Abstract
Platelet-expressed GPCRs are critical regulators of platelet function. Pharmacological blockade of these receptors forms a powerful therapeutic tool in the treatment and prevention of arterial thrombosis associated with coronary atherosclerosis and ischaemic stroke. However, anti-thrombotic drug therapy is associated with high inter-patient variability in therapeutic response and adverse bleeding side effects. In order to optimize the use of existing anti-platelet drugs and to develop new therapies, more detailed knowledge is required relating to the molecular mechanisms that regulate GPCR and therefore platelet function. One approach has been to identify rare, function-disrupting mutations within key platelet proteins in patients with bleeding disorders. In this review, we describe how an integrated functional genomics strategy has contributed important structure-function information about platelet GPCRs with specific emphasis upon purinergic and thromboxane A2 receptors. We also discuss the potential implications these findings have for pharmacotherapy and for understanding the molecular basis of mild bleeding disorders.
Tables of Links
| Targets | |
|---|---|
| GPCRsa | Purinergic P2Y1 receptors |
| 5-HT2A receptors | Purinergic P2Y12 receptors |
| Adenosine A2A receptors | Catalytic receptorsb |
| α2A-Adrenoceptor | Integrin α2β1 |
| Proteinase-activated receptors PAR1 | Integrin αIIbβ3 |
| Proteinase-activated receptors PAR4 | Enzymesc |
| Prostanoid IP1 receptor | CYP2C19 |
| Prostanoid EP3 receptor | PKA |
| Prostanoid TP-α receptor |
| Ligands | |
|---|---|
| 5-HT | Collagen |
| Adenosine | Prasugrel |
| ADP | Prostacyclin, PGI2 |
| Arachidonic acid | Thromboxane A2, TxA2 |
| AR-C69931MX (cangrelor) | U46619 |
| Aspirin | Vorapaxar |
| Clopidogrel | vWF, von Willebrand factor |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,b,c).
Introduction
Platelets are small anucleate cells derived from megakaryocytes in the bone marrow that circulate in the bloodstream and play a key role in haemostasis. Under physiological conditions, platelets do not adhere to the vessel wall because of the continuous release of inhibitory PGs and NO, which prevent platelet activation. When damage to the vasculature occurs, platelets bind to exposed collagen, become activated and release a number of stimulatory (pro-aggregatory) mediators which feedback in an autocrine manner to cause further platelet activation, recruitment of more platelets to the site of injury and the formation of a stable clot, preventing further blood loss (Kaplan and Jackson, 2011).
Platelet activation is a tightly regulated process with both increased and decreased platelet reactivity resulting in significant sequelae. Decreased platelet activity is associated with an increased risk of bleeding. Conversely increased platelet activity, for example in response to atherosclerotic plaque rupture, can lead to the build-up of vessel-occluding thrombi, resulting in myocardial infarction or stroke. The majority of drugs that target platelets inhibit platelet activation. However a key challenge of pharmacotherapy in this area is to achieve sufficient inhibition of platelet activity to prevent thrombus formation while maintaining the haemostatic properties of platelets in order to avoid excessive bleeding. Although the main signalling pathways underlying platelet activation are now well defined (Stegner and Nieswandt, 2011) further understanding of how platelet receptors are regulated, together with the identification of novel platelet proteins will aid the development of anti-platelet drugs with a better therapeutic index. Furthermore, with the development of rapid and detailed phenotypic and genotypic analyses, there is also scope for tailoring therapy to the individual.
The aim of this review was to focus on rare, function-disrupting variants of platelet GPCRs and to discuss the significance of these findings in relation to GPCR structure/function and their potential implications for pharmacological therapy.
Platelet GPCRs and platelet activation pathways
There are a number of GPCRs present on the platelet cell surface including two purinergic receptors, P2Y1 and P2Y12; two proteinase-activated receptors, PAR1 and PAR4; the thromboxane A2 (TXA2) receptor, TP-α; the 5HT2A receptor; the prostacyclin receptor, IP1; a PGE2 receptor (EP3) and the α2A-adrenoceptor (Offermanns, 2006). There is also some evidence for the expression of other GPCRs in human platelets at the mRNA level although this is not backed by conclusive evidence either through pharmacological or protein expression studies (Rowley et al., 2011). Of the GPCRs present on the platelet surface the key receptors involved in platelet activation are PAR1, PAR4, P2Y1, P2Y12 and TP-α. Of these the P2Y12 receptor is an established anti-thrombotic drug target while the PAR1 antagonist, vorapaxar has recently been approved for use in a subset of patients with myocardial infarction and peripheral arterial disease (Poole and Elkinson, 2014).
Platelet activation is a multi-step process, consisting of platelet adhesion, shape change, granule secretion and aggregation. These processes are mediated by a number of cell surface receptors, including integrins and ion channels as well as GPCRs. Activation of these platelet surface receptors triggers multiple signalling cascades that synergize to bring about a rapid co-ordinated response to prevent excessive bleeding. The signalling events underlying platelet activation have been comprehensively reviewed elsewhere (Stegner and Nieswandt, 2011) and are only very briefly outlined here focussing on the contribution of GPCRs to this process. Platelet adhesion is initiated by binding of sub-endothelial von Willebrand factor (VWF) to platelet GPIbα, which causes transient interaction between platelets and the vasculature. Firm adhesion is then mediated by the collagen receptors, α2β1 and GPVI, activation of which promotes platelet spreading and aggregation. Furthermore, thrombin generated at the site of vascular injury activates PAR receptors causing further shape change, aggregation, TxA2 generation and secretion of platelet granule cargo. Positive feedback loops are initiated following ADP release from dense granules and TxA2 generation, which act via P2Y and TP-α receptors, respectively, to potentiate platelet activation. These platelet activation pathways converge to promote activation of the integrin αIIbβ3, which then is able to bind fibrinogen. Signalling via activated αIIbβ3 is the final step of platelet activation, and results in cytoskeletal reorganization and the formation of large, stable platelet aggregates, leading to the generation of stable thrombi (Watson et al., 2005; Rivera et al., 2009).
GPCRs as targets for anti-platelet drugs
GPCRs remain the most widely and successfully targeted proteins for therapeutics and a number of anti-platelet drugs act, directly or indirectly at GPCRs expressed on the platelet cell surface. Alongside aspirin, which reduces Tx generation and hence reduces TP-α receptor stimulation, P2Y12 antagonists such as clopidogrel and prasugrel remain frontline therapy for a variety of acute coronary syndromes. Interestingly, drugs that inhibit these ADP and Tx pathways have proved to be more successful anti-platelet drugs, with less adverse bleeding effects compared with integrin αIIbβ3 antagonists and PAR1 antagonists, which are associated with significant bleeding (Cox et al., 2000; Wiviott et al., 2011; Tricoci et al., 2012). This is probably because ADP and TxA2 are feedback mediators that contribute to activation via potentiation of other agonists. By contrast inhibition of PAR1 and αIIbβ3 integrin, which are activated by major platelet agonists, may cause a more significant inhibition of platelet activity and therefore an increased risk of bleeding.
Genetic variations associated with anti-platelet therapies targeting GPCRs
A key issue associated with both aspirin and P2Y12 antagonists is a high degree of variability in inter-patient responsiveness to drug therapy, and that a proportion of patients display ‘drug resistance’. For example, a third of patients taking clopidogrel are non-responsive as assessed by platelet function testing (Cattaneo, 2011b). This significant proportion of non-responders to aspirin and P2Y12 receptor antagonists has led to considerable research into the underlying genetic causes of these variations. In the case of aspirin, this has largely focused on COX-1 polymorphisms, a number of which have been associated with reduced responsiveness to aspirin (Feher et al., 2009). Clopidogrel resistance meanwhile is largely associated with poor metabolism of the prodrug to the active form.
