Abstract
In pharmaceutical research, understanding the biodistribution, accumulation and metabolism of drugs in tissue plays a key role during drug discovery and development. In particular, information regarding pharmacokinetics, pharmacodynamics and transport properties of compounds in tissues is crucial during early screening. Historically, the abundance and distribution of drugs have been assessed by well-established techniques such as quantitative whole-body autoradiography (WBA) or tissue homogenization with LC/MS analysis. However, WBA does not distinguish active drug from its metabolites and LC/MS, while highly sensitive, does not report spatial distribution. Mass spectrometry imaging (MSI) can discriminate drug and its metabolites and endogenous compounds, while simultaneously reporting their distribution. MSI data are influencing drug development and currently used in investigational studies in areas such as compound toxicity. In in vivo studies MSI results may soon be used to support new drug regulatory applications, although clinical trial MSI data will take longer to be validated for incorporation into submissions. We review the current and future applications of MSI, focussing on applications for drug discovery and development, with examples to highlight the impact of this promising technique in early drug screening. Recent sample preparation and analysis methods that enable effective MSI, including quantitative analysis of drugs from tissue sections will be summarized and key aspects of methodological protocols to increase the effectiveness of MSI analysis for previously undetectable targets addressed. These examples highlight how MSI has become a powerful tool in drug research and development and offers great potential in streamlining the drug discovery process.
Tables of Links
| Targets | |
|---|---|
| Enzymes | |
| Prostaglandin E synthase 1 (mPGES1) |
| Ligands |
|---|
| Imatinib |
| Oxaliplatin |
| PaclitaxelTiotropium |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
The goal of pharmaceutical research is to develop new chemical entities that are efficacious, but also safe. In recent years, significantly fewer new drugs have been licensed, with escalating costs of research and development (Scannell et al., 2012). Notably, many compounds fail later in development, incurring substantial expense. Although technological advances have streamlined the discovery and development of new therapeutics, some important fundamental aspects of monitoring the fate of drugs in vivo remain inadequately addressed, contributing to high attrition rates.
Early knowledge of tissue distribution of drugs and their metabolites is extremely important in understanding pharmacological responses [pharmacokinetics (PK), pharmacodynamics (PD), drug transport, toxicity], and in predicting undesirable off-target effects (safety, drug–drug interactions) (Lanao and Fraile, 2005). While the physico-chemical properties of a compound (membrane permeability, protein binding, lipophilicity etc.) can be measured and modelled to predict biodistribution, tissue exposure has historically been inferred from surrogate measures such as concentrations of drug in plasma or tissue homogenates (Monro, 1990). While such methods enable high throughput screening during the discovery phase, reliance on circulating concentrations can prove erroneous when assessing tumour or blood–brain barrier penetration or highly localized delivery within multi-cellular tissues (Langer and Muller, 2004). Therefore techniques allowing histological assignment of drug distribution within tissue are required.
To date, drug distribution in tissues has been largely ascertained using whole-body autoradiography (WBA) or autoradioluminography. Such techniques detect radioisotopes integrated into drugs, typically 14C or 3H (Hahn, 1979); the radiolabel is quantified in dissected or whole animal tissue sections. However, as detection is based on the presence of a radioisotope, the techniques cannot distinguish parent drug from any metabolites retaining the label. Furthermore, during metabolism cleavage and ring scission may occur, so the radiolabel may no longer be associated with the phamacophore of the parent molecule and hence intensity of signal cannot be interpreted as a reflection of amounts of active drug. These factors restrict the value of WBA in predicting pharmacological and toxicological responses (Solon et al., 2010; Castellino et al., 2011). The associated cost in time and resources for synthesis also means that radio-isotopologues are not commonly generated during the discovery phase, but typically only once a drug progresses into development. Despite these limitations, the technique offers good specificity for quantifying ‘drug-related material’. Its high sensitivity and large dynamic range make this approach the industry gold standard in compliance with the regulatory authorities (Solon, 2007). For most WBA studies, time-dependent appearance of drug-related material in tissue is quantified, with good spatial resolution (50–100 μm). Higher resolution (down to 0.05 μm) can be achieved using micro-autoradiography, even locating tracers between cells, but is technically challenging and not standardized (Stumpf, 2013).
When an antibody to the drug is available, immunostaining can be highly specific, but does not achieve high throughput, with typically only one analyte measured per histological section, albeit at high (near cellular) spatial resolution. Similarly, fluorescent tags allow highly specific visualization of one analyte with high spatial resolution (Giepmans et al., 2006), although some dual-staining approaches exist. However, adding a fluorescence tag to the molecule may change distribution of the analyte into tissues. The use of clinical imaging techniques, such as PET and MRI to evaluate drug localization is very limited. (Artemov et al., 1995; Aboagye and Bhujwalla, 1999; Glunde et al., 2002;,2011; Giepmans et al., 2006; Ackerstaff et al., 2007; Glunde and Bhujwalla, 2007). MRI and PET target specific classes of molecules, but with low specificity and sensitivity. The spatial resolution of MRI varies from 1 cm3 to 1 mm3 (Zierhut et al., 2010) and new PET instrumentation can achieve less than 1 mm3 isotropic volume resolution (Lewellen, 2008). However, although these techniques are uniquely useful for in vivo imaging, it is again the time and resources required to generate probes or labels and lack of multiplexed analysis that limit the applicability of such techniques.
Mass spectrometry (MS) offers a label-free, multiplex alternative to evaluate the abundance of drugs in tissues, usually achieved by LC–MS/MS using sample homogenates. This commonly used technique can be quantitative, discriminates between parent and metabolites by mass and chromatographic retention time and is accepted by regulatory authorities. However, the main drawback of LC-MS/MS lies in the assumption of tissue homogeneity, which ignores the distinct compartments and functions within multi-cellular tissues (Mouton et al., 2008). Mass spectrometry imaging (MSI) has come to prominence for PK/PD and toxicological studies by merging the benefits of the spatial resolution of autoradiography, with the specificity of tandem MS. In addition, concomitant assessment of spatial distribution of many analytes, across a wide mass range, means that both targeted (compound specific) and untargeted (toxicological investigations of biomolecules) studies can be routinely performed. Table 1 summarizes the relative merits of MSI versus other techniques.
Table 1.
Comparison of advantages and disadvantages of techniques assessing drug distribution
| Methodology | Question answered | Advantages | Disadvantages |
|---|---|---|---|
| Autoradiography | Where and how much radioactivity? | Very high spatial resolution; reliable quantitation. | Ex vivo; requires radio-labelled drug; does not distinguish drug from metabolites. |
| Inmunohistochemistry | Where | Short processing time; easy interpretation; inexpensive | Ex vivo; requires antibodies, which vary in sensitivity and specificity; difficulties assigning; detection threshold; lack of standard scoring system |
| Fluorescence | Where | In vivo possible; reasonable cost | Not quantitative; poor resolution; autofluorescent interference |
| PET | Where, what and activity | In vivo possible; good resolution; can be coupled to CT X-ray, gamma camera | Expensive; short-lived isotopes; need cyclotron to produce isotopes |
| Coherent anti-Stokes Raman scattering microscopy (CARS) | Where and what | Label-free; sub-cellular spatial resolution | Not quantitative; poor selectivity; high background noise |
| Electrochemical atomic force microscopy (AFM) | Where and what | Label-free imaging; high resolution | Not quantitative; poor reproducibility; high background |
| MSI | Where and what | Multiplex; label-free imaging; good spatial resolution | Semi-quantitative; ion-suppression effects; complex analysis |
CT, computed tomography.
Applications of MSI with a pharmaceutical focus are reviewed here, highlighting its benefits and associated technical challenges. The paper primarily addresses the most commonly employed MSI technology, matrix-assisted laser desorption ionization (MALDI), but reference is given to alternative methodologies coming online. Examples of applications for both exogenous and endogenous compound are presented to highlight its effectiveness to monitor both drug distribution and also the biological changes caused by drug target engagement or disease progression.
