Abstract
Some studies suggest that the signalling pathway of neuregulin 1 (NRG1), a protein involved in the regulation of skeletal muscle metabolism, could be altered by nutritional and exercise interventions. We hypothesized that diet-induced obesity could lead to alterations of the NRG1 signalling pathway and that chronic exercise could improve NRG1 signalling in rat skeletal muscle. To test this hypothesis, male Wistar rats received a high fat/high sucrose (HF/HS) diet for 16 weeks. At the end of this period, NRG1 and ErbB expression/activity in skeletal muscle was assessed. The obese rats then continued the HF/HS diet or were switched to a well-balanced diet. Moreover, in both groups, half of the animals also performed low intensity treadmill exercise training. After another 8 weeks, NRG1 and ErbB expression/activity in skeletal muscle were tested again. The 16 week HF/HS diet induced obesity, but did not significantly affect the NRG1/ErbB signalling pathway in rat skeletal muscle. Conversely, after the switch to a well-balanced diet, NRG1 cleavage ratio and ErbB4 amount were increased. Chronic exercise training also promoted NRG1 cleavage, resulting in increased ErbB4 phosphorylation. This result was associated with increased protein expression and phosphorylation ratio of the metalloprotease ADAM17, which is involved in NRG1 shedding. Similarly, in vitro stretch-induced activation of ADAM17 in rat myoblasts induced NRG1 cleavage and ErbB4 activation. These results show that low intensity endurance training and well-balanced diet activate the NRG1-ErbB4 pathway, possibly via the metalloprotease ADAM17, in skeletal muscle of diet-induced obese rats.
Key points
Some studies suggest that neuregulin 1 (NRG1) could be involved in the regulation of skeletal muscle energy metabolism in rodents.
Here we assessed whether unbalanced diet is associated with alterations of the NRG1 signalling pathway and whether exercise and diet might restore NRG1 signalling in skeletal muscle of obese rats.
We show that diet-induced obesity does not impair NRG1 signalling in rat skeletal muscle.
We also report that endurance training and a well-balanced diet activate the NRG1 signalling in skeletal muscle of obese rats, possibly via a new mechanism mediated by the protease ADAM17.
These results suggest that some beneficial effects of physical activity and diet in obese rats could be partly explained by stimulation of the NRG1 signalling pathway.
Introduction
Neuregulin 1 (NRG1) is a protein that belongs to the epidermal growth factors family (Meyer et al. 1997). Proteolytic cleavage of membrane-bound NRG1 is mediated by metalloproteases, among which A disintegrin and metalloprotease 17 (ADAM17) is the most studied (Horiuchi et al. 2005). NRG1 cleavage results in the release of its extracellular epidermal growth factor-like domain that can activate the type I tyrosine kinase receptors called erythroblastic leukaemia viral oncogene homologues (ErbBs) (Talmage, 2008). NRG1 is a direct ligand for ErbB3 and ErbB4 (Plowman et al. 1993; Carraway et al. 1994). Homo- or hetero-dimerization of ErbB2, ErbB3 and ErbB4 induced by NRG1 leads to auto- and trans-phosphorylation of ErbB intracellular domains, which mediate downstream signalling events. Although most studies have focused on NRG1’s role in the morphogenesis of cardiac, muscle and nerve cells, it has been suggested that NRG1 could also be involved in the regulation of energy metabolism in adult rodents (Guma et al. 2010). Indeed, in vitro studies using the C2C12 and L6E9 muscle cell lines have shown that NRG1 may modulate oxidative capacity (Canto et al. 2007) and glucose metabolism (Suarez et al. 2001; Canto et al. 2004, 2006). Moreover, in vivo, NRG1 chronic treatment reduces food intake and weight gain in mice (Ennequin et al. 2015). Although NRG1 may be involved in energy metabolism regulation in vivo, little is known about the potential mechanisms that regulate the NRG1/ErbBs signalling pathway.
It has been postulated that nutrition could affect NRG1 signalling. For instance, palmitate treatment impairs NRG1-induced activation of Akt in isolated rat cardiac myocytes, while the mono-unsaturated fatty acid oleate reverses this effect (Miller et al. 2009). In ageing rats, caloric restriction restores cardiac NRG1 signalling through ErbB up-regulation (Rohrbach et al. 2006). Physical activity might also be involved in NRG1 signalling regulation. Indeed, increased intracellular calcium concentration upon skeletal muscle contraction triggers NRG1 cleavage, and release (Canto et al. 2006). It has been postulated that calcium-induced NRG1 cleavage during skeletal muscle contraction could be mediated by ADAM17, the main protease involved in NRG1 cleavage (Canto et al. 2006). Moreover, an acute bout of both sciatic nerve stimulation and treadmill running increase NRG1 cleavage and activate ErbB receptors (Lebrasseur et al. 2003; Canto et al. 2006). In humans, NRG1 serum level is correlated with cardiopulmonary exercise capacity (Moondra et al. 2009), and progressive resistance training leads to increased ErbB3 content in skeletal muscle (LeBrasseur et al. 2005).
Therefore, the aim of this study was to explore the effect of diet and exercise training on skeletal muscle NRG1/ErbB signalling in rats. We hypothesized that an unbalanced diet could result in impaired skeletal muscle NRG1 signalling through inhibition of ErbB expression and/or activation. Conversely, return to a normal diet and/or exercise training could improve NRG1 signalling via up-regulation of ErbB receptor and/or activation, possibly mediated by activation of the metalloprotease ADAM 17. Finally, in vitro experiments in rat primary myoblasts were also performed to further explore the link between ADAM17 activation and induction of the NRG1/ErbB signalling pathway.
Materials and methods
Animals and in vivo experimental design
Animal husbandry and experimental procedures were in accordance with the current legislation on animal experimentation and were approved by the local ethics committee (CREEA Auvergne, CE1-09). Seventy-two male Wistar rats (7 months old) were randomly assigned to two groups: 12 rats received a well-balanced diet (Control) and 60 rats a high fat/high sucrose (HF/HS group) diet for 16 weeks to induce obesity (T0 to T1, Fig.1). At T1, all control animals and 12 rats from the HF/HS group were killed. The other 48 rats of the HF/HS group received one of the following interventions for 8 weeks: (1) HF/HS diet alone (HF/HS), (2) HF/HS diet with exercise (HF/HS+E), (3), well-balanced diet alone (normal diet, ND) and (4) ND with exercise (ND+E) (T1 to T2, see Fig.3).
Figure 1.