Clopidogrel is a prodrug that requires metabolism by cytochrome P450 enzymes to form an active metabolite that subsequently binds irreversibly to the P2Y12 receptor. A number of studies have shown that clopidogrel does not effectively inhibit platelet activity in healthy volunteers and patients with acute coronary syndrome who have loss-of-function polymorphisms in CYP2C19 (Hulot et al., 2006; Giusti et al., 2007). Interestingly, loss-of-function variation in the cytochrome P450 2C19 gene (CYP2C19), caused by a single-nucleotide polymorphism (SNP) (rs4244285; c.681 G < A in NM_000769.1) within the coding region that creates a new splice site and thus a truncated and catalytically inactive protein, the CYP2C19*2 genotype, accounts for 12% of the variability in P2Y12 receptor inhibition seen with clopidogrel (Shuldiner et al., 2009). These variations in responsiveness led to the development of prasugrel, a structural analogue of clopidogrel. The hepatic metabolism of prasugrel, which produces the same active metabolite that irreversibly antagonizes the P2Y12 receptor, is less complex and therefore less susceptible to genetic variations in cytochrome P450 enzymes.
Responsiveness to anti-platelet agents is also affected by genetic variation in other key platelet genes, for example gain of function single-nucleotide variations have been described in platelet integrins (GP1α and αIIbβ3) (Cambria-Kiely and Gandhi, 2002). Therefore, platelet reactivity in the general population is significantly influenced by genetic differences in a number of genes that reduce drug responsiveness and potentially contribute to an increased risk of adverse cardiovascular events.
Genetic variation in platelet GPCRs
In addition to exploring common genetic variations within the general population, an alternative and successful approach to understanding pathophysiological disease mechanisms is the study of patients with bleeding disorders. For example, the description of Glanzmann's thrombasthenia (Glanzmann, 1918) led to the identification of the key platelet integrin, αIIbβ3 (Nurden and Caen, 1974). Similarly, the P2Y12 receptor was cloned in a study that described a patient with a bleeding history and a loss-of-function variation in the P2Y12R gene that encodes this receptor (Hollopeter et al., 2001). These examples illustrate how the study of rare function-disrupting variations affecting key platelet proteins can provide important information regarding platelet regulation and allow study of variant native proteins in vivo. This is especially important in platelets that are anucleate and therefore cannot be subjected to easy genetic approaches to change protein expression.
As part of the Genotyping and Phenotyping of Platelets consortium (GAPP), we have developed an approach for the rapid identification and characterization of rare genetic variations causing defects within platelet proteins, including GPCRs (Watson et al., 2010, 2013). This study has recruited a cohort of patients who attend UK haemophilia centres with symptoms of mild, lifelong bleeding, but who have no demonstrable defect in platelet number or coagulation factors. For each study subject, platelets were isolated from blood samples and were analysed by light transmission aggregation and secretion assays using a wide panel of agonists at different concentrations. Test results were compared with reference intervals for each agonist concentration determined by measurement of platelet aggregation and secretion in a cohort of healthy volunteer controls without bleeding symptoms (Dawood et al., 2012). The aim of this detailed analysis was to identify the defective signalling pathway responsible for a loss of platelet function, thus allowing targeted sequencing of a subset of genes that form the signalling pathway in which loss-of-function variants may underlie the platelet dysfunction. This approach has recently been validated following the description of a novel change in the HPS4 gene in a patient with reduced hair, eye and skin pigmentation, bleeding phenotype, and platelet dysfunction (Jones et al., 2012).
If a nucleotide variation is identified that predicts loss of protein function, then the structure-function of the variant protein is studied in detail in heterologous cell systems and by further analysis of platelets from the study subject. This approach has been highly successful in identifying loss-of-function variants in a number of GPCRs including the P2Y12 and TP-α receptors (detailed in Tables 2013a and 2013b), and has provided significant information regarding structure–function relationships of these important anti-thrombotic drug targets. The findings from these and other studies are discussed in further detail later.
Table 1.
Variants of TBXA2R
| Description | Variation in coding DNA | Inheritance | Region | Defect | Reference |
|---|---|---|---|---|---|
| Receptor deficiency | c.167dupG | Heterozygous | Reduced receptor expression | Kamae et al., 2011 | |
| R60L | c.179G<T | Homozygous or heterozygous | ICL1 | Reduced receptor coupling to Gq | Hirata et al., 1994 & Higuchi et al., 1999 |
| D304N | c.190G<A | Heterozygous | TMD7 | Reduced ligand binding | Mumford et al., 2010 |
| W29C | c.87G<C | Heterozygous | TMD1 | Reduced surface expression | Mumford et al., 2012 |
| N42S | c.125A<G | Heterozygous | TMD1 | Reduced surface expression | Nisar et al., submitted |
The numbering used to describe coding region variants relates to the Ref Seq transcript NM_001060.5.
Table 2.
Variants of P2RY12
| Description | Variation in coding DNA | Inheritance | Region | Defect | Reference |
|---|---|---|---|---|---|
| Receptor deficiency | c.717_718delCA | Heterozygous | no protein | No signalling via P2Y12 | Nurden et al. 1995 & Hollopeter et al. 2001 |
| Receptor deficiency | c.2T < G | Homozygous | no protein | No signalling via P2Y12 | Shiraga et al., 2005 |
| Receptor deficiency | c.378delC | Haploinsufficiency: only one variant P2RY12 allele detected | no protein | No signalling via P2Y12 | Fontana et al., 2009 |
| Partial receptor deficiency | none detected | Haploinsufficiency: only one WT P2RY12 allele detected | Reduced receptor function | Fontana et al., 2009 | |
| R256Q & R265W | c.767G < A; 793C < T | Compound heterozygous | TMD6; ECL3 | Reduced signalling via Gi | Cattaneo et al., 2003 |
| P258T | c.772C < A | Heterozygous | ECL3 | Reduced receptor function | Remijn et al., 2007 |
| K174E | c.520A < G | Heterozygous | ECL2 | Reduced ligand binding | Daly et al., 2009 |
| P341A | c.1021C < G | Heterozygous | C-term | Defective receptor trafficking | Nisar et al., 2011 |
| R122C | c.365G< | Homozygous | ICL2 DRY motif | Enhanced constitutive activity | Patel et al., 2014 |
Rare variants of the thromboxane receptor
Deficiency of the thromboxane TP receptor (MIM #614009) is inherited in an autosomal recessive or dominant manner and has been identified in several individuals from different kindreds who display mild mucocutaneous bleeding symptoms (Kamae et al., 2011). To date, one quantitative defect causing reduced TP-α receptor expression and four qualitative defects caused by TP receptor amino acid substitutions have been reported. These naturally occurring variants are listed in Table 2013a and the findings of these studies summarized later.
A nucleotide variation that caused loss of TP-α receptor expression was first described in a patient with a history of mucocutaneous bleeding (Kamae et al., 2011). Sequence analysis of TBXA2R in the patient and her father revealed that these individuals were heterozygous for a single-nucleotide duplication at c.167 (c.167dupG in NM_001060.5) resulting in a frame shift from amino acid 58. Corresponding cell lines studies showed that this caused significantly reduced receptor expression.