MSI
MSI owes its origins to the field of physics, where it was used to study semiconductor surfaces (Benninghoven and Sichtermann, 1978). The introduction of MALDI-MSI in 1997 by Caprioli (et al.) triggered significant development and optimization of methodologies, instrumentation, and software for bioanalysis. The technologies were rapidly applied to pharmaceutical biodistribution analysis to align molecular maps of drug distribution with histology (Stoeckli et al., 2007; Heeren and Chughtai, 2010; Nilsson et al., 2010), and are now making important contributions in fields such as molecular histology, drug distribution and proteomics (Fletcher et al., 2011). The ability to localize compounds allows MSI to tackle questions relating to complex distribution patterns such as blood–brain barrier penetration (Liu et al., 2013).
MSI can detect endogenous and exogenous analytes across a wide mass range, from low molecular weight drugs and endogenous compounds through to lipids and large proteins, directly from tissue samples (Pierson et al., 2004; Skold et al., 2006; Groseclose et al., 2008; Castellino et al., 2011; Parson et al., 2012; Weaver and Hummon, 2013). Importantly, MSI can, by mass measurement, differentiate between drugs and metabolites formed by Phase 1 or 2 metabolism, overcoming the limitations of non-specificity encountered with autoradiography. In addition, as labelled compounds are not required, animals may be dosed with multiple compounds of different mass (cassette dosing) (White and Manitpisitkul, 2001; Smith et al., 2007). This offers the combined benefits of increasing throughput of compounds in discovery, enabling study of combination therapies and reducing the numbers of animals required in research and development.
To successfully, accurately and reproducibly analyse the abundance and distribution of a drug directly from tissue sections, and for the resultant data to be acceptable to regulatory authorities, a robust workflow is required. Here, the steps in sample preparation, processing and analysis required for successful pharmaceutical MSI are briefly described, with reference to pertinent in-depth reviews. Consideration is also given to commonly available mass analysers and ionization techniques.
Principles and instrumentation
MSI is a multi-step process involving sample preparation, analyte desorption and ionization, mass analysis, and image registration (Figure 1A). Briefly, tissue cryosections are placed, commonly under vacuum, into an MS instrument and analyte molecules desorbed and ionized from the surface (Monroe et al., 2008). With ionization being performed from known positions, the collated mass spectra allow reconstruction of the distribution of molecules present, typically presented as 2D ion images showing relative abundance of selected molecular masses. The sample processing pipeline for MSI, while simple, offers multiple stages for optimization and modification (comprehensively reviewed, Goodwin, 2012).
Figure 1.
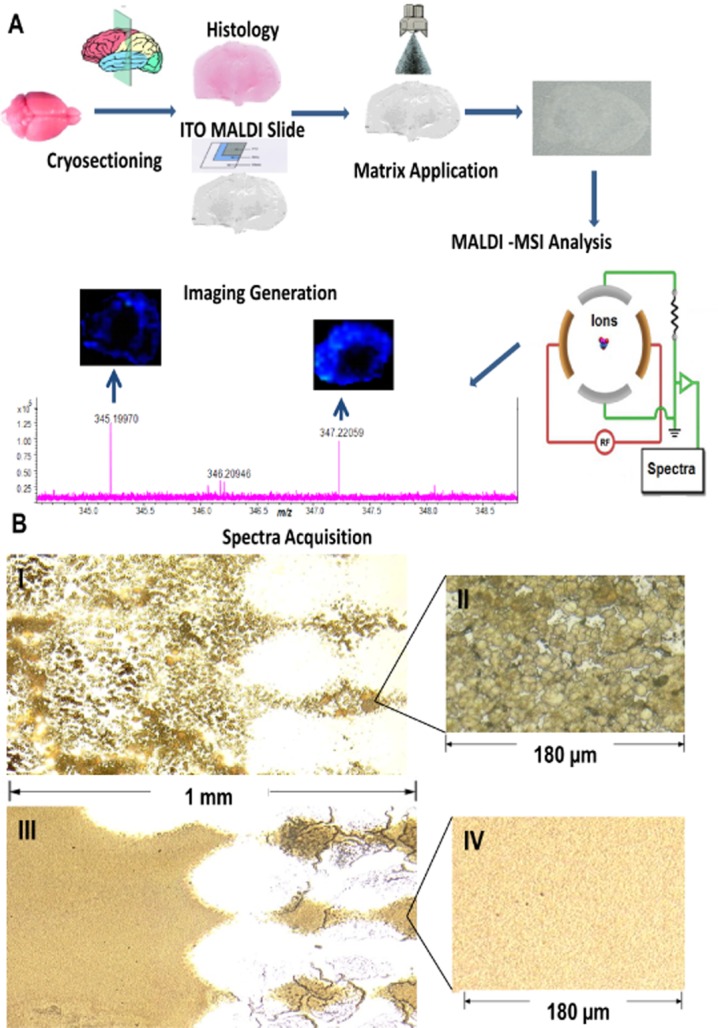
(A) MSI workflow. MSI involves sample preparation, analyte desorption and ionization, mass analysis and image registration. In brief, cryo-sections of tissue are coated with a suitable MALDI matrix and then introduced into an MSI instrument. A specified area of the tissue section is analysed and mass spectral information collected. The resulting ion distributions are presented as ion images. ITO, indium titanium oxide. (B) Matrix deposition by manual spraying and sublimation. Sublimation leads to deposition of smaller crystals (sub-μm) compared with a standard spray-coating, the latter achieving crystal sizes ∼10–20 μm. Photograph (×25 magnifications) of a rat adrenal gland tissue section mounted on a conductive glass slide and coated with α-cyano-4-hydroxycinnamic acid (CHCA) matrix by (I) a pneumatic thin layer chromatography sprayer (2.61 mg, whole slide) (III) sublimation (2.74 mg, whole slide). Optical image of CHCA crystals (×40 magnification) (II) formed by spraying or (IV) sublimation.
Tissue preparation for MALDI-MSI analysis
Protocols for sample collection must ensure that histological integrity of tissue sections is retained so that spatial localization of target molecules is uncorrupted by analyte degradation/diffusion. Rapid proteolytic activity has been reported in tissue sections (Goodwin et al., 2008; 2012a). Put simply, the quality of MSIs are only as good as the starting tissue section. To achieve the highest quality sections, the following steps need to be optimized for specific target tissues.
Preparation of tissue sections
MSI is commonly conducted using snap-frozen cryosections cut from dissected organs rapidly frozen in liquid nitrogen, rather than conventionally embedded/fixed tissues. For analysis of small drug molecules, processes that delocalize the analytes must be avoided, specifically lengthy soaking in fixative or washing with ethanol, although these processes may be compatible with analysis of structural molecules, for example proteins. Even so, proteomic analysis from formalin-fixed paraffin-embedded tissues is fraught with difficulty, involving multistage approaches to reverse cross-linking and remove paraffin (Groseclose et al., 2008; Djidja et al., 2009; Casadonte and Caprioli, 2011).
Consecutive tissue sections for traditional histology are commonly collected allowing MSI and histology images to be aligned. If the morphological assessment is to be performed using the MSI section, histological stains used must be compatible with MSI, such as methylene blue or cresyl violet (Chaurand et al., 2004), that is stains not causing ion suppression. ‘Ion suppression’ is a common problem encountered in biological MS, whereby high abundance analytes (e.g. fixatives, embedding agents) ionize preferentially to low abundance species (drug of interest), depleting the signal and swamping mass analyser. Haematoxylin and eosin staining can also be performed after MSI analyses by washing off the MALDI matrix (described later) with a suitable solvent, as many histological features remain intact (Chughtai and Heeren, 2010).
Tissue sections are usually cut at 10–20 μm, comparable with the thickness of a mammalian cell, so that the majority of cells are cut open. This allows MALDI matrix to co-crystallize with cell contents. This depth is also suitable for histological assessment, although thicker than required for some techniques for example electron microscopy. In conventional histology, tissues are usually embedded using an optimal cutting temperature (OCT) polymer to allow easier and precise cryosectioning. However, the presence of OCT suppresses the MS ion signal (Todd et al., 2001). If samples cannot be mounted with just a small drop of water on the reverse side of the sample, then mounting on gelatine can be attempted. An alternative procedure using RCL2/CS100, a non-volatile and non-cross-linking fixative reagent introduced by Mange et al. (2009), can be used for embedding to permit precise cryosectioning.