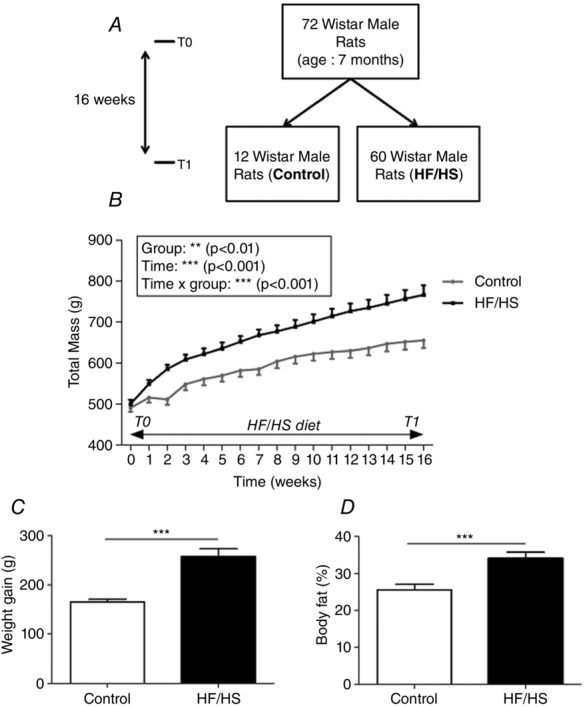
Body weight changes during the 16 weeks of HF/HS diet (T0 to T1)
A, 72 male Wistar rats (7 months old) received either a normal diet (n = 12, control) or a high fat and high sucrose diet (n = 60, HF/HS) for 16 weeks (T0 to T1). At T1, all control rats and 12 HF/HS animals were killed to analyse the effects of HF/HS diet-induced obesity. The other 48 HF/HS diet animals followed the study (see Fig.4, T1 to T2). B, body weight variation between T0 and T1. C, weight gain; D, body fat percentage at the end of the diet period (T1). Values are the mean ± SEM (n = 12 per condition). ***P < 0.001 compared to control (normal diet).
Figure 3.
Body weight changes from T0 to T2
A, the 48 male Wistar rats that were not killed at T1 (HF/HS) were assigned to one of the following groups for another 8 weeks (T1 to T2): HF/HS diet alone (n = 12, HF/HS group), HF/HS diet and endurance training (n = 12, HF/HS+E), normal diet alone (n = 12, ND), normal diet and endurance training (n = 12, ND+E). B, body weight changes between T0–T1 (HF/HS diet alone) and T1–T2 (diet ± training); C, weight variation (gain/loss); and D, body fat percentage between T1 and T2 (end of the interventions). Values are the mean ± SEM. HF/HS, high fat/high sucrose diet; HF/HS+E, high fat/high sucrose and exercise training; ND, normal diet; NE+E, normal diet and exercise training (n = 12 rats per group). NS, not significant.
Muscle precursor cell culture and stretch
Primary myoblasts were prepared from neonatal rats (2–4 days old) as previously described (Zhan et al. 2007). Cells were placed in fresh growth medium for 2 h before a constant 10% global stretch was initiated using the FlexcellH FX- 5000 TM Tension System (Flexcell International, Hillsborough, NC, USA). Sets of non-stretched myoblasts were used as controls. Stretch was maintained for 30 min in normal cell culture conditions (37°C with 5% CO2 in a humidified incubator). When indicated, the Src family tyrosine kinase (SFK) inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4 days]pyrimidine (PP2), dissolved in DMSO, was added to the medium (final concentration 10 mm) 30 min prior to the stretch and also during the stretch. DMSO alone (0.1% final concentration) was added to the medium for the negative control.
Diet, training programme and body composition
The two diets (ND and HF/HS), which have already been described (Gerbaix et al. 2012; Masgrau et al. 2012), were isoenergetic (95 kcal day−1), but differed qualitatively. The HF/HS diet was enriched in sucrose, saturated fatty acids and cholesterol (provided by lard). To ensure that all rats consumed equal amounts of calories each day, meals were prepared in individual ramekins and removed daily. The HF/HS+E and ND+E groups performed an endurance training protocol on a treadmill at 0°C five times a week for 8 weeks. This low intensity exercise training programme has been described previously (Gerbaix et al. 2013). Speed was progressively increased from 6 to 10 m min−1 and duration from 15 to 50 min. Based on literature data, this speed might represent around 55–60% of 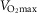 (Bedford et al. 1979; Kim et al. 2013; Chavanelle et al. 2014). One week before the animals were killed, total body composition was evaluated by dual X-ray absorptiometry (DXA) (Hologic QDR 4500 DXA, Bedford, MA, USA).
(Bedford et al. 1979; Kim et al. 2013; Chavanelle et al. 2014). One week before the animals were killed, total body composition was evaluated by dual X-ray absorptiometry (DXA) (Hologic QDR 4500 DXA, Bedford, MA, USA).
Animal dissection
Rats were anaesthetized with isofluorane and killed by decapitation after 12 h of fasting. Exercise training sessions were stopped 2 days before the animals were killed. Skeletal muscles (gastrocnemius) were dissected, weighed and frozen in liquid nitrogen for later biochemical analysis.
Real-time quantitative PCR (RT-qPCR)
Total RNA was extracted from frozen gastrocnemius muscles using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). First strand cDNA was synthetized using the High Capacity CDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) and gene expression was assessed by qPCR on a Rotor Gene cycler (Qiagen, Courtaboeuf, France) using the Rotor-Gene SYBR Green PCR Kit (Qiagen) and specific primer pairs (available on request). Gene expression levels were calculated using the absolute quantification method and a cDNA calibration curve.
Protein extraction
Frozen whole gastrocnemius muscles were finely pulverized in liquid nitrogen using a ball mill (Dangoumeau, Prolabo, Paris, France). Powdered samples (50 mg) were homogenized at 4°C in 400 μl lysis buffer (20 mm Hepes, 350 mm NaCl, 20% (v/v) glycerol, 1% (v/v) Nonidet P-40, 1 mm MgCl2, 0.5 mm EDTA, 0.1 mm EGTA, pH 7.9) supplemented with a protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA). Myoblast lysates were homogenized at 4°C in RIPA buffer (50 mm Tris-HCl, 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mm sodium fluoride, 2 mm EDTA, and protease/phosphatase inhibitor cocktails (Sigma-Aldrich)). Homogenates were then centrifuged at 10 000 g for 5 min and supernatants stored at –80°C for further analysis.