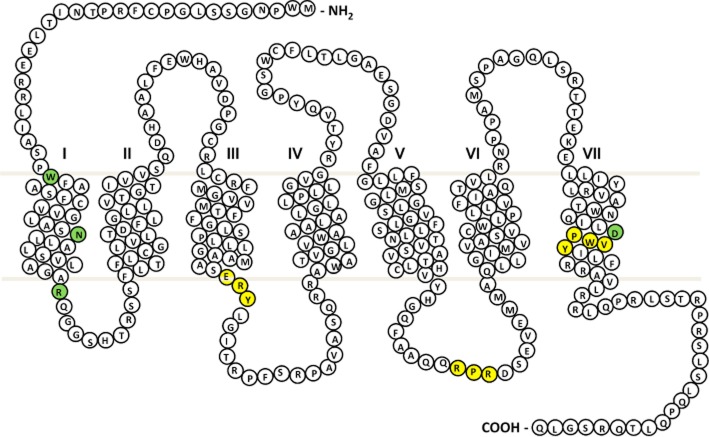
The first qualitative defect in the TP-α receptor caused by a missense nucleotide variation in the TBX2R gene was reported by Hirata et al. (1994). This resulted from an Arg60Leu amino acid substitution at the start of the first intracellular loop (ICL) (Figure 1) and occurred in a patient with a history of post-surgical bleeding (Hirata et al., 1994), but has also since been described in another kindred with a history of mild bleeding (Fuse et al., 1996; Higuchi et al., 1999). The platelets from these affected individuals show absent or reduced aggregation to the synthetic TxA2 analogue U46619. In patients homozygous for the Arg60Leu amino acid substitution, the defect in aggregation was also accompanied by a reduction in TxA2-induced IP3 generation and Ca2+ mobilization. Interestingly, heterozygous Arg60Leu patients whose platelets also showed reduced aggregation to TP-α receptor agonists, showed apparently normal calcium mobilization, suggesting a possible additional pro-aggregatory effect of TP-α receptor activation that is independent of calcium signalling. Initial expression studies in CHO cells showed that co-expression of both the wild-type (WT) receptor with the variant Arg60Leu TP-α receptor was associated with a reduction in Ca2+ mobilization although the signalling ability of the variant receptor alone was not evaluated (Hirata et al., 1994). These conflicting data in cell lines and human platelets merit further investigation. More recent studies have shown that when expressed alone, the Arg60Leu TP-α receptor variant has dramatically attenuated receptor responses when compared with WT, despite comparable ligand-binding affinities and receptor surface expression (Chakraborty et al., 2013). Molecular modelling indicates that Arg60 interacts via hydrogen bonds with Met126 and Arg130 in transmembrane domain (TMD) 3, and that this interaction is lost when the Arg is substituted for Leu (Chakraborty et al., 2013). Importantly, Arg130 is part of the D/ERY (Asp/Glu-Arg-Try) motif (Figure 1), which is highly conserved among GPCRs (Rovati et al., 2007) and is critical for receptor activation. Therefore, it is likely that the variant Arg60Leu TP-α receptor is unable to undergo the required conformational changes required to promote efficient G-protein coupling. This recent modelling data correlate with previous mutagenesis studies of the ERY motif within TP-α receptors, which confirmed the involvement of Arg130 in receptor coupling to Gq (Capra et al., 2004). Mutation of the Arg130 to Val resulted in a loss in inositol phosphate accumulation in response to U46619, a consequence of defective PLC activation via Gq.
Figure 1.
TxA2 (TP-α) receptor snake plot. Sites of naturally occurring variants found in patients with a bleeding history are highlighted in green. Key amino acid regulatory motifs are highlighted in yellow (specifically RxR ER retention motif; D/NPXXY motif, E/DRY motif).
The remaining TBXA2R gene rare variants causing amino acid substitutions in the TP-α receptor protein sequence (Table 2013a) have been described using the GAPP approach outlined above. In 2010, Mumford et al. identified a patient with a history of bruising and prolonged epistaxes since infancy (Mumford et al., 2010). Platelet aggregation and secretion were reduced in response to arachidonic acid and U46619, whereas responses to other agonists were within normal range. Sequencing of the TBXA2R gene showed that the patient was heterozygous for a c.190G < A variation predicting an Asp304Asn substitution in the seventh TMD of the receptor (Figure 1). Ligand-binding studies in platelets revealed a 50% reduction in maximal binding to the variant Asp304Asn TP-α receptor compared with WT, without a change in binding affinity. Further studies in CHO cells also showed that the variant Asp304Asn TP-α receptor had a significantly impaired ability to bind radioligand despite expression at the cell surface being comparable with WT. These observations suggested that the reduction in TxA2-mediated platelet activation in the patient may be due to impaired ligand binding. Interestingly, this Asp304Asn substitution occurred in the highly conserved NPXXY motif (Figure 1), where the Asn at position 1 is substituted for Asp in 21% of class A GPCRs (Mirzadegan et al., 2003). High-resolution structural studies suggest that this motif, which is located near the cytoplasmic end of TMD7, interacts with a network of water molecules to weakly stabilize the inactive state of the receptor and also allows rapid conformational changes to occur for activation (Rosenbaum et al., 2009).
The observation that an Asn residue at position 1 of the NPXXY motif (P 7.49 as identified by Ballesteros–Weinstein numbering) might suggest that the substitution of Asp to Asn observed in this study would not be function disrupting. However, data from two separate mutagenesis studies suggest that the Asp and Asn are not interchangeable. In the human gonadotrophin-releasing hormone receptor (GnRH), an Asp318Asn mutation impairs G-protein coupling (Zhou et al., 1994). In addition, Johnson and co-workers showed that there were differences in binding to ADP-ribosylation factor isoforms among GPCRs that possess the NPXXY versus the DPXXY motif (Johnson et al., 2006). It is unclear why the Asp304Asn substitution causes a decrease in ligand binding as the majority of studies of variations within the NPXXY motif have found differences in receptor activation. The GnRH Asp318Asn substitution showed no alterations in ligand binding (Zhou et al., 1994), which suggests that this effect may be specific for the Tx receptor, although there is some evidence that residue 7.49 (Ballesteros–Weinstein numbering) interacts via hydrogen bonding with the ligand-binding pocket (Li et al., 2004; Pardo et al., 2007).
More recently, two further function-disrupting TBXA2R gene variations have been identified, both predicting amino acid substitutions within the TMD1 (Figure 1) (Mumford et al., 2013; Nisar et al., 2014). Interestingly, both these variations reduce TP-α receptor expression at the cell surface, suggesting an important role for TMD1 in the regulation of anterograde receptor traffic. The Trp29Cys substitution was identified in a patient who displayed abnormal post-surgical bleeding and whose platelets showed reduced aggregation and secretion in response to arachidonic acid and U46619. Ligand-binding studies in both patient platelets and in HEK293 cells expressing the variant receptor showed a reduction in Bmax and Kd, indicating a reduction in receptor surface expression and ligand-binding affinity. Further studies showed no change in total receptor expression, but a significant reduction in cell surface expression, which was accompanied by a reduced ability to signal via Gq. The Trp29 residue (1.37 Ballesteros–Weinstein numbering), alongside other residues within TMD1 has previously been shown to be important for the formation of heterodimers between TP-α and the alternative receptor isoform TP-β (Fanelli et al., 2011). Interestingly, this study showed that a variant TP-α receptor in which a number of key residues within TMD1 (including Trp29 in combination with Ile25, Cys35, Val36, Leu39, Leu43, Leu44 and Ser47) were replaced with alanine, resulted in reductions in receptor signalling and ligand binding that were comparable with the Trp29Cys variant reported by Mumford and colleagues. Taken together, these findings suggest that Trp29 of the TP-α receptor is a key residue contributing to surface expression seen with the TMD1 mutant studied by Fanelli and co-workers, possibly by reducing the ability of TP-α to form functional dimers at the cell membrane. While expression of TP-β in platelets at the protein level is not clear, the first 343 residues are shared between the two isoforms, it is therefore likely the same residues are involved in homodimer formation. Although the ability of the Trp29Cys variant receptor to dimerize was not directly studied in the Mumford et al. (2012) study, subsequent work has shown that the variant Trp29Cys TP-α receptor does not interact with the WT TP-α at the cell surface (S.P. Nisar and S.J. Mundell, unpubl. obs.).