For some instruments, the sample must be ionized from a conductive surface; a metal plate, a metal-coated microscope slide or adhesive double-sided conductive tape may be used (Schwartz et al., 2003; Crecelius et al., 2005). Cell debris and salts from the tissue surface (Todd et al., 2001) that can cause ion suppression or formation of unstable adducts can be removed by washing. Several solvents have been used, ethanol being preferred because of its fixative dehydration properties (Lemaire et al., 2007). This approach is suitable for proteins or structural components of tissue, but may lead to the removal of small molecules, particularly hydrophobic ones (e.g. steroids). Therefore, the washing step is target compound-dependent and usually avoided for small molecule analysis unless optimized specifically (Shariatgorji et al., 2012). Sections should be stored at −80°C. Prior to matrix application, samples should be dried under vacuum.
Matrix deposition
The role of the MALDI matrix is to absorb laser energy in the MALDI source, leading to the explosive desorption of analytes (often neutral species) held within matrix crystals, into the gas-phase without significant degradation (Karas, 1996). Uniform matrix application plays a critical role in the quality of reproducible, high-resolution MSI data and the process must be controlled to enable homogenous co-crystallization of the analytes from the tissue within the matrix on the section surface, without causing diffusion or disruption of morphology.
The matrix solution consists of: (i) an organic solvent (commonly methanol or acetonitrile), whose function is to rapidly extract target compounds from the tissue, allowing co-crystallization of the molecules of interest within the growing crystals of the matrix at the tissue surface (Amstalden van Hove et al., 2010); (ii) trifluoroacetic acid or another strong organic acid (when performing positive mode ionization) promoting the ionization of analytes by enriching the media with available protons; and (iii) an organic acid (matrix). Together these are chosen to achieve a suitable coverage capacity, homogeneity of crystallization and crystal sizes, suitable time of crystallization, manageable duration of analysis (in terms of vacuum stability), resistance to laser irradiation especially for high-frequency lasers, ionic yield in negative and positive mode and, where appropriate, enough fragmentation to use the post-source decay to collate structural information.
Those matrices most commonly used are in Table 2 (Heeren and Chughtai, 2010). In terms of ionization modes, the well-known proton donor matrices such as α-cyano-4-hydroxycinnamic acid and 2,5-dihydroxybenzoic acid promote ionization by forming proton cluster ions in positive ion mode. In negative mode, basic matrices such as 9-amino acridine (Hercules, 2002) and norharmane are good proton acceptors, favouring the formation of deprotonated analyte ions (Scott et al., 2014). The choice of matrix is influenced by the size of the analyte, as interference from the matrix by isobaric clusters/adducts in the same mass window can occur: most conventional matrices have molecular weights in the same range as many drug-like molecules (100–300 Da). This issue can be overcome using high molecular weight (Ayorinde et al., 1999), inorganic matrices (Chen and Chen, 2004) or novel deuterium-labelled matrices (Shariatgorji et al., 2012).
Table 2.
Matrices used to facilitate laser desorption ionization
| Abbreviation | Applications | References | |
|---|---|---|---|
| Matrix for positive ion mode | |||
| α-Cyano-4-hydroxycinnamic acid | CHCA | Therapeutic peptides and proteins; small molecules | Reyzer et al., 2003 Cornett et al., 2008 |
| Sinapinic acid | SA | Large peptides and proteins (>10 kDa) | Meetani and Voorhees, 2005 |
| 2-(4-Hydroxyphenylazo)benzoic acid | HABA | Huwiler et al., 2003 | |
| 2,4,6-Trihydroxyacetophenone | THAP | Oligonucleotide mass < 3.5 kDa | Streletskii et al., 2005 |
| 3-Hydroxypicolinic acid | HPA | Oligonucleotide mass > 3.5 kDa | Wu et al., 1993 |
| 2,5-Dihydroxybenzoic acid | DHB | Therapeutic peptides and proteins; small molecules | Goodwin et al., 2012a,b Gonnet et al., 2003 |
| Porphyrins | TPP | Small molecules | Ayorinde et al., 1999 |
| Fluorine C60 | Small molecules | Liu et al., 2012 | |
| Matrix for negative ion mode | |||
| 9-Aminoacridine | 9AA | Small molecules/lipids | Hercules, 2002 |
| 9H-Pyrido[3,4-b]indole | norharmane | Lipids and small molecules | Scott et al., 2014 |
| 2-Mercaptobenzothiazole | MBT | Therapeutic peptides and small molecules | Zhou et al., 2010 |
| Titanium dioxide | TiO2 | Cyclodextrins | Chen and Chen, 2004 |
| Matrix for dual mode | |||
| 1,8-bis(Dimethylamino)naphthalene | DMAN | Small molecules | Shroff and Svatos, 2009 |
| Liquid ionics | Therapeutic peptides, small molecules and oligosaccharides | Crank and Armstrong, 2009 | |
| Solid ionics | Therapeutic proteins and big peptides | Crank and Armstrong, 2009 | |
| Nicotinic hydrazine | Oligonucleotides | Jiao et al., 2014 |
Size of droplets and subsequent crystals formed by the different matrices influences the spatial resolution of imaging possible (Figure 1B). Matrix deposition can be performed as individual droplets (spotted) or as a homogeneous layer (coated). Coating can be performed, either spray-based (e.g. thin layer chromatography sprayer) or solvent-free (e.g. sublimation) depending on the spatial resolution that is required (Figure 2B). To limit diffusion of the analyte molecules during matrix deposition, alternative solvent-free matrices have been developed and successfully applied for small molecule MSI analysis (Goodwin et al., 2010a,b). However, the main drawback of ‘dry-coating’ is lack of sensitivity because of poor extraction of analytes from the tissue (Goodwin et al., 2011). The advantages and disadvantages of the most common deposition techniques are summarized in Table 3 (Kaletaş et al., 2009).
Figure 2.
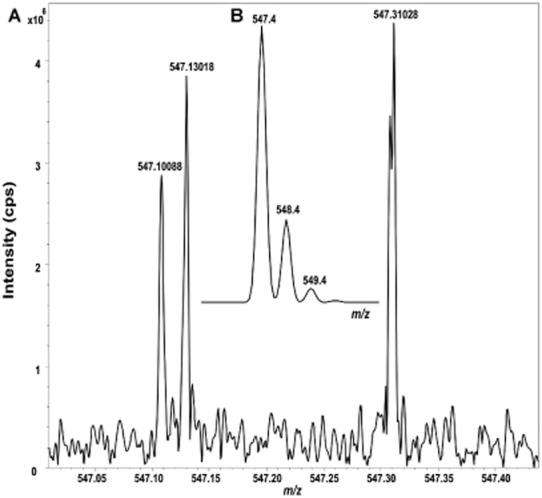
High-resolution versus low-resolution MS. High mass resolution is particularly important in tissue imaging because of the presence of many background ions. High resolving power allows distinction of low molecular weight analytes at low concentration from isobaric matrix interferents. Mass spectra of endogenous metabolites in a section of mouse brain, collected by (A) Fourier transform ion cyclotron resonance MS (FTICRMS) with high mass resolution, showing a mass window of 0.45 Da, with three distinct ionic species clustering at m/z 547 or (B, inset): a quadrupole MS with low mass resolution, showing a mass window of 6 Da; the same ions at m/z 547 cannot be distinguished. cps, count per second.
Table 3.
Matrix deposition techniques
| Technique | Advantages | Disadvantages |
|---|---|---|
| Acoustic multi-spotter | Uniform, fast, good reproducibility | Droplet application, limited spatial resolution |
| Electrospray deposition | Homogenous | Limited time for analyte matrix interaction |
| Pneumatic sprayer | Homogenous | Droplet size not constant |
| Image Prep® | Controlled conditions, automated, homogenous | Droplet size not constant, expensive |
| Dry-coating | Cheap, high purity matrix | Poor analyte–matrix extraction |
| Sublimation | Homogenous, reproducible, fast | Poor analyte–matrix extraction |
| Desktop inkjet printer | Uniform droplets (multichannel) | Slow, poor solvent compatibility, clogging |
MSI techniques
Many techniques are capable of performing MSI, with MALDI the most commonly used. MALDI is a development of laser desorption/ionization MS introduced in 1988 by Karas and Hillenkamp (1988). In traditional MALDI, the analyte is mixed with excess of chemical matrix at a molar ratio of 1:100–1000 (Dreisewerd, 2003), whereas for MALDI-MSI, the matrix is coated as an even layer as described earlier. MALDI is highly sensitive (attomole) and can ionize molecules across a wide mass range lending itself to applications from large biomolecules down to small molecular weight drugs, and as such, has been used for analysis of proteins, peptides, lipids and pharmaceutical compounds (Franck et al., 2009). Best results are achieved with molecules with masses up to 25 kDa; imaging for proteins >25 kDa is challenging with few published examples (Kislinger et al., 2005).