Western blotting
Protein samples were separated on Criterion Stain-Free precast gels in a BioRad Mini PROTEAN Tetra-Cell unit and transferred to nitrocellulose membranes using a BioRad Trans Blot Turbo transfer. Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline (pH 7.5) containing 0.1% Tween 20 (TBST) at room temperature for 1 h. Then, membranes were incubated in 2% BSA with the relevant primary antibodies at 4°C overnight. Anti-ErbB3 (1:200) and anti-p-ErbB3 (1:200) antibodies were purchased from Cell Signaling (Beverly, MA, USA). Anti-ErbB-2 (1:200), anti-ErbB-4 (1:200), anti-p-ErbB-2 (1:200), anti-p-ErbB4 (1:200) and anti-TIMP3 (tissue inhibitor of metalloproteinase 3; 1:1000) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The anti-NRG1 N120A/9 antibody (1:1000) was from Millipore (Merck Millipore, Billerica, MA, USA) and the anti-ADAM17 (1:1000) antibody from Abcam (Cambridge, MA, USA). The rabbit antibody against phosphorylated ADAM17 was produced using a peptide with phosphorylated Y702 (K696 KLDKQYESL705) by New England Peptide (Gardner, MA, USA) (Niu et al. 2013). After incubation with the appropriate horseradish peroxidase-conjugated secondary antibody in TBST at room temperature for 45 min, enhanced chemiluminescence (ECL) reagents (Bio-Rad, Hercules, CA, USA) were used to detect interactions and digital images were acquired using the Molecular Imager ChemiDoc XRS System (Bio-Rad). Signals were quantified using the Image Lab 4.1 software (BioRad) and normalized using the Total Protein Normalization (TPN) method provided by Stain Free Blot technology (Gilda & Gomes, 2015). When phosphorylated proteins were quantified, we first normalized the results to the total protein content before calculating the phosphorylation ratio and the result was finally expressed relative to control.
Statistical analysis
Data are presented as mean ± SEM. Student’s t test was used for between-group comparisons. Body weight changes were assessed using a two-way repeated-measures ANOVA. The effects of diet (HF/HS or ND) and the effect of exercise (trained or untrained) were investigated with a 2 × 2 ANOVA. When a significant effect was found, post hoc multiple comparisons were made using Tukey’s post hoc analysis. Statistical significance was set at 0.05.
Results
weeks of HF/HS diet induces obesity
To test the effects of an unbalanced diet on the NRG1/ErbB pathway, first rats were fed either the normal diet (n = 12) or the HF/HS diet (n = 12) for 16 weeks (T0–T1; Fig.1A). Progressively, animals that received the HF/HS diet became heavier than controls (Fig.1B), with significantly higher total weight gain (147.7 ± 2.7 vs. 224.1 ± 16.1 g; Fig.1C) after 16 weeks. Prior to the animals being killed, body fat percentage was also significantly higher in the HF/HS group compared to controls (25.6 ± 1.5 vs. 34.1 ± 1.7%; Fig.1D).
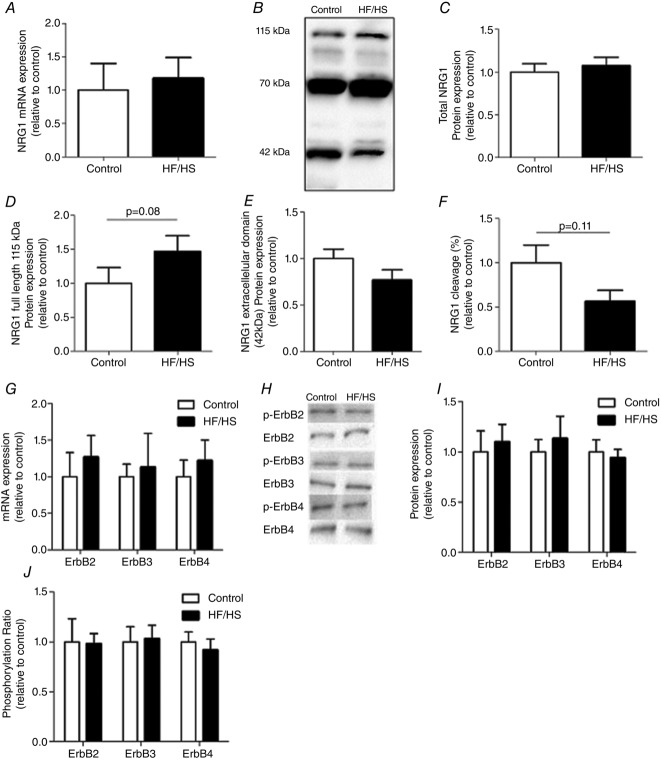
weeks of HF/HS diet does not significantly affect NRG1/ErbB mRNA and protein expression
To investigate whether the HF/HS diet affected NRG1 signalling, NRG1 mRNA/protein level (quantitative modification) and cleavage index (qualitative change) were evaluated. NRG1 mRNA expression remained unchanged in skeletal muscle of rats after 16 weeks of HF/HS diet compared to controls (Fig.2A). Western blot analysis of rat skeletal muscle extracts showed several bands mainly around 115, 70 and 42 kDa (Fig.2B). These molecular sizes correspond to the known masses of recombinant NRG1 proteins (Montero et al. 2000; Aguilar & Slamon, 2001; Shirakabe et al. 2001; Wang et al. 2001). We chose to specifically focus on the 115 and 42 kDa bands because several studies have shown that the 115 kDa band corresponds to full length NRG1 and the 42 kDa to the cleaved, active form of NRG1 (Shirakabe et al. 2001; Wang et al. 2001). Their quantification allowed us to define a ‘cleavage index’ (the ratio of cleaved 42 kDa to full length 115 kDa). Total NRG1 protein expression level was based on the sum of the intensities of the 115, 70 and 42 kDa bands. The levels of total NRG1 protein (Fig.2C), of full length 115 kDa NRG1 (Fig.2D) and of the extracellular cleaved active form of NRG1 (Fig.2E) were not significantly affected by the HF/HS diet compared to controls. Accordingly, the NRG1 cleavage index was also not significantly changed, although a trend towards a lower cleavage in the HF/HS group compared to controls was observed (Fig.2F; P = 0.11). Moreover, the 16 week HF/HS diet did not induce any significant change in the mRNA and protein levels of ErbB receptors compared to controls (Fig.2G–I). Finally, in agreement with the absence of clear modifications in the cleaved NRG1 level, the phosphorylation ratio of ErbB2, 3 and 4 was comparable in diet-induced obese rats and controls (Fig.2J), thus strengthening the finding that the HF/HS diet did not significantly affect the NRG1/ErbB pathway.
Figure 2.
