar]
- Pierce BL, Neuhouser ML, Wener MH, Bernstein L, Baumgartner RN, Ballard-Barbash R, Gilliland FD, Baumgartner KB, Sorensen B, McTiernan A. Ulrich CM. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Res Treat. 2009;114:155–67. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C, Albergaria A, Paredes J, Sousa B, Dufloth R, Vieira D, Schmitt F. Baltazar F. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology. 2010;56:860–867. doi: 10.1111/j.1365-2559.2010.03560.x. [DOI] [PubMed] [Google Scholar]
- Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, Schmitt F. Baltazar F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumors and their association with CD147 and CD44. J Biomed Biotechnol. 2010;2010:427694. doi: 10.1155/2010/427694. ; doi: 10.1155/2010/427694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C, Sousa B, Albergaria A, Paredes J, Dufloth R, Vieira D, Schmitt F. Baltazar F. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol. 2011;26:1279–1286. doi: 10.14670/HH-26.1279. [DOI] [PubMed] [Google Scholar]
- Schmitz KH, Warren M, Rundle AG, Williams NI, Gross MD. Kurzer MS. Exercise effect on oxidative stress is independent of change in estrogen metabolism. Cancer Epidemiol Biomarkers Prev. 2008;17:220–223. doi: 10.1158/1055-9965.EPI-07-0058. [DOI] [PubMed] [Google Scholar]
- Schneiderhan W, Scheler M, Holzmann KH, Marx M, Gschwend JE, Bucholz M, Gress TM, Seufferlein T, Adler G. Oswald F. CD147 silencing inhibits lactate transport and reduces malignant potential of pancreatic cancer cells in in vivo and in vitro models. Gut. 2009;58:1391–1398. doi: 10.1136/gut.2009.181412. [DOI] [PubMed] [Google Scholar]
- Schwoch C. Pasoow H. Preparation and properties of human erythrocyte ghosts. Mol Cell Biochem. 1984;2:197–218. doi: 10.1007/BF01795474. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Tumor metabolism: cancer cells give and take lactate. J Clin Invest. 2008;12:3835–3847. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JL, Davis M, McClellan JL, Enos RT. Murphy EA. Effects of voluntary exercise on tumorigenesis in the C3(1)/SV40Tag transgenic mouse model of breast cancer. Int J Oncol. 2013;42:1466–1472. doi: 10.3892/ijo.2013.1827. [DOI] [PubMed] [Google Scholar]
- Summermatter S, Santos G, Pérez-Schindler J. Handschin C. Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci USA. 2013;110:8738–8743. doi: 10.1073/pnas.1212976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich CM, Wiskemann J. Steindorf K. Physiologic and molecular mechanisms linking physical activity to cancer risk and progression. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:3–9. doi: 10.1007/s00103-011-1400-4. [DOI] [PubMed] [Google Scholar]
- Végran F, Boidot R, Michiels C, Sonveaux P. Feron O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011;71:2550–2560. doi: 10.1158/0008-5472.CAN-10-2828. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Loo TY, Shen JG, Wang N, Wang DM, Yang DP, Mo SL, Guan XY. Chen JP. LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress-mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat. 2012;131:791–800. doi: 10.1007/s10549-011-1466-6. [DOI] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Yuasa Y, Nagasaki H, Akiyama Y, Hashimoto Y, Takizawa T, Kojima K, Kawano T, Sugihara K, Imai K. Nakachi K. DNA methylation status is inversely correlated with green tea intake and physical activity in gastric cancer patients. Int J Cancer. 2009;11:2677–2682. doi: 10.1002/ijc.24231. [DOI] [PubMed] [Google Scholar]
- Zeng H, Irwin ML, Lu L, Risch H, Mayne S, Mu L, Deng Q, Scarampi L, Mitidieri M, Katsaros D. Yu H. Physical activity and breast cancer survival: an epigenetic link through reduced methylation of a tumor suppressor gene L3MBTL1. Breast Cancer Res Treat. 2012;133:127–135. doi: 10.1007/s10549-011-1716-7. [DOI] [PubMed] [Google Scholar]