MALDI-MSI can detect multitudes of biomolecules generated as intact ions directly from localized pulsed laser spots; the laser beam is rastered across the matrix-covered tissue surface (Karas and Kruger, 2003). Matrix molecules absorb the laser energy, resulting in explosive desorption of matrix and analytes. The depth of ablation craters is estimated around 1 μm or more, depending on laser fluence (Knochenmuss, 2014). Although a traditionally slow process, the introduction of N2 (337 nm) or neodymium-doped yttrium aluminium garnet (Nd:YAG) (355 nm) lasers with repetition rates of 200–5000 Hz and typical pulse lengths of 3 ns or less has shortened the data acquisition process (Dreisewerd, 2003; 2014). To attempt near cellular imaging, laser spot sizes have been reduced from >100 to <20 μm, with focusing to the diameter of a single cell (∼7 μm) reported (Holle et al., 2006). However, although such improvements greatly enhance spatial resolution, they dramatically decrease sensitivity and are only suitable for highly abundant species (e.g. membrane lipids, Schober et al., 2012; Anderson et al., 2013).
Mass analysers
The ions generated by the approaches mentioned earlier are detected by their mass/charge ratios (m/z) and a range of mass analysers is available, each with benefits and limitations (Table 4; Jaroslav et al., 2010). A single MSI experiment can yield multiple ion images within a narrow mass range of only a few hundred Da and therefore analysers with high mass accuracy and resolving power are desirable. This contrasts with tandem quadrupole systems commonly used for targeted analysis, but only resolving to unit mass [for implications of varying mass resolution, see Figure 2 (Heeren and Chughtai, 2010)].
Table 4.
Properties of mass analysers used in MSI
| Analyser | MRP | Mass range | Detection | PAF (Hz) |
|---|---|---|---|---|
| Time of flight | 103–104 | 0–300 KDa | Parallel | >10 |
| FTICR-MS | 104–106 | 20 Da–10 KDa | Parallel | >1 |
| Linear ion traps | 102–103 | 50 Da–5 KDa | Sequential | <10 |
| Triple quadrupole | 102–103 | 0–5 KDa | MRM/sequential | >100 |
| Magnetic sector | 102–103 | 0–5 KDa | Single ion/array | <1 |
FTICR, Fourier transform ion cyclotron resonance; MRM, multiple reaction monitoring; MRP, mass resolving power; PAF, pixel acquisition frequency; ToF, time of flight.
The majority of MALDI imaging measurements are performed using time-of-flight (ToF) mass analysers because of their high detection efficiency and parallel detection, leading to high sensitivity. Ion-traps are gaining popularity, with three main trapped-ion mass analysers in existence; three-dimensional quadrupole ion traps (QIT) (Paul traps), Penning traps as used in Fourier ion cyclotron resonance (FTICR) mass spectrometers and Kingdon trap as used in the Orbitrap® mass spectrometers (Thermo Fisher, Palo Alto, CA, USA). All operate by storing ions in the trap and manipulating them using electrostatic, magnetic fields and radiofrequency in a series of carefully timed events (Douglas et al., 2005). The traditional strategy for MALDI-MSI of pharmaceutical compounds is to perform the imaging experiment in MS/MS mode. All ions in the range of the targeted precursor are fragmented and the abundance of the target compound is determined from the measured intensity of one or more of its structurally significant fragment ions. High-performance instruments such as FTICR-MS offer new strategies not reliant on fragment ions, as their superior accurate mass resolving power allows identification by elemental composition (Grange et al., 1996; Tolmachev et al., 2006). Subsequent MS/MS measurements can be made at discrete pixel locations by complementary techniques to add insight into molecular structure and to determine if multiple isomers contribute to the image. This is only possible if each isomer has a unique MS/MS fragmentation pattern.
Recently, Bruker has introduced continuous accumulation of selected ions (CASI®) (Fuchser et al., 2014), a technique showing very promising results with an increase in sensitivity up to 10 times in comparison with the broadband acquisition mode. This continuous mode of operation successfully separates the targeted ions (e.g. drug, metabolite, etc.) from the intense chemical background generated from the tissue, thereby lowering the limit of detection for the targeted species.
Data processing from spectra to pixels
Specialized software programs, including Biomap® (Novartis, Basel, Switzerland) and FlexImaging® (Bruker Daltonics, GmbH, Bremen, Germany), generate distribution images of ions of specific masses from MSI data. Data from each laser spot on the irradiated tissue, contribute mass spectral information about the species desorbed (Caprioli, 2007), and the relative intensity of a specific target molecule is plotted by pixel, producing a molecular map. The maps can then be aligned with histological information, commonly using an adjacent tissue section.
Quantitation by MSI
MSI has been generally considered a qualitative method (Chen et al., 2008), although fundamentally, the signal generated by MSI is proportional to the relative abundance of the analyte. The challenges to quantitation arise in robust sample preparation and homogenous matrix application as both factors influence signal intensity. Application of a calibration curve onto control tissue, processed and analysed concomitantly, is the simplest way to obtain quantitative information (Nilsson et al., 2010). However, as ion suppression of analyte signal can change across a complex tissue because of varying regional composition, analysis may be improved by inclusion of an internal standard (e.g. stable isotopomer) within the matrix (Atkinson et al., 2007; Kallback et al., 2012). Using this approach, tissue-specific correction factors can be invoked (Hamm et al., 2012), allowing correction of analyte signal in every pixel sampled. Currently, data generated by MSI are cross-referenced to a gold standard quantitative technique. Concentrations measured by LC-MS/MS in adjacent tissue sections following homogenization have been shown to correlate well with data generated by MALDI-MSI (Reyzer et al., 2003; Hankin and Murphy, 2010; Nilsson et al., 2012; Cobice et al., 2013).
It is important to emphasize that there is not currently a requirement to know tissue concentrations of drugs or metabolites for regulatory submission. WBA is used during regulatory submissions, to agencies such as the FDA, to assess fetal exposure and to compare data of animal biodistribution to mass balance studies of absorption, distribution metabolism and excretion (ADME) of radio-labelled drug in humans. Therefore, MSI data collected during drug discovery and development is and will be used in an explanatory and exploratory way. Clinical MSI-based diagnostic approaches will require technique, instrumentation and database approval from regulatory agencies. While we cannot provide references for MSI-based methods currently achieving this status, aligned MS profiling and tissue-based assays are progressing into this area (Schafer et al., 2009; Marko et al., 2012).
Application of MSI in drug development
Application of MSI in biomedical science has expanded significantly since first described by Caprioli et al. in 1997 with many diverse publications ranging from disease pathology, single-cell analysis and drug distribution (Chaurand et al., 2001; Cornett et al., 2008; Schober et al., 2012). Here, examples are chosen to highlight the significant impact MSI has made to small molecule drug discovery and development.
Drug distribution
PK analysis to assess ADME of drug-like molecules in either animals or human is mandatory in new drug applications submitted for final approval by the regulatory authorities (Rohner et al., 2005; Rubakhin et al., 2005; Greer et al., 2011). The first publication of MSI to assess tissue distribution of small drug-like molecules was by Troendle (et al., 1999) in which the anti-neoplastic drug paclitaxel was imaged in human ovarian tumour. The spatial distribution of the parent drug and its endogenous metabolites was assessed; these species could not have been distinguished by WBA. Since then, several applications of imaging of drugs and their metabolites by MSI have been reported (Reyzer et al., 2003; Goodwin and Pitt, 2010; Prideaux and Stoeckli, 2012).