NRG1 expression, cleavage ratio and ErbB activation in rat gastrocnemius samples from control and HF/HS rats after 16 weeks of HF/HS diet
A, Nrg1 mRNA expression (n = 10 per group) was assessed by RT-qPCR. B, Western blot analysis of NRG1 expression in rat gastrocnemius using the N120A/9 antibody detected three main bands of 115, 70 and 42 kDa that correspond to the masses of recombinant NRG1 proteins (Montero et al. 2000; Aguilar & Slamon, 2001; Shirakabe et al. 2001; Wang et al. 2001). C, total NRG1 protein level is the sum of the intensities of the 115 , 70 and 42 kDa bands detected with the N120A/9 antibody. D, full length (115 kDa); F, cleaved NRG1 (the 42 kDa active form); and E, NRG1 cleavage index (the ratio of cleaved 42 kDa to full length 115 kDa). F, ErbB mRNA expression (n = 10 per group) was assessed by RT-qPCR. G, Western blot analysis of ErbB expression and phosphorylation in rat gastrocnemius (n = 10 per group). H, quantification of ErbB2, ErbB3 and ErbB4 protein expression in control and HF/HS rats. I, phosphorylation ratios (ratio between phosphorylated form and total expression of a given ErbB) were then calculated (relative to control condition). All Western blot results were first normalized to the total protein content and then expressed relative to control. Results are the mean ± SEM (n = 10 per group). HF/HS, high fat/high sucrose diet.
Exercise training and return to normal diet induce weight loss in obese rats
Although rats of the HF/HS group were divided into four sub-groups at T0, the specific interventions began only at T1 after the 16 weeks of HF/HS diet (Fig.3A). Body weight assessment showed that during the first period (T0–T1: only HF/HS diet) there was a significant time effect (P < 0.001), but no group effect and no group × time interaction (Fig.4B, left part). During the second period (T1–T2; Fig.3B, right part), we observed not only a significant time effect (P < 0.001), but also a significant time × diet interaction (P < 0.001) and a time × exercise interaction (P < 0.001). Analysis of the weight changes between T1 and T2 (Fig.3C) showed that, as expected, only the HF/HS group gained weight (+14.75 ± 4.7 g) whereas the HF/HS+E, ND and ND+E groups lost weight (–48.07 ± 7.2, –12.07 ± 7.1 and –103.40 ± 10.1 g, respectively). The effects of return to normal diet and exercise training on body weight changes seemed to be cumulative. However, two-way ANOVA showed a significant effect of diet (P < 0.001) and exercise (P < 0.001), but not of the diet × exercise interaction (P = 0.07). Overall, only exercise significantly decreased the body fat percentage (P < 0.001). Indeed, at the end of the intervention period, body fat percentage was 10% lower in the ND+E group and about 5% lower in the HF/HS+E group compared to the HF/HS group (26.2 ± 1.4, 30.1 ± 1.4 and 36.4 ± 1.3%, respectively, Fig.3D). Conversely, no significant difference was observed between the ND and HF/HS groups.
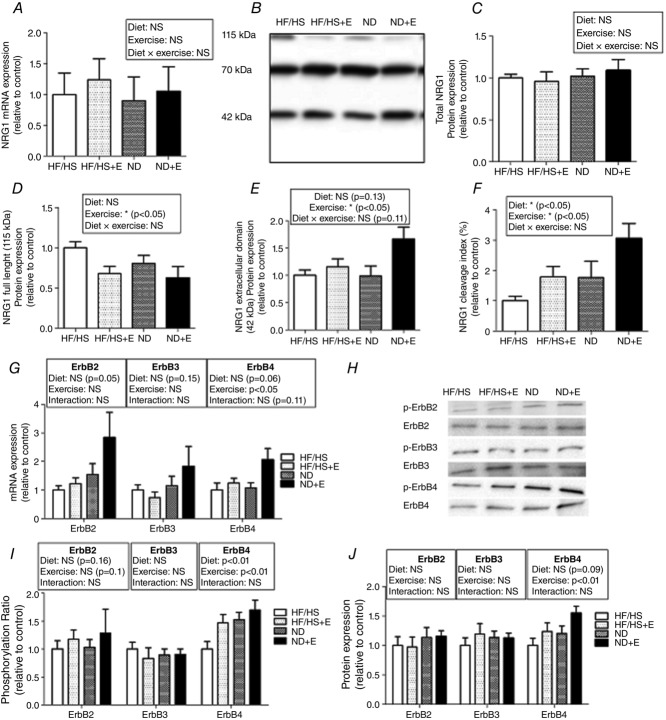
Figure 4.
NRG1 cleavage and ErbB4 activation are increased in rat gastrocnemius after well-balanced diet and exercise training
A, Nrg1 mRNA expression (n = 10 per group) was assessed by RT-qPCR. B, Western blot analysis of NRG1 expression in rat gastrocnemius using the N120A/9 antibody. Quantification of: C, total NRG1 protein level (the sum of the intensities of the 115, 70 and 42 kDa bands); D, full length (115 kDa) NRG1; E, cleaved NRG1 (the 42 kDa active form); and F, NRG1 cleavage index (the ratio of the cleaved 42 kDa form to the full length 115 kDa form). G, ErbB mRNA expression (n = 10 per group) assessed by RT-qPCR. H, Western blot analysis of ErbB expression and phosphorylation in rat gastrocnemius. I, quantification of ErbB2, ErbB3 and ErbB4 protein levels in the different groups at the end of the intervention period. J, phosphorylation ratios (phosphorylated expression divided by the total expression of the corresponding ErbB) were then calculated (relative to control condition). All Western blot results were first normalized to the total protein content and then expressed relative to control. Results are the mean ± SEM (n = 10 animals per group). HF/HS, high fat/high sucrose diet; HF/HS+E, high fat/high sucrose diet and exercise training; ND, normal diet; NE+E, normal diet and exercise training.
Exercise training and return to a normal diet increase the level of cleaved active NRG1 and activate ErbB4
NRG1 mRNA and protein levels were not altered in response to exercise training (HF/HS+E), normal diet (ND) or combined interventions (ND+E) compared to untreated animals (HF/HS group) (Fig.4A–C). Nevertheless, specific quantification of the full length 115 kDa form (Fig.4D) and the cleaved 42 kDa active form of NRG1 (Fig.4E) showed that exercise (HF/HS+E) or return to normal diet (ND) decreased full length NRG1 and increased cleaved NRG1 levels and consequently also the NRG1 cleavage index (by 1.5-fold) compared to controls (HF/HS diet) (Fig.4F). In rats in which return to normal diet was combined with exercise training (ND + E), a 3-fold increase in NRG1 cleavage index was observed compared to the HF/HS group (Fig.4F). ErbB4 phosphorylation ratio (Fig.4H and I) was also increased by physical training or return to normal diet, in accordance with the increased NRG1 cleavage index. Moreover, ErbB4 mRNA (Fig.4G) and protein levels (Fig.4J) were significantly higher in the trained groups, while diet alone did not have a significant effect.