A further variant of the TP-α receptor, Asn42Ser, has also been described (Nisar et al., 2014). Like the Trp29Cys variant, this substitution also occurs within TMD1 and results in reduced surface receptor expression. This patient had a history of menorrhagia, excessive post-operative bleeding and easy bruising, a phenotype indicative of a platelet-function disorder. The Asn42 residue (1.5 Ballesteros–Weinstein numbering) is the most conserved residue in class A GPCRs and is involved in hydrogen bonding with Gly51, Ala299 and Asp83 in bovine rhodopsin (Smith, 2010), all of which are conserved in the human TP-α receptor. Cell line studies show that the Asn42Ser variant TP-α receptor is retained intracellularly, probably in a trans Golgi network (TGN)/ER compartment. Therefore, it is likely that interactions with the Asn42 residue are required for correct processing and transport of this receptor to the cell surface. Another notable feature of the protein sequence of the TP-α receptor is the potential presence of an arginine-based ER retention motif (RxR; Figure 1) within ICL3 of the receptor. These motifs regulate anterograde traffic of proteins including GPCRs (Michelsen et al., 2005; Cunningham et al., 2012). The predominantly intracellular localization of this Asn42Ser variant and the ability of this site to interact with other amino acids through hydrogen bonding mean that this substitution has the potential to affect conformational rearrangement of the receptor. Such rearrangement may expose motifs such as the RxR motif present in ICL3, which may otherwise be masked in native WT form. The consequence of unmasking through conformational rearrangement may result in impaired export from the ER to the plasma membrane, as observed for the Asn42Ser variant. This remains to be explored in more detail.
The description of the Asn42Ser mutation takes the number of TP receptor variants identified in patients with abnormal bleeding to a total of five, indicating that these remain rare contributors to bleeding risk even in selected populations. However, study and characterization of these mutants have provided key insights into TP receptor structure/function and in particular, highlighted the role of TMD1 in the regulation of TP cell surface expression.
Rare variants of the P2Y12 receptor
P2Y12 receptor deficiency (MIM #609821) has been identified as an autosomal dominant or recessive disorder characterized by mild to moderate mucocutaneous bleeding and excessive bleeding in response to trauma or after surgery (Cattaneo et al., 2003). The first description of genetic variation in P2RY12 occurred in the report describing the cloning of the gene and was a heterozygous dinucleotide deletion within the coding region (c.717_718delCA) (Hollopeter et al., 2001) in a previously described patient (Nurden et al., 1995), which resulted in a significant loss of P2RY12 expression. Platelets from patients who are heterozygous for a variation that causes loss of P2Y12 receptor expression (see Table 2013b) display reduced and reversible aggregation to ADP and reduced aggregation to submaximal concentrations of other agonists. Platelet secretion is also reduced because of the positive feedback role of P2Y12 in amplification. Therefore, the phenotype is similar to the effects seen in patients with a primary secretion defect. A number of further patients have now been described with P2Y12 receptor deficiency, which have been the subject of several comprehensive reviews (Cattaneo, 2011a,c) and are summarized in Table 2013b.
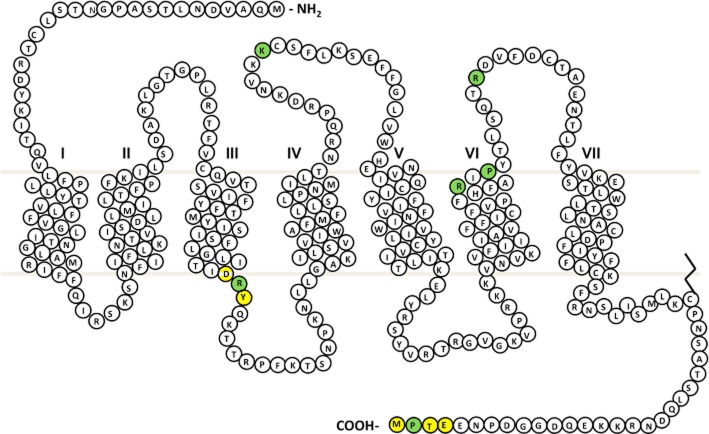
The first P2Y12 receptor defect that caused an alteration in receptor function (as opposed to absent expression) was reported by Cattaneo and colleagues who described an individual who was compound heterozygous for two amino acid substitutions (Arg256Gln and Arg265Trp) in the sixth TMD and the extracellular loop 3 (ECL3) of the P2Y12 receptor respectively (Cattaneo et al., 2003) (Figure 2). The patient had a lifelong history of easy bruising and excessive post-surgical bleeding. Platelet studies showed reduced and reversible aggregation to high concentrations of ADP, while shape change remained intact. Signalling studies showed a reduced ability to inhibit PGE1-induced cAMP generation despite normal ligand binding. Further analysis was also carried out on the two children of the index case, both of whom were heterozygous only for the Arg265Trp substitution. Interestingly, these showed a reduction in aggregation and signalling at low concentrations of ADP, but at higher ADP concentrations, there was no difference compared with healthy controls. Cell line studies, in which the variant Arg256Gln and Arg265Trp P2Y12 receptors were individually expressed in CHO cells, revealed that both amino acid substitutions caused a similar reduction in receptor activation. In subsequent studies, in cell line models, the Arg256 residue has also been shown to be important for ligand interactions and antagonist recognition (Hoffmann et al.,2008;2009; Mao et al., 2010; Chen et al., 2011; Schmidt et al., 2013). The study by Mao and co-workers showed that both the variant Arg256Gln and Arg265Trp P2Y12 receptor expressed in CHO cells were significantly more sensitive to blockade by the P2Y12 antagonist AR-C69931MX (cangrelor) than the WT receptor. Furthermore, the recent description of the P2Y12 crystal structure also showed that Arg256, alongside other residues, forms the ligand-binding pocket and is involved in both antagonist and agonist binding (Zhang et al., 2014a,b). The discovery of genetic variants that result in alterations in sensitivity to receptor blockade is clearly advantageous to aiding rational drug design.
Figure 2.
P2Y12 receptor snake plot. Sites of naturally occurring variants found in patients with a bleeding history are highlighted in green. Key amino acid regulatory motifs are highlighted in yellow (specifically E/DRY motif and type 1 PDZ ligand).
A similar platelet phenotype was observed by Remijn and co-workers who reported a Pro258Thr substitution in the P2Y12 receptor in a patient with a history of epistaxis, easy bruising and post-traumatic bleeding (Remijn et al., 2007). This patient's platelets also showed reduced and reversible aggregation in response to high ADP concentrations; however, the exact mechanism by which this the Pro258Thr change within the third extracellular loop (Figure 2) affects P2Y12 receptor function is not known.
Two further amino acid substitutions in P2Y12 were identified in a cohort of patients who had been previously been recruited to the European Molecular and Clinical Markers for the Diagnosis and Management of type 1 von Willebrand Disease (MCMDM-1VWD) study. Type 1 VWD is caused by a quantitative reduction in the plasma concentration of VWF, which is required for platelet adhesion. The bleeding symptoms associated with type 1 VWD are indistinguishable from those seen in patients with mild platelet function disorders and show significant inter-individual variation, even among members of the same family. Data from the MCMDM-1VWD study have confirmed the contribution of genetic loci outside of the VWF locus to the pathogenesis of bleeding in type 1 VWD (Goodeve et al., 2007). This raises the possibility that loss-of-function variations in genes that affect platelet function may contribute to the severity of bleeding in some individuals with type I VWD.