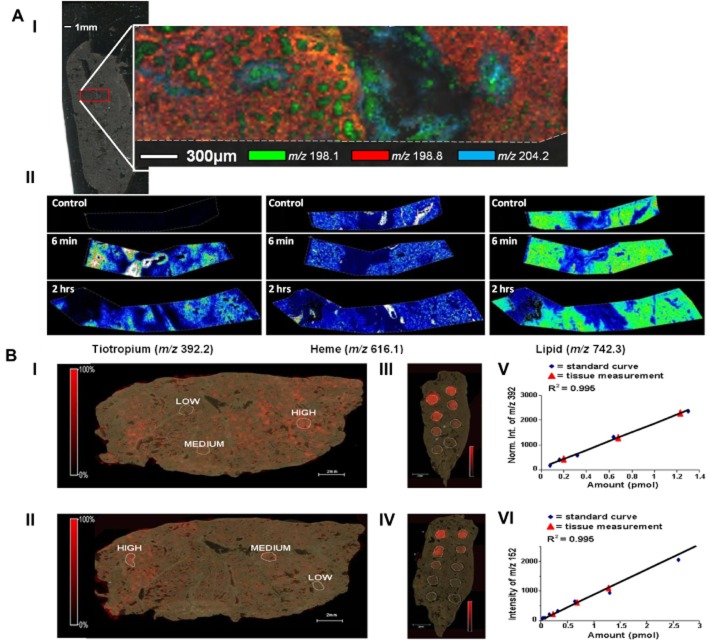
The report by Nilsson et al. (2012) clearly demonstrates the added value of MSI in PK analysis. In vivo transport of tiotropium (an anticholinergic bronchodilator drug) into rat lung was studied using MALDI-ToF MSI. Analysis at high spatial resolution allowed accurate localization of drug to specific histological tissue compartments, achieved by systematic point by point MS and MS/MS sampling at 200 μm intervals (Figure 3A). A recent extension of this work (A. Nilsson, unpublished), presented here shows tiotropium distribution monitored at near cellular resolution (20 μm rastering) at 6 and 120 min following inhalation (Figure 3). At the earliest time point, tiotropium was localized to the major airways and especially airway walls, whereas already at 120 min post inhalation, the drug is distributed more widely into the parenchyma. Co-registration of images from histologically stained sections with MSI data allows superimposition of drug distribution with endogenous markers of specific compartments such as bronchioles, blood vessels, airways and epithelial layers (Fehniger et al., 2014).
Figure 3.
(A) Tiotropium distribution in lung analysed at 20 μm spatial resolution using MALDI-ToF/ToF. Optical insert shows area of lung analysed. (I) Multiple endogenous masses simultaneously displayed to produce a molecular histological images that helps define structure of tissue and airways, (II) masses associated with drug, haem (marker for blood) and endogenous lipid (marker for tissue) from tissues sections at 6 and 120 min post inhalation of drug. (B) Quantitation and analysis of distribution of tiotropium in lung tissue from rats dosed with the drug (I, II) by comparison with drug-standard samples spotted on control tissue (III, IV). Amounts measured by quantitative experiments performed in MS mode (I, III, V) matched very well with analyses in MS/MS mode monitoring the product ion of m/z 152 (II, IV, VI) even though sensitivity was greater using MS/MS. The abundance of tiotropium quantified by MSI also matched very well with amounts measured by conventional LC-MS/MS analysis of tissue extracts. Adapted from Nilsson et al. (2010).
Localization of drugs in tumours by MSI has been particularly important in oncology research, demonstrating targeted delivery. MALDI-FTICR-MSI enabled the anti-tumour drug, imatinib, and its des-methyl metabolite to be localized in a mouse glioma (Cornett et al., 2008). Another anti-cancer agent, oxaliplatin, was imaged in rat liver, spleen and muscle (Bouslimani et al., 2010), and in another study, a pro-drug banoxatrone (AQ4N) and its active form were imaged by MALDI-MSI in lung tumour xenografts (Atkinson et al., 2007).
Lastly the technique has also allowed efficient assessment of brain penetration of drugs, for example clozapine distributes in rat brain and other tissues, including the lungs (Hsieh et al., 2006; Wiseman et al., 2008; Yanes et al., 2009), kidneys and testes (Goodwin et al., 2010a,b) in close agreement with WBA.
Investigatory toxicology
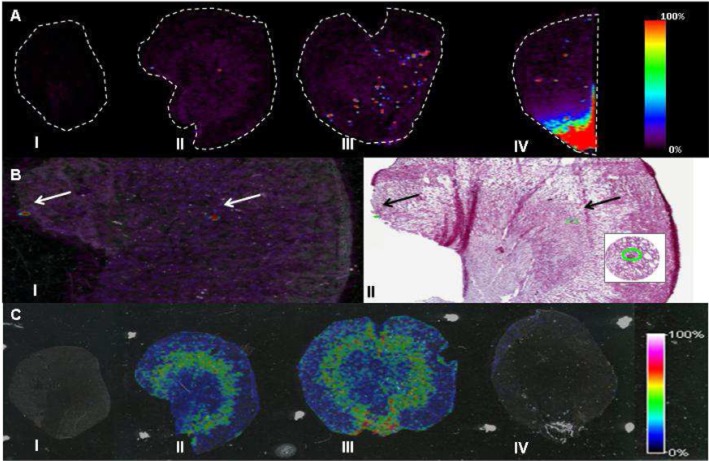
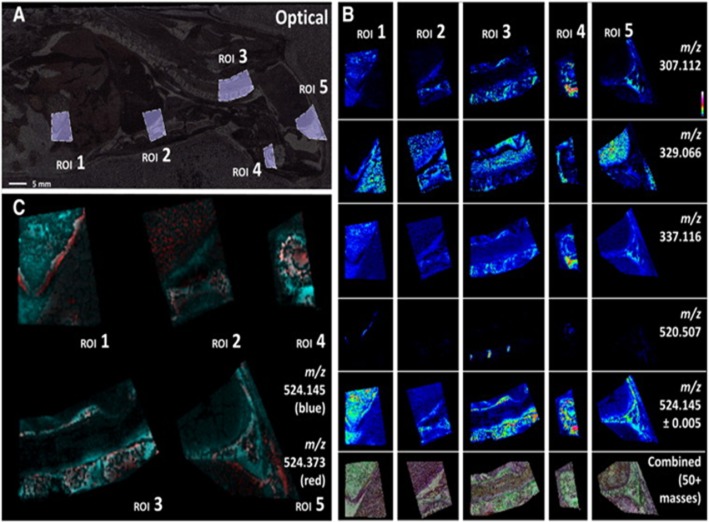
In contrast to targeted analysis of specific drugs, MALDI MSI also has great potential to reveal endogenous molecular changes associated with toxicological or pharmacological events (Lalowski et al., 2013) in a ‘hypothesis–free’ manner. The use for MSI for investigatory toxicology is highlighted by Nilsson et al. (2012) who studied biomarkers of renal damage following treatment with inhibitors of prostaglandin E synthase 1. The composition of renal crystalline deposits was identified when other bioanalysis methods failed. This untargeted MSI analysis aligned to histology also revealed molecular changes that co-localized to pathologically defined, damaged regions of the kidney (Figure 4). Indeed off-target effects of drugs may be predicted using whole-body MSI offering the possibility to localize both parent and metabolites in every organ within a whole-body section (Stoeckli et al., 2007). This technique permits comparison and cross-validation of quantitative WBA. Sample preparation of whole-body sections is challenging and has been optimized by Goodwin et al. (2010a) using conductive carbon tape for mounting the large specimen. Figure 5 shows an example of use of MSI during whole adult rat tissue sectioning of animals embedded in carboxymethyl cellulose.
Figure 4.
Detection of crystalline deposits in kidney by MALDI-TOF MSI. (A) Distribution of the drug metabolite, bisulphonamide at m/z 235.11. (i) vehicle control; (ii) tissue from animal receiving drug with low crystal load; (iii) tissue from animal receiving drug with high crystal load; (iv) tissue from animals receiving further drug with high crystal load. (B) (i) Localization of bisulphonamide matched the locations of crystals annotated by pathologists; (ii) sections stained with haematoxylin and eosin post MALDI analysis. (C) Un-targeted analysis by MSI revealed molecular changes associated with areas in the kidney described as damaged by the pathologists (i, iv) optical images; (ii, iii) endogenous metabolite m/z 437.31. Data were acquired at 100 μm spatial resolution. Adapted from Nilsson et al. (2012).