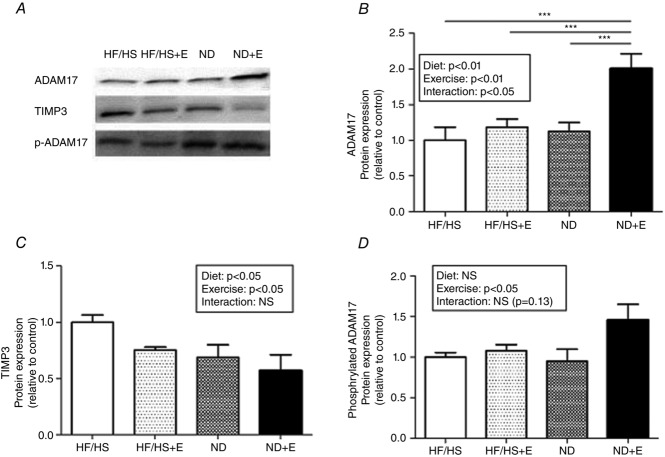
Exercise training and return to normal diet result in higher ADAM17 and decreased TIMP3 protein levels
The impact of exercise training and diet on the protein level of ADAM 17, the main protease involved in NRG1 cleavage, was then assessed by Western blotting (Fig.5A). Exercise and return to normal diet significantly increased ADAM17 protein level (Fig.5B) and this effect was even stronger when exercise training was combined with normal diet (ND+E group: 2-fold increase compared to the HF/HS group). Expression of the endogenous inhibitor TIMP3, which regulates ADAM17 activity in vivo (Mahmoodi et al. 2005; Cardellini et al. 2009), was also measured. TIMP3 protein level was significantly decreased upon exercise or return to normal diet (Fig.5C), suggesting an increased basal activity of ADAM17 in skeletal muscle following these interventions. Quantification of ADAM17 phosphorylation on Tyr702, which mediates ADAM17 activation in rat skeletal muscle (Niu et al. 2013), showed that the ratio between Tyr702-phosphorylated ADAM17 and total ADAM17 was significantly increased in skeletal muscles of trained rats (Fig.5D).
Figure 5.
Exercise training combined with normal diet results in higher protein level of ADAM17
A, expression of TIMP3, ADAM17 and phosphorylated ADAM17 in rat gastrocnemius by Western blotting at the end of the intervention period (T1 to T2). B–D, quantification of TIMP3 (B) ADAM17 (C) and ADAM17 (D) phosphorylation ratios (p-ADAM17 divided by ADAM17). All Western blot results were first normalized to the total protein content and then expressed relative to control. Results are the mean ± SEM (n = 10 rats per group). ***P < 0.001. HF/HS, high fat/high sucrose diet; HF/HS+E, high fat/high sucrose diet and exercise training; ND, normal diet; NE+E, normal diet and exercise training.
Stretch-induced ADAM17 activation increases the level of cleaved active NRG1 and ErbB4 phosphorylation ratio in rat skeletal muscle cells
In vitro stretch of rat primary myoblasts induces ADAM17 activation via Tyr702 phosphorylation (Niu et al. 2013). We therefore used this model to test the hypothesis that ADAM17 activation leads to increased NRG1 cleavage and ErbB4 phosphorylation in rat skeletal muscle. First, we confirmed that stretch of rat primary myoblasts promotes ADAM17 Tyr702 phosphorylation (Fig.6A and B). As hypothesized, this was associated with increased NRG1 cleavage and ErbB4 phosphorylation (Fig.6C and D). Moreover, when ADAM17 phosphorylation on Tyr702 was inhibited with PP2, an inhibitor of Src kinases that mediate stretch-induced ADAM17 phosphorylation (Niu et al. 2013), the increase of NRG1 cleavage and ErbB4 phosphorylation was no longer observed in stretched myoblasts (stretch + PP2) (Fig.6C and D).
Figure 6.

Stretch-induced increases of ADAM17 activity promote the expression of cleaved active NRG1 and increase ErbB4 phosphorylation ratio in rat skeletal muscle cells
A, Western blot analysis of ADAM17, p-ADAM17, NRG1, ErbB4 and p-ErbB4 expression in control and stretched rat primary myoblasts, with and without PP2 inhibitor. B–D, ADAM17 phosphorylation ratio (B, p-ADAM17 divided by ADAM17), NRG1 cleavage index (C, the 42 kDa active form divided by full length 115 kDa isoform) and ErbB4 phosphorylation ratio (D, p-ErbB4 divided by ErbB4) were then calculated. All Western blot results were first normalized to the total protein content and then expressed relative to control. Results are the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine.
Discussion
Our study shows that HF/HS diet does not alter the NRG1/ErbB pathway in rat skeletal muscle. On the other hand, 8 weeks of endurance exercise training led to increased ADAM17 protein expression in skeletal muscle of HF/HS diet-induced obese rats that were switched to a normal diet, and the ADAM17 endogenous inhibitor TIMP3 was down-regulated by both diet and exercise interventions. This effect was associated with increased NRG1 cleavage index and increased ErbB4 phosphorylation ratio. These in vivo results suggest that endurance exercise training and return to a well-balanced diet result in ADAM17-mediated NRG1 cleavage and consequently ErbB4 activation in skeletal muscle of HF/HS diet-induced obese rats. This hypothesis is reinforced by in vitro results showing that stretch-induced activation of ADAM17 in rat primary myoblasts leads to NRG1 cleavage and ErbB4 activation.