In order to test this hypothesis, P2Y12R from the MCMDM-1VWD index cases and geographically matched healthy controls were sequenced as part of the GAPP study. The first of the two variant receptors identified was a Lys174Glu substitution in the second extracellular loop of the P2Y12 receptor (Daly et al., 2009). Platelets from the patient and two related individuals showed reduced aggregation and secretion to ADP. Ligand-binding studies revealed a significant reduction in the ability of the variant P2Y12 Lys174Glu receptor to bind radioligand compared with WT receptor, while total and surface expression were not affected. The Lys174 residue is adjacent to a cysteine residue (Cys175; Figure 2), which has previously been shown to be involved in ligand binding (Costanzi et al., 2004; Savi et al., 2006). Interestingly, the P2Y12 crystal structure suggests that Lys174 does not directly interact with the agonist, but instead stabilizes the agonist-bound conformation via the formation of a salt bridge with Glu273 in the TMD7 (Zhang et al., 2014a).
A further patient from the MCMDM-1VWD cohort was found to be heterozygous for a Pro341Ala substitution in the extreme C terminus of the P2Y12 receptor. Platelet studies on the mother of the patient, who did not have VWD, but was heterozygous for the Pro341Ala P2Y12 receptor substitution, revealed a reduced ability to signal via Gi at low ADP concentrations and a reduction in maximal P2Y12 ligand binding (Nisar et al., 2011). Although there was no effect on aggregation to ADP or other agonists, further studies revealed that the variant receptor was unable to correctly recycle back to the surface following receptor stimulation and internalization. Studies in cells transfected with the variant Pro341Ala P2Y12 receptor revealed that the receptor was retained intra-cellularly in the TGN and Rab–7-positive compartments, and was not able to recycle and resensitize following agonist exposure (Nisar et al., 2011; Cunningham et al., 2013). The description of the Pro341Ala substitution was particularly interesting because of its location within the PDZ ligand of the P2Y12 receptor (Figure 2). This PDZ ligand consists of a short amino acid sequence that is found at the extreme C terminus of a large number of proteins including more than 30 GPCRs (Marchese et al., 2008). GPCR PDZ ligands can bind to a number of PDZ domain-containing proteins and these interactions regulate various aspects of receptor regulation including stabilization, signalling and trafficking (Weinman et al., 2006). Indeed, the P2Y12 receptor has been shown to interact basally with the NHERF1 in a PDZ-dependent manner (Nisar et al., 2012). Despite a vast number of cell line studies that have highlighted the importance of PDZ interactions, this was the first report of a naturally occurring mutation that demonstrated the in vivo importance of a GPCR PDZ ligand.
More recently, the in vivo importance of the P2Y12 DRY motif was also demonstrated by the description of a naturally occurring variant Arg122Cys P2Y12 receptor identified in a patient who had a lifelong history of spontaneous bleeding, and haemorrhage upon surgical challenge (Patel et al., 2014). Importantly, this patient was homozygous for the Arg122Cys substitution in the P2Y12 receptor and displayed significantly reduced platelet aggregation in response to ADP as a result of reduced receptor expression at the platelet cell surface. Further analysis in cell lines suggested that this was due to enhanced agonist-independent receptor internalization and accumulation in lysosomes. The DRY motif (Figure 2) is a highly conserved region found in almost all GPCRs and is known to play a critical role in regulating receptor conformational states. The recent resolution of the P2Y12 crystal structure shows that P2Y12 is atypical for a class A GPCR because of the absence of interactions between the DRY motif and helix VI, which may explain high constitutive receptor activity (Zhang et al., 2014b). Data from the R122C study suggest that disruption of this motif further prevents stabilization of the P2Y12 receptor in an inactive conformation in the absence of agonist, resulting in increased constitutive internalization (Patel et al., 2014). Interestingly, platelet phenotyping of this patient along with related family members also showed a reduction in PAR1 peptide-induced platelet activation (Patel et al., 2014). Subsequent sequencing analysis of the F2R gene, which encodes the PAR1 receptor found that the patient was also homozygous for a SNP – PAR1 rs168753 – which had previously been shown to reduce PAR1 expression in platelets (Dupont et al., 2003; Smith et al., 2005). This is the first description of two GPCRs being affected by loss-of-function mutations that combine to reduce platelet activity. As in the cohort of VWD patients described earlier, this demonstrates the multifactorial nature of mild bleeding disorders.
Genetic variants of other platelet GPCRs
To date, there have been no reported function-disrupting rare variants for the P2Y1, PAR1 and PAR4 receptors, although common population variations in the genes encoding the PAR1 and P2Y1 receptors have been associated with weak alterations in platelet activity. For example, the rs168753 variation in F2R, that encodes the PAR1 receptor, is associated with reduced platelet activity. The P2Y1 SNP (1622 G/G genotype) has been associated with a reduced anti-platelet effect of aspirin (Lordkipanidze et al., 2011). The GAPP consortium has found a single missense PAR1 rare variant and one missense PAR4 variant (J. Stockley et al., unpublished) although no change in receptor function has yet been detected. The lack of rare mutations in these receptors (that are widely expressed and have key non-platelet functions) suggests that substitutions that significantly alter function of these receptors may be incompatible with life. An inherited defect in the α2A-adrenoceptor that is associated with reduced receptor aggregation to adrenaline has been described (Rao et al., 1988); however, the causative gene defect is unknown.
There are also a number of inhibitory GPCRs expressed on the platelet surface, such as the prostanoid receptors, (EP2 and IP) and the adenosine A2A receptor. These receptors couple to Gs, raise intracellular cAMP levels and inhibit platelet activation via PKA. Although variants of the relevant gene (ADORA2A) have been associated with a variety of diseases (Deckert et al., 1996; Hohoff et al., 2007), loss-of-function changes in inhibitory receptors are unlikely to contribute to an increased bleeding risk. However, cAMP is a vital regulatory pathway in platelets and loss-of-function mutations in genes involved in this pathway could contribute to increased platelet reactivity and therefore increased risk of adverse cardiovascular events.
Implications of receptor mutations for mild bleeding
While these studies have provided useful information regarding receptor function, it is important to note that the presence of a heterozygous variation in a GPCR gene does not always correlate with a clinical bleeding phenotype. For example, in the previously reported kindreds with mutations in the TP-α receptors due to Asp304Asn and Trp29Cys substitutions, the index cases presented with mild bleeding. However, in both kindreds, there were other first-degree relatives who were also heterozygous for these loss-of-function substitutions, but were asymptomatic (Mumford et al., 2010, 2012). Similarly, patients with P2Y12 receptor deficiency display bleeding symptoms of variable severity that does not correlate well with genotype, or the presence of homozygous versus heterozygous changes. These findings highlight the multifactorial nature of mild bleeding symptoms and suggest that there may be further unidentified defects in the index cases of these kindreds that contribute to the bleeding diathesis. For example the index case with the Pro341Ala P2Y12 receptor substitution also had type 1 VWD and a more severe bleeding history than his mother who had an identical platelet P2Y12 receptor defect, but did not have VWD (Nisar et al., 2011). The requirement for a closed high-pressure cardiovascular system for life necessitates multiple pathways for haemostasis with a high degree of redundancy. Thus, while studies using a detailed phenotyping approach combined with targeted genotyping (Dawood et al., 2012), such as described earlier can be used to diagnose platelet function disorders, wider speculation on the causes of bleeding should be avoided in the absence of whole genome sequencing and validation of various non-platelet genes that are involved in haemostasis.