Figure 5.
Whole-body MALDI-MSI using conductive adhesive carbon tape to maintain electric conductivity required for some mass spectrometers. High spatial (100 μm resolution) and spectral distribution mapping of a selection of regions of interest (ROI), 1 (abdominal area), 2–3 (thoracic area), 4–5 (head) from carbon tape mounted rat tissue section on conductive plastic, analysed by FTICR-MSI. (A) Optical image with ROI marked. (B) MSI distributions of multiple ROI for a range of endogenous compounds (C) multiple masses displayed simultaneously. Reprinted from Goodwin et al. (2012b), with permission.
Broadening the scope of MSI by on-tissue derivatization
Ionization of the drug/metabolites by desorption and ionization process is an absolute requirement for effective detection of compounds in tissue sections by MALDI-MSI. Careful matrix selection and solvent composition can help achieve the desired sensitivity; however, some drugs are recalcitrant to these processes, lacking chargeable moieties, for example many steroids do not readily ionize and are difficult to detect by MS.
Chemical derivatization is an extensively used strategy for improving the detection of poorly ionizable molecules like steroids for both atmospheric pressure chemical ionization and electrospray analysis in LC/MS analysis (Quirke and Van Berkel, 2001; Higashi et al., 2005). However, the exciting possibility of MSI in combination with on-tissue chemical derivatization (OTCD) is still in its infancy with few cases reported (Chacon et al., 2011; Manier et al., 2011). Chacon et al. (2011) demonstrated the use of OTCD with a low MW scavenger of levuglandins (LGs), 3-methoxysalicylamine (3-MoSA). LGs are highly reactive towards primary amines, forming adducts with proteins and DNA; a process linked to oxidative injury, inflammation and the progression of Alzheimer's disease (Boutaud et al., 2006). The study of the distribution of 3-MoSA on intact tissue by MALDI-MSI is impeded by matrix interference and low sensitivity. Derivatization of 3-MoSA with 1,1′-thiocarbonyldiimidazole (TCDI) resulted in a readily ionizable oxothiazolidine derivative. TCDI treatment of tissue from mice dosed with 3-MoSA allowed successful PK profiling of this drug in multiple organs.
A recent study by Cobice et al. (2013) applied OTCD in conjunction with MSI, to image ketosteroids, mediators of diverse cellular responses in stress, reproduction and metabolism and common pharmaceutical targets. The approach, using Girard T as a derivatization reagent (Wheeler and Rosado-Lojo, 1962), offered exciting advances in mapping the distribution of ketosteroids in rodent tissues, including brain and adrenal gland. Signal intensity was considerably increased (104-fold) after derivatization, allowing MSI to be applied during PK/PD analysis of an inhibitor of the enzyme 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1). This enzyme generates active glucocorticoid hormones in brain and metabolic tissues and is an attractive target to protect against glucocorticoid-induced neuronal damage and metabolic disease, respectively, with ageing (Sooy et al., 2010). Using MSI time-dependent alterations, the ratio of active to inactive glucocorticoids were demonstrated in brain and liver following dosing with a novel 11β-HSD1 inhibitor (Figure 6). Relative quantitation was achieved using ratio of substrate and product of the enzyme and the data correlated well with measurements by LC-MS/MS in whole-brain homogenates (Figure 6). Further OTCD approaches are required to broaden the spectrum of analytes currently readily detected by MALDI-MSI.
Figure 6.

Effect of pharmacological inhibition of the enzyme, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), with a low MW inhibitor (UE2316) in C57BL6 mice. Girard T (GirT) derivatives [corticosterone (CORT, active steroid and enzyme product) and 11-dehydrocorticosterone (11DHC, inert metabolite and enzyme substrate) ] were distributed across the cortex, hippocampus (HPC) and amygdala (Am) (A) Histological image of horizontal cryosection of murine brain stained with haematoxylin and eosin (H&E). (B–E) MSI heat map distribution of m/z 390.084 ± 0.025 Da representing UE2316 in brain over a 6 h time course in mice receiving vehicle (V) or UE2316. (F) Amounts of UE2316 in whole brain measured by liquid chromatography tandem MS (LC-MS/MS) demonstrating good agreement with MSI. (G) Histological image of coronal cryosection of murine brain stained with H&E showing the outline of the MSI regions of interest (ROIs) (cortex, HPC, Am). (H, J) MSI heat map of GirT-CORT at m/z 460.317 ± 0.005 Da brain from mice receiving Vehicle (H) or UE2316 (D; 1 h post dose) (J). (I, K) GirT-11DHC at m/z 458.301 ± 0.025 Da in brain from mice receiving vehicle (I) or UE2316 (K). Signal intensity is depicted by colour on the scale shown. Scale bar (2 mm). (L) A significant decline (P < 0.01, overall between groups) in CORT/11DHC ratios was observed across the ROIs by MSI in the brain after administration of UE2316, showing good agreement with data generated by LC-MS/MS in whole brain. Data are mean ± SEM; n = 12. *P < 0.05, **P < 0.01; two-way anova for MSI and Student's t-test for LC-MS/MS. Reprinted with permission from Cobice et al., 2013.
Future perspectives
MALDI-MSI is an emerging tool to enhance the understanding of the distribution of drug, metabolite and endogenous biomarkers directly in tissue sections. Its ability to co-localize drug/metabolite distribution with histological information has been transformational in drug development and can be achieved at meaningful levels of spatial resolution. The power to differentiate parent drug from metabolites concomitantly without the need for labels offers significant improvements over alternative bioassays and may help answer questions about access in target and off-target tissues early in the drug development process, highlighting potential toxicological problems. Despite the extensive use of MSI in pharmaceutical companies, the reported use of MSI for pharmaceutical research is limited as publication requires full disclosure of compound structures. Many papers report only method development using non-proprietary compounds (Prideaux and Stoeckli, 2012). While it may be many years before related publications appear, data from MSI experiments are significantly influencing industrial project decisions and it is only a matter of time before MSI experiments support regulatory submission.
However, before MALDI-MSI is accepted as a routine quantitative tool, issues such as regional ion-suppression, analyte or post-mortem tissue degradation and reproducibility of sample preparation still need to be addressed. Salt and lipid removal prior to the analysis of peptides and proteins simplifies the chemical environment and efforts in this direction continue (Lemaire et al., 2007; Mange et al., 2009). Unfortunately, these protocols are not suitable for many low MW compounds and other approaches such as heat stabilization are currently being explored (Goodwin et al., 2012b). The problem of ion suppression is exacerbated with poorly ionizable molecules, where MSI is seriously hindered by poor limits of detection. For robust data, tissue preparation must be consistent and matrix coverage uniform. Conventional manual deposition techniques such as pneumatic TLC sprayer or artistic airbrush may lead to incorrect heat maps generation because of uneven deposition or hot spots. However, uniform coating can be achieved using the highly precise automatic matrix deposition systems now available.
In addition, industry and researchers will further develop and refine hardware and software to make MSI experiments more robust and easier to implement in high-throughput. Iterative technical improvements in ionization and mass analysers will increase the sensitivity and speed of MSI experiments. However, managing sample size and desired spatial resolution can be slow, requiring substantial computing resources. Dataset sizes can approach or exceed several gigabytes, with processing times reaching several hours. Nevertheless, if the mass range of the compounds and region of interest are known, the dataset may be vastly simplified. Because of these restrictions, it is often impractical to perform many MALDI MSI experiments and it can be advantageous initially to rapidly profile in lower mass resolution or low-density raster patterns to specify a region for confirmatory high-density imaging.
Limitations of spatial resolution also need to be addressed if cellular imaging is to be achieved. The alternative ionization methods, secondary ion MS (SIMS) and desorption electrospray ionization (DESI) may hold the key and are proving advantageous under specific circumstances depending on the target analyte and its abundance. Nano-structure initiator MS (NIMS) (Yanes et al., 2009) and laser ablation electrospray ionization (LAESI) (Nemes and Vertes, 2010), among others, may also broaden the scope for application of the MSI in drug development. More detailed technical reviews have been provided by Amstalden van Hove et al. (2010) and Vickerman (2011).