We first hypothesized that increased amounts of saturated fatty acids in the diet might disturb the NRG1/ErbB signalling pathway. In skeletal muscle, saturated fatty acids have deleterious effects on the insulin signalling pathway, which shares numerous steps with the NRG1 pathway (Canto et al. 2004). Moreover, it has already been observed in vitro in cardiomyocytes that saturated fatty acids disturb the NRG1/ErbB signalling pathway (Miller et al. 2009). To test this hypothesis, Wistar rats received an HF/HS diet for 16 weeks. As already reported (Lomba et al. 2010), this obesity-inducing diet was effective, leading to an increase in weight and fat mass compared to the isoenergetic well-balanced diet of control animals. Conversely, NRG1 and ErbB mRNA and protein levels were not changed significantly in the HF/HS group compared to controls. In addition, also the activity of the NRG1/ErbB pathway was not altered by the HF/HS diet, as indicated by the absence of significant changes in NRG1 cleavage index and ErbB phosphorylation level. Our results demonstrate that the HF/HS diet used does not alter NRG1/ErbB signalling in skeletal muscle, refuting our initial hypothesis. Nevertheless, we cannot rule out the possibility that other pathogenic diets may alter the NRG1/ErbB signalling pathway. In the present study, our HF/HS diet contained 45% lipids (with 32.9% saturated fatty acids), compared to 14% in the control diet (with 12.6% saturated fatty acids). The increased amount of lipids in the HF/HS diet derived from lard incorporation. Lard is rich in saturated (about 40%) but also in monounsaturated (about 48%) fatty acids. In cardiac cells, oleate can reverse the deleterious effects of palmitate on the NRG1 pathway (Miller et al. 2009), and thus we postulated that the monounsaturated fatty acids in our HF/HS diet (49.3% of the total lipids) protected skeletal muscle fibres from the potential deleterious effect of saturated fatty acids. Further studies are thus required to assess the effects of diets with different amounts and proportions of fatty acids on the NRG1 pathway.
Physical activity and a well-balanced diet are the cornerstones of the therapeutic management of overweight and obesity (Laddu et al. 2011). We hypothesized that endurance training, combined or not with a return to a well-balanced diet, might stimulate the NRG1/ErbB signalling pathway in obese rats. After 16 weeks of HF/HS diet, exercise training for 8 weeks significantly reduced body weight in obese rats. A similar effect was also observed by switching from the HF/HS diet to a well-balanced diet. These results are consistent with previous literature data in obese rodents (Paulino et al. 2010). Concerning the effects of these interventions on the NRG1/ErbB signalling pathway, our results show that exercise and diet have only a modest quantitative impact on NRG1 expression levels. Only one study assessed the influence of exercise training on the expression of different NRG1 variants in human skeletal muscle (LeBrasseur et al. 2005) and, like us, the authors did not find any significant change at the end of the training protocol. On the other hand, we found that ErbB4 mRNA and protein levels were increased by exercise training. Although this increase was more pronounced in the group combining endurance exercise and well-balanced diet, the independent effect of diet did not reach statistical significance. Higher ErbB expression after exercise training has already been reported in human skeletal muscle, but only for ErbB3 (LeBrasseur et al. 2005). This discrepancy could be explained by species-related (rats in the present study and humans in the quoted work) and muscle-related differences (rat gastrocnemius in the present study vs. human vastus lateralis), but also by the intervention protocols (progressive resistance training three times per week for 8 weeks in the human study, and low intensity treadmill endurance training five times per week for 8 weeks in our study). To date, no study has evaluated the impact of nutritional intervention on NRG1/ErbB signalling in skeletal muscle. Nevertheless, energy restriction has been shown to increase ErbB expression and restore mitochondrial metabolism in the heart of old rats, suggesting that ErbB receptors are sensitive to both intervention strategies, at least in cardiac tissue (Rohrbach et al. 2006). In the present study, we did not modify the total amount of caloric intake (95 kcal day−1 in each group). Further studies are thus needed to explore the effects of different chronic exercise protocols (e.g. with higher intensity) and also of different nutritional interventions (with qualitative and quantitative changes) on skeletal muscle NRG1 and ErbB expression.
In addition and in line with the quantitative changes, significant functional modifications (i.e. concerning the activity of the NRG1/ErbB pathway) were induced by endurance training in obese rats switched, or not, to a well-balanced diet. ADAM17 expression was increased by both interventions. Moreover, two-way ANOVA showed a significant interaction between diet and exercise and the post hoc analysis indicated that ADAM17 protein increase was only significant in the group combining endurance training and normal diet (ND+E). The ADAM17 inhibitor TIMP3 was decreased by both diet and exercise interventions, while only exercise training significantly increased phosphorylation of ADAM17 on Tyr702, which has been associated with increased activity of this protease in rat skeletal muscle in vitro (Niu et al. 2013). Taken together, these results strongly suggest increased basal activity of ADAM17 in skeletal muscle of obese rats following exercise training. This effect is potentiated by the concomitant return to a well-balanced diet. To date, no study has explored the effects of endurance training on ADAM17 in skeletal muscle, but ADAM17 expression is sensitive to nutritional interventions and particularly to fat modulation in the diet (Konstantinidou et al., 2009; Junyent et al. 2010). Indeed, acute ingestion of virgin olive oil in men up-regulates ADAM17 gene expression (Konstantinidou et al., 2009). Moreover, in humans, a specific ADAM17 polymorphism may contribute to the obesity risk and this risk could be further increased by dietary n-6 polyunsaturated fatty acids, suggesting a potential role of this protease in overweight pathogenesis (Junyent et al. 2010). In line with the increased ADAM17 expression and activity in the trained obese rats, we also found a significant increase in NRG1 cleavage in these rats, suggesting a link between these phenomena. Acute exercise is known to stimulate NRG1 cleavage in rats and humans (Lebrasseur et al. 2003, 2005). Conversely, no effect of chronic resistance exercise has been shown on NRG1 cleavage in human skeletal muscle (LeBrasseur et al. 2005). We thus hypothesize that endurance training might be more effective than resistance training to induce significant changes in NRG1 cleavage in skeletal muscle. This discrepancy could also be explained by species-related and muscle-related differences, as already discussed above. We can also hypothesize that a higher treadmill exercise intensity could induce greater effects on the NRG1/ErbB signalling pathway. More studies comparing different exercise modalities in the same model are needed to confirm this assumption. Concomitantly with higher NRG1 cleavage, we also observed a significant increase of ErbB4 phosphorylation in skeletal muscle of obese rats following both exercise training and return to normal diet. This result shows that the NRG1/ErbB4 pathway is activated in response to these interventions, the effect of which appears to be cumulative. It has already been shown that ErbB2 and ErbB4 can be phosphorylated in response to acute contraction in rat skeletal muscle (Canto et al. 2006). This is the first study showing that chronic endurance training can activate the NRG1/ErbB pathway in vivo. As ErbB4 activation has been implicated in glucose transport regulation in skeletal muscle by mediating GLUT4 translocation (Canto et al. 2004), further studies are needed to assess the potential consequences of the increased activity of the NRG1/ErbB4 pathway induced by chronic exercise on glucose regulation in vivo.