Discussion and future perspectives
Variation in GPCR genes results in the disruption of receptor function in a wide variety of human genetic diseases, including mild platelet bleeding disorders. The description and characterization of a number of rare TP-alpha and P2Y12 receptor variants has provided key insights into the physiological significance of GPCR subdomains. Although there have been a number of studies that have undertaken mutation analysis of these receptors, these naturally occurring variants provide the opportunity to study the effect of changes such as amino acid substitutions in endogenously expressed receptors in human platelets. Platelets have a clear advantage over other cell types as they can be taken repeatedly and with relative ease from patients and family members. While murine knock-out models have been important for delineating roles of individual surface receptors in platelet activation and in vivo thrombosis, more subtle study of receptor function at the molecular level in platelets is associated with a number of technical challenges. For example, there is an inherent lack of receptor-specific antibodies able to detect receptor expression. Furthermore, there are often difficulties in translating findings in cell expression studies into platelets because of differences in cell machinery, membrane trafficking and receptor expression levels.
Importantly the rare receptor variants described earlier validate findings found in the vast number of cell expression and modelling studies that have previously assigned structure–function relationships to particular receptor motifs. For example, rare receptor variants that disrupt the NPXXXY, PDZ and DRY motifs (Figures 2) have all been shown to affect endogenous receptor function in platelets. The description of these rare variants that affect ligand binding, surface expression, G-protein coupling and intracellular trafficking highlights the critical importance of each of these processes upon GPCR function, and for the P2Y12 and TP-α receptors, maps these functions to specific domains and residues. This is significant because these receptors remain at the forefront of anti-platelet drug therapy. The descriptions of variant receptors such as the Arg256Gln/ Arg265Trp P2Y12 receptor that affect antagonist sensitivity may aid targeted rational drug design in this area. Recent data from phase III clinical trials of PAR1 antagonists (Capodanno et al., 2012; Tricoci et al., 2012) confirm the need for more subtle approaches to combat the bleeding associated with anti-platelet therapy. Greater insight into how platelet GPCRs are regulated may also provide novel mechanisms to prevent excessive platelet activation while preserving haemostasis.
Genome-wide analysis has shown that variants of platelet receptors, including ADRA2 and P2Y12R, are associated with increased platelet reactivity in the normal population (Jones et al., 2009; Johnson et al., 2010), a finding that has confirmed previous candidate gene studies (Kunicki et al., 2012). Recent advances in second-generation sequencing technologies mean that large numbers of individuals can be rapidly tested for genetic changes in their whole exome or through pre-selected candidate genes (Jones et al., 2012). However, it is clear that an integrated approach involving detailed phenotyping and cell validation studies are required in order to truly dissect out and then demonstrate meaningful changes in platelet activity. The study of patients with mild bleeding has been highly successful in identifying a number of rare GPCR mutants that affect receptor function. It is hoped that the extension of this candidate gene-led approach to whole exome analysis will provide further information regarding known platelets genes and to identify novel regulators of platelet function.
Acknowledgments
We would like to thank Professors Steve Watson and Eamonn Kelly for critical reading of the manuscript.
Glossary
- ECL
extracellular loop
- ICL
intracellular loop
- TMD
transmembrane domain
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic Receptors. Br J Pharmacol. 2013b;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambria-Kiely JA, Gandhi PJ. Aspirin resistance and genetic polymorphisms. J Thromb Thrombolysis. 2002;14:51–58. doi: 10.1023/a:1022066305399. [DOI] [PubMed] [Google Scholar]
- Capodanno D, Bhatt DL, Goto S, O'Donoghue ML, Moliterno DJ, Tamburino C, et al. Safety and efficacy of protease-activated receptor-1 antagonists in patients with coronary artery disease: a meta-analysis of randomized clinical trials. J Thromb Haemost. 2012;10:2006–2015. doi: 10.1111/j.1538-7836.2012.04869.x. [DOI] [PubMed] [Google Scholar]
- Capra V, Veltri A, Foglia C, Crimaldi L, Habib A, Parenti M, et al. Mutational analysis of the highly conserved ERY motif of the thromboxane A2 receptor: alternative role in G protein-coupled receptor signaling. Mol Pharmacol. 2004;66:880–889. doi: 10.1124/mol.104.001487. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. Bleeding manifestations of congenital and drug-induced defects of the platelet P2Y12 receptor for adenosine diphosphate. Thromb Haemost. 2011a;105(Suppl. 1):S67–S74. doi: 10.1160/THS10-11-0742. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. The clinical relevance of response variability to antiplatelet therapy. Hematology Am Soc Hematol Educ Program. 2011b;2011:70–75. doi: 10.1182/asheducation-2011.1.70. [DOI] [PubMed] [Google Scholar]
- Cattaneo M. The platelet P2Y(1)(2) receptor for adenosine diphosphate: congenital and drug-induced defects. Blood. 2011c;117:2102–2112. doi: 10.1182/blood-2010-08-263111. [DOI] [PubMed] [Google Scholar]
- Cattaneo M, Zighetti ML, Lombardi R, Martinez C, Lecchi A, Conley PB, et al. Molecular bases of defective signal transduction in the platelet P2Y12 receptor of a patient with congenital bleeding. Proc Natl Acad Sci U S A. 2003;100:1978–1983. doi: 10.1073/pnas.0437879100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty R, Pydi SP, Gleim S, Bhullar RP, Hwa J, Dakshinamurti S, et al. New insights into structural determinants for prostanoid thromboxane A2 receptor- and prostacyclin receptor-G protein coupling. Mol Cell Biol. 2013;33:184–193. doi: 10.1128/MCB.00725-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Dong X, Zhou M, Shi H, Luo X. Docking-based virtual screening of potential human P2Y12 receptor antagonists. Acta Biochim Biophys Sin (Shanghai) 2011;43:400–408. doi: 10.1093/abbs/gmr023. [DOI] [PubMed] [Google Scholar]
- Costanzi S, Mamedova L, Gao ZG, Jacobson KA. Architecture of P2Y nucleotide receptors: structural comparison based on sequence analysis, mutagenesis, and homology modeling. J Med Chem. 2004;47:5393–5404. doi: 10.1021/jm049914c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Smith R, Quinn M, Theroux P, Crean P, Fitzgerald DJ. Evidence of platelet activation during treatment with a GPIIb/IIIa antagonist in patients presenting with acute coronary syndromes. J Am Coll Cardiol. 2000;36:1514–1519. doi: 10.1016/s0735-1097(00)00919-0. [DOI] [PubMed] [Google Scholar]
- Cunningham MR, McIntosh KA, Pediani JD, Robben J, Cooke AE, Nilsson M, et al. Novel role for proteinase-activated receptor 2 (PAR2) in membrane trafficking of proteinase-activated receptor 4 (PAR4) J Biol Chem. 2012;287:16656–16669. doi: 10.1074/jbc.M111.315911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MR, Nisar SP, Cooke AE, Emery ED, Mundell SJ. Differential endosomal sorting of a novel P2Y(12) purinoreceptor mutant. Traffic. 2013;14:585–598. doi: 10.1111/tra.12054. [DOI] [PubMed] [Google Scholar]