SIMS can achieve higher spatial resolution than MALDI without matrix application, hence reducing opportunities for diffusion. SIMS employs a primary ion beam (e.g. metal ions) to produce secondary ions from the sample surface (Jones et al., 2007), focussed as sharply as 50 nm, depending on the primary ion beam current, molecular weight, and its charge state (Altelaar et al., 2005). With higher spatial resolution and precision than laser-based imaging, SIMS offers exciting opportunities in molecular pharmacology (Nygren et al., 2007), for example, imaging of abundant elements and small organic molecules in cellular organelles (Altelaar et al., 2007). Ion suppression plays a minor role for absolute quantitation in comparison with MALDI. However, the main disadvantages are the energetic desorption process resulting in significant fragmentation, the mass range is limited to ions lower than 1000 Da and access to this technology is still very limited.
DESI, an atmospheric pressure ionization method developed by Cooks in 2004 (Takats et al., 2004), is derived from a combination of two MS ionization methods: electrospray and desorption ionization. Although improvements are still required, it holds promise and is already being applied to study endogenous molecules and drug metabolites (Wiseman et al., 2006). DESI uses energetic, charged electrosprayed solvent droplets to release the molecules from the surface (Takats et al., 2004), offering the unique possibility of in situ and real-time analysis (Dill et al., 2009). As a matrix-free technique, DESI does not suffer from matrix-analyte co-crystallization issues and serves as an alternative platform when compounds do not ionize in MALDI. DESI can routinely achieve at least 400 μm spatial resolution (Ifa et al., 2007). However, a recent spray design theoretically predicts lateral resolution of 40 μm (Ifa et al., 2007) and a new development in nano-DESI is said to resolve down to 10 μm (Laskin et al., 2012). The VAMAS (Versailles Project on Advanced Materials and Standards) study of 20 laboratories from 10 countries showed that reproducibility of measurements could be achieved within 20%. However variability in spray and sample stage design played a significant factor in causing larger inter-laboratory differences (Gurdak et al., 2014) and further work is required to improve the robustness of the technique. Reproducibility and robustness of any analytical technique is significant when undertaking multi-site analysis or if the study is performed over an extended period. However, emerging technologies can readily be utilized in standard investigatory studies where two samples are compared side by side. Findings can then be further validated with more stringent techniques.
To conclude, pharmaceutical companies are increasingly aware of the disconnection between outcomes in clinical programmes based on preclinical data. MSI can aid the refinement of pre-clinical studies and an increasing use of in situ MSI for analysis of clinical samples is predicted.
Acknowledgments
We are grateful to the British Heart Foundation Centre of Research Excellence and the EPSRC for funding.
Glossary
- 11β-HSD1
11β-hydroxysteroid dehydrogenase 1
- ADME
absorption, distribution metabolism and excretion
- DESI
desorption electrospray ionization
- MALDI
matrix-assisted laser desorption ionization
- MSI
mass spectrometry imaging
- PD
pharmacodynamics
- PK
pharmacokinetic
- SIMS
secondary ion mass spectrometry
- WBA
whole-body autoradiography
Conflict of interest
The authors do not have any conflict of interest. The company employing R. G., P. E. and A. N. does not sell any of the devices or drugs mentioned in this paper.
References
- Aboagye EO, Bhujwalla ZM. Malignant transformation alters membrane choline phospholipid metabolism of human mammary epithelial cells. Cancer Res. 1999;59:80–84. [PubMed] [Google Scholar]
- Ackerstaff E, Gimi B, Artemov D, Bhujwalla ZM. Anti-inflammatory agent indomethacin reduces invasion and alters metabolism in a human breast cancer cell line. Neoplasia. 2007;9:222–235. doi: 10.1593/neo.06673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altelaar AF, van Minnen J, Jimenez CR, Heeren RM, Piersma SR. Direct molecular imaging of Lymnaea stagnalis nervous tissue at subcellular spatial resolution by mass spectrometry. Anal Chem. 2005;77:735–741. doi: 10.1021/ac048329g. [DOI] [PubMed] [Google Scholar]
- Altelaar AF, Luxembourg SL, McDonnell LA, Piersma SR, Heeren RM. Imaging mass spectrometry at cellular length scales. Nat Protoc. 2007;2:1185–1196. doi: 10.1038/nprot.2007.117. [DOI] [PubMed] [Google Scholar]
- Amstalden van Hove ER, Smith DF, Heeren RM. A concise review of mass spectrometry imaging. J Chromatogr A. 2010;1217:3946–3954. doi: 10.1016/j.chroma.2010.01.033. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Mills D, Spraggins J, Lambert WS, Calkins DJ, Schey KL. High-resolution matrix-assisted laser desorption ionization-imaging mass spectrometry of lipids in rodent optic nerve tissue. Mol Vis. 2013;19:581–592. [PMC free article] [PubMed] [Google Scholar]
- Artemov D, Bhujwalla ZM, Maxwell RJ, Griffiths JR, Judson IR, Leach MO, et al. Pharmacokinetics of the 13C labeled anticancer agent temozolomide detected in vivo by selective cross-polarization transfer. Magn Reson Med. 1995;34:338–342. doi: 10.1002/mrm.1910340310. [DOI] [PubMed] [Google Scholar]
- Atkinson SJ, Loadman PM, Sutton C, Patterson LH, Clench MR. Examination of the distribution of the bioreductive drug AQ4N and its active metabolite AQ4 in solid tumours by imaging matrix-assisted laser desorption/ionisation mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:1271–1276. doi: 10.1002/rcm.2952. [DOI] [PubMed] [Google Scholar]
- Ayorinde FO, Hambright P, Porter TN, Keith QL., Jr Use of meso-tetrakis (pentafluorophenyl) porphyrin as a matrix for low molecular weight alkylphenol ethoxylates in laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 1999;13:2474–2479. doi: 10.1002/(SICI)1097-0231(19991230)13:24<2474::AID-RCM814>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Benninghoven A, Sichtermann WK. Detection, identification and structural investigation of biologically important compounds by secondary ion mass spectrometry. Anal Chem. 1978;50:1180–1184. doi: 10.1021/ac50030a043. [DOI] [PubMed] [Google Scholar]
- Bouslimani A, Bec N, Glueckmann M, Hirtz C, Larroque C. Matrix-assisted laser desorption/ionization imaging mass spectrometry of oxaliplatin derivatives in heated intraoperative chemotherapy (HIPEC)-like treated rat kidney. Rapid Commun Mass Spectrom. 2010;24:415–421. doi: 10.1002/rcm.4408. [DOI] [PubMed] [Google Scholar]
- Boutaud O, Montine TJ, Chang L, Klein WL, Oates JA. PGH2-derived levuglandin adducts increase the neurotoxicity of amyloid beta1-42. J Neurochem. 2006;96:917–923. doi: 10.1111/j.1471-4159.2005.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli R. MALDI mass spectrometry for direct tissue analysis: a new tool for biomarker discovery. J Proteome Res. 2007;4:1138–1142. doi: 10.1021/pr050095+. [DOI] [PubMed] [Google Scholar]
- Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-ToF MS. Anal Chem. 1997;69:4751–4760. doi: 10.1021/ac970888i. [DOI] [PubMed] [Google Scholar]
- Casadonte R, Caprioli RM. Proteomic analysis of formalin-fixed paraffin-embedded tissue by MALDI imaging mass spectrometry. Nat Protoc. 2011;6:1695–1709. doi: 10.1038/nprot.2011.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino S, Groseclose MR, Wagner D. MALDI imaging mass spectrometry: bridging biology and chemistry in drug development. Bioanalysis. 2011;3:2427–2441. doi: 10.4155/bio.11.232. [DOI] [PubMed] [Google Scholar]