Taken together, the in vivo results strongly suggest a mechanism in which exercise training promotes ADAM17 activity, resulting in NRG1 cleavage and ErbB4 activation. The in vitro results on rat myoblasts further support the link between these phenomena. Indeed, this hypothesis is reinforced by the findings that stretch-induced activation of ADAM17 induced NRG1 cleavage and ErbB4 phosphorylation and that these effects were abolished when SRC-mediated activation of ADAM17 was blocked by the SRC inhibitor PP2. Further studies are needed to explore the isolated and concomitant effects of stretch and some nutrients, such as fatty acids, on the ADAM17/NRG1/ErbB pathway to better understand the mechanisms involved in the effects of nutritional and exercise interventions on this pathway.
In conclusion, our findings show that the HF/HS diet did not induce major NRG1/ErbB signalling pathway changes in rat skeletal muscle. On the other hand, endurance training up-regulated ADAM17 in skeletal muscle of HF/HS diet-induced obese rats switched to a well-balanced diet. ADAM17 could, in turn, stimulate NRG1 cleavage and increase the level of active NRG1 and ErbB4 phosphorylation. This mechanism may contribute to the improved energy metabolism regularly observed following diet and exercise interventions.
Acknowledgments
We thank Chistophe Del’homme, Catherine Besson and Philippe Denis for animal husbandry and skilful technical assistance. We thank Elisabetta Andermarcher for language help and writing assistance and Anna Guma for helpful discussions and suggestions.
Glossary
- ADAM17
A disintegrin and metalloprotease 17
- DXA
dual X-ray absorptiometry
- ErbB
erythroblastic leukaemia viral oncogene homologue
- HF/HS
high fat/high sucrose
- ND
normal diet
- NRG1
neuregulin 1
- PP2
4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- RT-qPCR
quantitative PCR
- TIMP3
tissue inhibitor of metalloproteinase 3
Additional information
Competing interests
No competing interests.
Author contributions
G.E.: data collection, analysis and interpretation, drafting and writing the manuscript. N.B.: drafting and critical reading of the manuscript. K.C.: data collection, analysis and interpretation. V.C.: data collection and analysis. M.G.: study conception and data collection. L.M.: study conception and data collection. M.E.: data collection and analysis. S.W: study conception and data collection. A.M.: study conception and data collection. C.G: study conception and data collection. D.C.: funding acquisition, study conception and data collection. A.N.: provision of study materials and data collection. Y.L.: provision of study materials and data collection. F.C.: data collection, analysis and interpretation. P.S: study conception, data analysis and interpretation, drafting and critical reading of the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by the Heart and Arteries Foundation (Fondation Coeur et Artères).
References
- Aguilar Z. Slamon DJ. The transmembrane heregulin precursor is functionally active. J Biol Chem. 2001;276:44099–44107. doi: 10.1074/jbc.M103442200. [DOI] [PubMed] [Google Scholar]
- Bedford TG, Tipton CM, Wilson NC, Oppliger RA. Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol Respir Environ Exerc Physiol. 1979;47:1278–1283. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- Canto C, Chibalin AV, Barnes BR, Glund S, Suarez E, Ryder JW, Palacin M, Zierath JR, Zorzano A. Guma A. Neuregulins mediate calcium-induced glucose transport during muscle contraction. J Biol Chem. 2006;281:21690–21697. doi: 10.1074/jbc.M600475200. [DOI] [PubMed] [Google Scholar]
- Canto C, Pich S, Paz JC, Sanches R, Martinez V, Orpinell M, Palacin M, Zorzano A. Guma A. Neuregulins increase mitochondrial oxidative capacity and insulin sensitivity in skeletal muscle cells. Diabetes. 2007;56:2185–2193. doi: 10.2337/db06-1726. [DOI] [PubMed] [Google Scholar]
- Canto C, Suarez E, Lizcano JM, Grino E, Shepherd PR, Fryer LG, Carling D, Bertran J, Palacin M, Zorzano A. Guma A. Neuregulin signaling on glucose transport in muscle cells. J Biol Chem. 2004;279:12260–12268. doi: 10.1074/jbc.M308554200. [DOI] [PubMed] [Google Scholar]
- Cardellini M, Menghini R, Martelli E, Casagrande V, Marino A, Rizza S, Porzio O, Mauriello A, Solini A, Ippoliti A, Lauro R, Folli F. Federici M. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes. 2009;58:2396–2401. doi: 10.2337/db09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway KL, 3rd, Sliwkowski MX, Akita R, Platko JV, Guy PM, Nuijens A, Diamonti AJ, Vandlen RL, Cantley LC. Cerione RA. The erbB3 gene product is a receptor for heregulin. J Biol Chem. 1994;269:14303–14306. [PubMed] [Google Scholar]
- Chavanelle V, Sirvent P, Ennequin G, Caillaud K, Montaurier C, Morio B, Boisseau N. Richard R. Comparison of oxygen consumption in rats during uphill (concentric) and downhill (eccentric) treadmill exercise tests. J Sports Sci Med. 2014;13:689–694. [PMC free article] [PubMed] [Google Scholar]
- Ennequin G, Boisseau N, Caillaud K, Chavanelle V, Etienne M, Li X, Montaurier C. Sirvent P. Neuregulin 1 affects leptin levels, food intake and weight gain in normal-weight, but not obese, db/db mice. Diabetes Metab. 2015 doi: 10.1016/j.diabet.2014.12.002. doi: 10.1016/j.diabet.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Gerbaix M, Metz L, Mac-Way F, Lavet C, Guillet C, Walrand S, Masgrau A, Linossier MT, Vico L. Courteix D. Impact of an obesogenic diet program on bone densitometry, micro architecture and metabolism in male rat. Lipids Health Dis. 2012;11:91. doi: 10.1186/1476-511X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbaix M, Metz L, Mac-Way F, Lavet C, Guillet C, Walrand S, Masgrau A, Vico L. Courteix D. A well-balanced diet combined or not with exercise induces fat mass loss without any decrease of bone mass despite bone micro-architecture alterations in obese rat. Bone. 2013;53:382–390. doi: 10.1016/j.bone.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Gilda JE. Gomes AV. Western blotting using in-gel protein labeling as a normalization control: stain-free technology. Methods Mol Biol. 2015;1295:381–391. doi: 10.1007/978-1-4939-2550-6_27. [DOI] [PubMed] [Google Scholar]