- Daly ME, Dawood BB, Lester WA, Peake IR, Rodeghiero F, Goodeve AC, et al. Identification and characterization of a novel P2Y 12 variant in a patient diagnosed with type 1 von Willebrand disease in the European MCMDM-1VWD study. Blood. 2009;113:4110–4113. doi: 10.1182/blood-2008-11-190850. [DOI] [PubMed] [Google Scholar]
- Dawood BB, Lowe GC, Lordkipanidze M, Bem D, Daly ME, Makris M, et al. Evaluation of participants with suspected heritable platelet function disorders including recommendation and validation of a streamlined agonist panel. Blood. 2012;120:5041–5049. doi: 10.1182/blood-2012-07-444281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert J, Nothen MM, Rietschel M, Wildenauer D, Bondy B, Ertl MA, et al. Human adenosine A2a receptor (A2aAR) gene: systematic mutation screening in patients with schizophrenia. J Neural Transm. 1996;103:1447–1455. doi: 10.1007/BF01271259. [DOI] [PubMed] [Google Scholar]
- Dupont A, Fontana P, Bachelot-Loza C, Reny JL, Bieche I, Desvard F, et al. An intronic polymorphism in the PAR-1 gene is associated with platelet receptor density and the response to SFLLRN. Blood. 2003;101:1833–1840. doi: 10.1182/blood-2002-07-2149. [DOI] [PubMed] [Google Scholar]
- Fanelli F, Mauri M, Capra V, Raimondi F, Guzzi F, Ambrosio M, et al. Light on the structure of thromboxane A(2) receptor heterodimers. Cell and Mol Life Sci. 2011;68:3109–3120. doi: 10.1007/s00018-010-0615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher G, Feher A, Pusch G, Lupkovics G, Szapary L, Papp E. The genetics of antiplatelet drug resistance. Clin Genet. 2009;75:1–18. doi: 10.1111/j.1399-0004.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- Fontana G, Ware J, Cattaneo M. Haploinsufficiency of the platelet P2Y12 gene in a family with congenital bleeding diathesis. Haematologica. 2009;94:581–584. doi: 10.3324/haematol.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse I, Hattori A, Mito M, Higuchi W, Yahata K, Shibata A, et al. Pathogenetic analysis of five cases with a platelet disorder characterized by the absence of thromboxane A2 (TXA2)-induced platelet aggregation in spite of normal TXA2 binding activity. Thromb Haemost. 1996;76:1080–1085. [PubMed] [Google Scholar]
- Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, et al. Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10 + 12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet Genomics. 2007;17:1057–1064. doi: 10.1097/FPC.0b013e3282f1b2be. [DOI] [PubMed] [Google Scholar]
- Glanzmann E. Hereditare hamorrhagische thrombasthenie: ein beitrag zur pathologie der blut plattchen. J Kinderkr. 1918;88:113–141. [Google Scholar]
- Goodeve A, Eikenboom J, Castaman G, Rodeghiero F, Federici AB, Batlle J, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, Molecular and Clinical Markers for the Diagnosis and Management of Type 1 von Willebrand Disease (MCMDM-1VWD) Blood. 2007;109:112–121. doi: 10.1182/blood-2006-05-020784. [DOI] [PubMed] [Google Scholar]
- Higuchi W, Fuse I, Hattori A, Aizawa Y. Mutations of the platelet thromboxane A2 (TXA2) receptor in patients characterized by the absence of TXA2-induced platelet aggregation despite normal TXA2 binding activity. Thromb Haemost. 1999;82:1528–1531. [PubMed] [Google Scholar]
- Hirata T, Kakizuka A, Ushikubi F, Fuse I, Okuma M, Narumiya S. Arg60 to Leu mutation of the human thromboxane A2 receptor in a dominantly inherited bleeding disorder. J Clin Invest. 1994;94:1662–1667. doi: 10.1172/JCI117510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K, Sixel U, Di Pasquale F, von Kugelgen I. Involvement of basic amino acid residues in transmembrane regions 6 and 7 in agonist and antagonist recognition of the human platelet P2Y(12)-receptor. Biochem Pharmacol. 2008;76:1201–1213. doi: 10.1016/j.bcp.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Hoffmann K, Baqi Y, Morena MS, Glanzel M, Muller CE, von Kugelgen I. Interaction of new, very potent non-nucleotide antagonists with Arg256 of the human platelet P2Y12 receptor. J Pharmacol Exp Ther. 2009;331:648–655. doi: 10.1124/jpet.109.156687. [DOI] [PubMed] [Google Scholar]
- Hohoff C, Marziniak M, Lesch KP, Deckert J, Sommer C, Mossner R. An adenosine A2A receptor gene haplotype is associated with migraine with aura. Cephalalgia. 2007;27:177–181. doi: 10.1111/j.1468-2982.2007.01254.x. [DOI] [PubMed] [Google Scholar]
- Hollopeter G, Jantzen HM, Vincent D, Li G, England L, Ramakrishnan V, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Yanek LR, Chen MH, Faraday N, Larson MG, Tofler G, et al. Genome-wide meta-analyses identifies seven loci associated with platelet aggregation in response to agonists. Nat Genet. 2010;42:608–613. doi: 10.1038/ng.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MS, Robertson DN, Holland PJ, Lutz EM, Mitchell R. Role of the conserved NPxxY motif of the 5-HT2A receptor in determining selective interaction with isoforms of ADP-ribosylation factor (ARF) Cell Signal. 2006;18:1793–1800. doi: 10.1016/j.cellsig.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Jones CI, Bray S, Garner SF, Stephens J, de Bono B, Angenent WG, et al. A functional genomics approach reveals novel quantitative trait loci associated with platelet signaling pathways. Blood. 2009;114:1405–1416. doi: 10.1182/blood-2009-02-202614. [DOI] [PubMed] [Google Scholar]
- Jones ML, Murden SL, Bem D, Mundell SJ, Gissen P, Daly ME, et al. Rapid genetic diagnosis of heritable platelet function disorders with next-generation sequencing: proof-of-principle with Hermansky–Pudlak syndrome. J Thromb Haemost. 2012;10:306–309. doi: 10.1111/j.1538-7836.2011.04569.x. [DOI] [PubMed] [Google Scholar]
- Kamae T, Kiyomizu K, Nakazawa T, Tadokoro S, Kashiwagi H, Honda S, et al. Bleeding tendency and impaired platelet function in a patient carrying a heterozygous mutation in the thromboxane A2 receptor. J Thromb Haemost. 2011;9:1040–1048. doi: 10.1111/j.1538-7836.2011.04245.x. [DOI] [PubMed] [Google Scholar]
- Kaplan ZS, Jackson SP. The role of platelets in atherothrombosis. Hematology Am Soc Hematol Educ Program. 2011;2011:51–61. doi: 10.1182/asheducation-2011.1.51. [DOI] [PubMed] [Google Scholar]
- Kunicki TJ, Williams SA, Nugent DJ. Genetic variants that affect platelet function. Curr Opin Hematol. 2012;19:371–379. doi: 10.1097/MOH.0b013e3283567526. [DOI] [PubMed] [Google Scholar]
- Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. J Mol Biol. 2004;343:1409–1438. doi: 10.1016/j.jmb.2004.08.090. [DOI] [PubMed] [Google Scholar]
- Lordkipanidze M, Diodati JG, Palisaitis DA, Schampaert E, Turgeon J, Pharand C. Genetic determinants of response to aspirin: appraisal of 4 candidate genes. Thromb Res. 2011;128:47–53. doi: 10.1016/j.thromres.2011.02.019. [DOI] [PubMed] [Google Scholar]
- Mao Y, Zhang L, Jin J, Ashby B, Kunapuli SP. Mutational analysis of residues important for ligand interaction with the human P2Y(12) receptor. Eur J Pharmacol. 2010;644:10–16. doi: 10.1016/j.ejphar.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelsen K, Yuan H, Schwappach B. Hide and run. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep. 2005;6:717–722. doi: 10.1038/sj.embor.7400480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadegan T, Benko G, Filipek S, Palczewski K. Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry. 2003;42:2759–2767. doi: 10.1021/bi027224+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford A, Nisar S, Darnige L, Jones M, Bachelot-Loza C, Gandrille S, et al. Platelet dysfunction associated with the novel Trp29Cys thromboxane A(2) receptor variant. J Thromb Haemost. 2012;11:547–554. doi: 10.1111/jth.12117. [DOI] [PubMed] [Google Scholar]