- Guma A, Martinez-Redondo V, Lopez-Soldado I, Canto C. Zorzano A. Emerging role of neuregulin as a modulator of muscle metabolism. Am J Physiol Endocrinol Metab. 2010;298:E742–E750. doi: 10.1152/ajpendo.00541.2009. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Zhou HM, Kelly K, Manova K. Blobel CP. Evaluation of the contributions of ADAMs 9, 12, 15, 17, and 19 to heart development and ectodomain shedding of neuregulins β1 and β2. Dev Biol. 2005;283:459–471. doi: 10.1016/j.ydbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Junyent M, Parnell LD, Lai CQ, Arnett DK, Tsai MY, Kabagambe EK, Straka RJ, Province M, An P, Smith CE, Lee YC, Borecki I. Ordovas JM. ADAM17_i33708A>G polymorphism interacts with dietary n-6 polyunsaturated fatty acids to modulate obesity risk in the Genetics of Lipid Lowering Drugs and Diet Network study. Nutr Metab Cardiovasc Dis. 2010;20:698–705. doi: 10.1016/j.numecd.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim SH, Kim WH. Moon CR. The effects of treadmill exercise on expression of UCP-2 of brown adipose tissue and TNF-α of soleus muscle in obese Zucker rats. J Exerc Nutrition Biochem. 2013;17:199–207. doi: 10.5717/jenb.2013.17.4.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou V, Khymenets O, Fito M, De La Torre R, Anglada R, Dopazo A. Covas MI. Characterization of human gene expression changes after olive oil ingestion: an exploratory approach. Folia Biol (Praha) 2009;55:85–91. [PubMed] [Google Scholar]
- Laddu D, Dow C, Hingle M, Thomson C. Going S. A review of evidence-based strategies to treat obesity in adults. Nutr Clin Pract. 2011;26:512–525. doi: 10.1177/0884533611418335. [DOI] [PubMed] [Google Scholar]
- Lebrasseur NK, Cote GM, Miller TA, Fielding RA. Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol. 2003;284:C1149–C1155. doi: 10.1152/ajpcell.00487.2002. [DOI] [PubMed] [Google Scholar]
- LeBrasseur NK, Mizer KC, Parkington JD, Sawyer DB. Fielding RA. The expression of neuregulin and erbB receptors in human skeletal muscle: effects of progressive resistance training. Eur J Appl Physiol. 2005;94:371–375. doi: 10.1007/s00421-005-1333-4. [DOI] [PubMed] [Google Scholar]
- Lomba A, Milagro FI, Garcia-Diaz DF, Marti A, Campion J. Martinez JA. Obesity induced by a pair-fed high fat sucrose diet: methylation and expression pattern of genes related to energy homeostasis. Lipids Health Dis. 2010;9:60. doi: 10.1186/1476-511X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoodi M, Sahebjam S, Smookler D, Khokha R. Mort JS. Lack of tissue inhibitor of metalloproteinases-3 results in an enhanced inflammatory response in antigen-induced arthritis. Am J Pathol. 2005;166:1733–1740. doi: 10.1016/S0002-9440(10)62483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masgrau A, Mishellany-Dutour A, Murakami H, Beaufrere AM, Walrand S, Giraudet C, Migne C, Gerbaix M, Metz L, Courteix D, Guillet C. Boirie Y. Time-course changes of muscle protein synthesis associated with obesity-induced lipotoxicity. J Physiol. 2012;590:5199–5210. doi: 10.1113/jphysiol.2012.238576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE. Birchmeier C. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [DOI] [PubMed] [Google Scholar]
- Miller TA, Icli B, Cote GM, Lebrasseur NK, Borkan SC, Pimentel DR, Peng X. Sawyer DB. Palmitate alters neuregulin signaling and biology in cardiac myocytes. Biochem Biophys Res Commun. 2009;379:32–37. doi: 10.1016/j.bbrc.2008.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero JC, Yuste L, Diaz-Rodriguez E, Esparis-Ogando A. Pandiella A. Differential shedding of transmembrane neuregulin isoforms by the tumor necrosis factor-α-converting enzyme. Mol Cell Neurosci. 2000;16:631–648. doi: 10.1006/mcne.2000.0896. [DOI] [PubMed] [Google Scholar]
- Moondra V, Sarma S, Buxton T, Safa R, Cote G, Storer T, Lebrasseur NK. Sawyer DB. Serum neuregulin-1β as a biomarker of cardiovascular fitness. Open Biomark J. 2009;2:1–5. doi: 10.2174/1875318300902010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu A, Wen Y, Liu H, Zhan M, Jin B. Li YP. Src mediates the mechanical activation of myogenesis by activating TNFα-converting enzyme. J Cell Sci. 2013;126:4349–4357. doi: 10.1242/jcs.125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino EC, Ferreira JC, Bechara LR, Tsutsui JM, Mathias W, Jr, Lima FB, Casarini DE, Cicogna AC, Brum PC. Negrao CE. Exercise training and caloric restriction prevent reduction in cardiac Ca2+-handling protein profile in obese rats. Hypertension. 2010;56:629–635. doi: 10.1161/HYPERTENSIONAHA.110.156141. [DOI] [PubMed] [Google Scholar]
- Plowman GD, Green JM, Culouscou JM, Carlton GW, Rothwell VM. Buckley S. Heregulin induces tyrosine phosphorylation of HER4/p180erbB4. Nature. 1993;366:473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- Rohrbach S, Niemann B, Abushouk AM. Holtz J. Caloric restriction and mitochondrial function in the ageing myocardium. Exp Gerontol. 2006;41:525–531. doi: 10.1016/j.exger.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Wakatsuki S, Kurisaki T. Fujisawa-Sehara A. Roles of Meltrin β/ADAM19 in the processing of neuregulin. J Biol Chem. 2001;276:9352–9358. doi: 10.1074/jbc.M007913200. [DOI] [PubMed] [Google Scholar]
- Suarez E, Bach D, Cadefau J, Palacin M, Zorzano A. Guma A. A novel role of neuregulin in skeletal muscle. Neuregulin stimulates glucose uptake, glucose transporter translocation, and transporter expression in muscle cells. J Biol Chem. 2001;276:18257–18264. doi: 10.1074/jbc.M008100200. [DOI] [PubMed] [Google Scholar]
- Talmage DA. Mechanisms of neuregulin action. Novartis Found Symp. 2008;289:84–93. doi: 10.1002/9780470751251.ch6. , 74–84; discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Miller SJ. Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem. 2001;276:2841–2851. doi: 10.1074/jbc.M005700200. [DOI] [PubMed] [Google Scholar]
- Zhan M, Jin B, Chen SE, Reecy JM. Li YP. TACE release of TNF-α mediates mechanotransduction-induced activation of p38 MAPK and myogenesis. J Cell Sci. 2007;120:692–701. doi: 10.1242/jcs.03372. [DOI] [PMC free article] [PubMed] [Google Scholar]