- Mumford AD, Dawood BB, Daly ME, Murden SL, Williams MD, Protty MB, et al. A novel thromboxane A2 receptor D304N variant that abrogates ligand binding in a patient with a bleeding diathesis. Blood. 2010;115:363–369. doi: 10.1182/blood-2009-08-236976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford AD, Nisar S, Darnige L, Jones ML, Bachelot-Loza C, Gandrille S, et al. Platelet dysfunction associated with the novel Trp29Cys thromboxane A(2) receptor variant. J Thromb Haemost. 2013;11:547–554. doi: 10.1111/jth.12117. [DOI] [PubMed] [Google Scholar]
- Nisar S, Daly ME, Federici AB, Artoni A, Mumford AD, Watson SP, et al. An intact PDZ motif is essential for correct P2Y12 purinoceptor traffic in human platelets. Blood. 2011;118:5641–5651. doi: 10.1182/blood-2011-02-336826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisar SP, Cunningham M, Saxena K, Pope RJ, Kelly E, Mundell SJ. Arrestin scaffolds NHERF1 to the P2Y12 receptor to regulate receptor internalization. J Biol Chem. 2012;287:24505–24515. doi: 10.1074/jbc.M112.347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisar SP, Lordkipanidze M, Jones ML, Dawood B, Murden S, Cunningham MR, et al. A novel thromboxane A2 receptor N42S variant results in reduced surface expression and platelet dysfunction. Thromb Haemost. 2014;111:923–932. doi: 10.1160/TH13-08-0672. [DOI] [PubMed] [Google Scholar]
- Nurden AT, Caen JP. An abnormal platelet glycoprotein pattern in three cases of Glanzmann's thrombasthenia. Br J Haematol. 1974;28:253–260. doi: 10.1111/j.1365-2141.1974.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Nurden P, Savi P, Heilmann E, Bihour C, Herbert JM, Maffrand JP, et al. An inherited bleeding disorder linked to a defective interaction between ADP and its receptor on platelets. Its influence on glycoprotein IIb–IIIa complex function. J Clin Invest. 1995;95:1612–1622. doi: 10.1172/JCI117835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- Pardo L, Deupi X, Dolker N, Lopez-Rodriguez ML, Campillo M. The role of internal water molecules in the structure and function of the rhodopsin family of G protein-coupled receptors. Chembiochem. 2007;8:19–24. doi: 10.1002/cbic.200600429. [DOI] [PubMed] [Google Scholar]
- Patel YM, Lordkipanidze M, Lowe GC, Nisar SP, Garner K, Stockley J, et al. A novel mutation in the P2Y12 receptor and a function-reducing polymorphism in protease-activated receptor 1 in a patient with chronic bleeding. J Thromb Haemost. 2014;12:716–725. doi: 10.1111/jth.12539. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole RM, Elkinson S. Vorapaxar: first global approval. Drugs. 2014;74:1153–1163. doi: 10.1007/s40265-014-0252-2. [DOI] [PubMed] [Google Scholar]
- Rao AK, Willis J, Kowalska MA, Wachtfogel YT, Colman RW. Differential requirements for platelet aggregation and inhibition of adenylate cyclase by epinephrine. Studies of a familial platelet alpha 2-adrenergic receptor defect. Blood. 1988;71:494–501. [PubMed] [Google Scholar]
- Remijn JA, IJsseldijk MJ, Strunk AL, Abbes AP, Engel H, Dikkeschei B, et al. Novel molecular defect in the platelet ADP receptor P2Y12 of a patient with haemorrhagic diathesis. Clin Chem Lab Med. 2007;45:187–189. doi: 10.1515/CCLM.2007.036. [DOI] [PubMed] [Google Scholar]
- Rivera J, Lozano ML, Navarro-Nunez L, Vicente V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica. 2009;94:700–711. doi: 10.3324/haematol.2008.003178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovati GE, Capra V, Neubig RR. The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Mol Pharmacol. 2007;71:959–964. doi: 10.1124/mol.106.029470. [DOI] [PubMed] [Google Scholar]
- Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savi P, Zachayus JL, Delesque-Touchard N, Labouret C, Herve C, Uzabiaga MF, et al. The active metabolite of Clopidogrel disrupts P2Y12 receptor oligomers and partitions them out of lipid rafts. Proc Natl Acad Sci U S A. 2006;103:11069–11074. doi: 10.1073/pnas.0510446103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P, Ritscher L, Dong EN, Hermsdorf T, Coster M, Wittkopf D, et al. Identification of determinants required for agonistic and inverse agonistic ligand properties at the ADP receptor P2Y12. Mol Pharmacol. 2013;83:256–266. doi: 10.1124/mol.112.082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraga M, Miyata S, Kato H, Kashiwaga H, Honda S, Kurata Y, et al. Impaired platelet function in a patient with P2Y12 deficiency caused by a mutation in the translation initiation codon. J Thromb Haemost. 2005;3:2315–2323. doi: 10.1111/j.1538-7836.2005.01554.x. [DOI] [PubMed] [Google Scholar]
- Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Judge HM, Peters G, Armstrong M, Dupont A, Gaussem P, et al. PAR-1 genotype influences platelet aggregation and procoagulant responses in patients with coronary artery disease prior to and during clopidogrel therapy. Platelets. 2005;16:340–345. doi: 10.1080/00207230500120294. [DOI] [PubMed] [Google Scholar]
- Smith SO. Structure and activation of the visual pigment rhodopsin. Annu Rev Biophysics. 2010;39:309–328. doi: 10.1146/annurev-biophys-101209-104901. [DOI] [PubMed] [Google Scholar]
- Stegner D, Nieswandt B. Platelet receptor signaling in thrombus formation. J Mol Med (Berl) 2011;89:109–121. doi: 10.1007/s00109-010-0691-5. [DOI] [PubMed] [Google Scholar]
- Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33. doi: 10.1056/NEJMoa1109719. [DOI] [PubMed] [Google Scholar]
- Watson S, Daly M, Dawood B, Gissen P, Makris M, Mundell S, et al. Phenotypic approaches to gene mapping in platelet function disorders – identification of new variant of P2Y12, TxA2 and GPVI receptors. Hamostaseologie. 2010;30:29–38. [PubMed] [Google Scholar]
- Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- Watson SP, Lowe GC, Lordkipanidze M, Morganon NV. Genotyping and phenotyping of platelet function disorders. J Thromb Haemost. 2013;11:351–363. doi: 10.1111/jth.12199. [DOI] [PubMed] [Google Scholar]
- Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, Shenolikar S. The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu Rev Physiol. 2006;68:491–505. doi: 10.1146/annurev.physiol.68.040104.131050. [DOI] [PubMed] [Google Scholar]
- Wiviott SD, Flather MD, O'Donoghue ML, Goto S, Fitzgerald DJ, Cura F, et al. Randomized trial of atopaxar in the treatment of patients with coronary artery disease: the lessons from antagonizing the cellular effect of Thrombin-Coronary Artery Disease Trial. Circulation. 2011;123:1854–1863. doi: 10.1161/CIRCULATIONAHA.110.001404. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang K, Gao ZG, Paoletta S, Zhang D, Han GW, et al. Agonist-bound structure of the human P2Y12 receptor. Nature. 2014a;509:119–122. doi: 10.1038/nature13288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Zhang J, Gao ZG, Zhang D, Zhu L, Han GW, et al. Structure of the human P2Y receptor in complex with an antithrombotic drug. Nature. 2014b;509:115–118. doi: 10.1038/nature13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Flanagan C, Ballesteros JA, Konvicka K, Davidson JS, Weinstein H, et al. A reciprocal mutation supports helix 2 and helix 7 proximity in the gonadotropin-releasing hormone receptor. Mol Pharmacol. 1994;45:165–170. [PubMed] [Google Scholar]