- Chacon A, Zagol-Ikapitte I, Amarnath V, Reyzer ML, Oates JA, Caprioli RM, et al. On-tissue chemical derivatization of 3-methoxysalicylamine for MALDI-imaging mass spectrometry. J Mass Spectrom. 2011;46:840–846. doi: 10.1002/jms.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurand P, DaGue BB, Pearsall RS, Threadgill DW, Caprioli RM. Profiling proteins from azoxymethane-induced colon tumors at the molecular level by matrix-assisted laser desorption/ionization mass spectrometry. Proteomics. 2001;1:1320–1326. doi: 10.1002/1615-9861(200110)1:10<1320::AID-PROT1320>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Chaurand P, Schwartz SA, Billheimer D, Xu BJ, Crecelius A, Caprioli RM. Integrating histology and imaging mass spectrometry. Anal Chem. 2004;76:1145–1155. doi: 10.1021/ac0351264. [DOI] [PubMed] [Google Scholar]
- Chen CT, Chen YC. Molecularly imprinted TiO2-matrix-assisted laser desorption/ionization mass spectrometry for selectively detecting alpha-cyclodextrin. Anal Chem. 2004;76:1453–1457. doi: 10.1021/ac034986h. [DOI] [PubMed] [Google Scholar]
- Chen Y, Allegood J, Liu Y, Wang E, Cachon-Gonzalez B, Cox TM, et al. Imaging MALDI mass spectrometry using an oscillating capillary nebulizer matrix coating system and its application to analysis of lipids in brain from a mouse model of Tay-Sachs/Sandhoff disease. Anal Chem. 2008;80:2780–2788. doi: 10.1021/ac702350g. [DOI] [PubMed] [Google Scholar]
- Chughtai K, Heeren RM. Mass spectrometric imaging for biomedical tissue analysis. Chem Rev. 2010;110:3237–3277. doi: 10.1021/cr100012c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobice DF, Mackay CL, Goodwin RJ, McBride A, Langridge-Smith PR, Webster SP, et al. Mass spectrometry imaging for dissecting steroid intracrinology within target tissues. Anal Chem. 2013;85:11576–11584. doi: 10.1021/ac402777k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett DS, Frappier SL, Caprioli RM. MALDI-FTICR imaging mass spectrometry of drugs and metabolites in tissue. Anal Chem. 2008;80:5648–5653. doi: 10.1021/ac800617s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crank JA, Armstrong DW. Towards a second generation of ionic liquid matrices (ILMs) for MALDI-MS of peptides, proteins, and carbohydrates. J Am Soc Mass Spectrom. 2009;20:1790–1800. doi: 10.1016/j.jasms.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Crecelius AC, Cornett DS, Caprioli RM, Williams B, Dawant BM, Bodenheimer B. Three-dimensional visualization of protein expression in mouse brain structures using imaging mass spectrometry. J Am Soc Mass Spectrom. 2005;16:1093–1099. doi: 10.1016/j.jasms.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Dill AL, Ifa DR, Manicke NE, Ouyang Z, Cooks RG. Mass spectrometric imaging of lipids using desorption electrospray ionization. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2883–2889. doi: 10.1016/j.jchromb.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djidja MC, Francese S, Loadman PM, Sutton CW, Scriven P, Claude E, et al. Detergent addition to tryptic digests and ion mobility separation prior to MS/MS improves peptide yield and protein identification for in situ proteomic investigation of frozen and formalin-fixed paraffin-embedded adenocarcinoma tissue sections. Proteomics. 2009;9:2750–2763. doi: 10.1002/pmic.200800624. [DOI] [PubMed] [Google Scholar]
- Douglas DJ, Frank AJ, Mao D. Linear ion traps in mass spectrometry. Mass Spectrom Rev. 2005;24:1–29. doi: 10.1002/mas.20004. [DOI] [PubMed] [Google Scholar]
- Dreisewerd K. The desorption process in MALDI. Chem Rev. 2003;103:395–426. doi: 10.1021/cr010375i. [DOI] [PubMed] [Google Scholar]
- Dreisewerd K. Recent methodological advances in MALDI mass spectrometry. Anal Bioanal Chem. 2014;406:2261–2278. doi: 10.1007/s00216-014-7646-6. [DOI] [PubMed] [Google Scholar]
- Fehniger TE, Suits F, Vegvari A, Horvatovich P, Foster M, Marko-Varga G. Queries of MALDI-imaging global datasets identifying ion mass signatures associated with tissue compartments. Proteomics. 2014;14:862–871. doi: 10.1002/pmic.201300431. [DOI] [PubMed] [Google Scholar]
- Fletcher JS, Lockyer NP, Vickerman JC. Developments in molecular SIMS depth profiling and 3D imaging of biological systems using polyatomic primary ions. Mass Spectrom Rev. 2011;30:142–174. doi: 10.1002/mas.20275. [DOI] [PubMed] [Google Scholar]
- Franck J, Arafah K, Elayed M, Bonnel D, Vergara D, Jacquet A, et al. MALDI imaging mass spectrometry: state of the art technology in clinical proteomics. Mol Cell Proteomics. 2009;8:2023–2033. doi: 10.1074/mcp.R800016-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchser J, Cornett S, Becker M. 2014. High resolution molecular imaging of pharmaceuticals at therapeutic levels. Application Note # FTMS-37.
- Giepmans BN, Adams SR, Ellisman MH, Tsien RY. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- Glunde K, Bhujwalla ZM. Choline kinase alpha in cancer prognosis and treatment. Lancet Oncol. 2007;8:855–857. doi: 10.1016/S1470-2045(07)70289-9. [DOI] [PubMed] [Google Scholar]
- Glunde K, Ackerstaff E, Natarajan K, Artemov D, Bhujwalla ZM. Real-time changes in 1H and 31P NMR spectra of malignant human mammary epithelial cells during treatment with the anti-inflammatory agent indomethacin. Magn Reson Med. 2002;48:819–825. doi: 10.1002/mrm.10295. [DOI] [PubMed] [Google Scholar]
- Glunde K, Jiang L, Moestue SA, Gribbestad IS. MRS and MRSI guidance in molecular medicine: targeting and monitoring of choline and glucose metabolism in cancer. NMR Biomed. 2011;24:673–690. doi: 10.1002/nbm.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnet F, Lemaitre G, Waksman G, Tortajada J. MALDI/MS peptide mass fingerprinting for proteome analysis: identification of hydrophobic proteins attached to eucaryote keratinocyte cytoplasmic membrane using different matrices in concert. Proteome Sci. 2003;1:2. doi: 10.1186/1477-5956-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RJ. Sample preparation for mass spectrometry imaging: small mistakes can lead to big consequences. J Proteomics. 2012;75:4893–4911. doi: 10.1016/j.jprot.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Goodwin RJ, Pitt AR. Mass spectrometry imaging of pharmacological compounds in tissue sections. Bioanalysis. 2010;2:279–293. doi: 10.4155/bio.09.180. [DOI] [PubMed] [Google Scholar]
- Goodwin RJ, Dungworth JC, Cobb SR, Pitt AR. Time-dependent evolution of tissue markers by MALDI-MS imaging. Proteomics. 2008;8:3801–3808. doi: 10.1002/pmic.200800201. [DOI] [PubMed] [Google Scholar]
- Goodwin RJ, Macintyre L, Watson DG, Scullion SP, Pitt AR. A solvent-free matrix application method for matrix-assisted laser desorption/ionization imaging of small molecules. Rapid Commun Mass Spectrom. 2010a;24:1682–1686. doi: 10.1002/rcm.4567. [DOI] [PubMed] [Google Scholar]
- Goodwin RJ, Scullion P, Macintyre L, Watson DG, Pitt AR. Use of a solvent-free dry matrix coating for quantitative matrix-assisted laser desorption ionization imaging of 4-bromophenyl-1,4-diazabicyclo(3.2.2)nonane-4-carboxylate in rat brain and quantitative analysis of the drug from laser microdissected tissue regions. Anal Chem. 2010b;82:3868–3873. doi: 10.1021/ac100398y. [DOI] [PubMed] [Google Scholar]
- Goodwin RJ, Pitt AR, Harrison D, Weidt SK, Langridge-Smith PR, Barrett MP, et al. Matrix-free mass spectrometric imaging using laser desorption ionisation Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun Mass Spectrom. 2011;25:969–972. doi: 10.1002/rcm.4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RJ, Iverson SL, Andren PE. The significance of ambient-temperature on pharmaceutical and endogenous compound abundance and distribution in tissues sections when analyzed by matrix-assisted laser desorption/ionization mass spectrometry imaging. Rapid Commun Mass Spectrom. 2012a;26:494–498. doi: 10.1002/rcm.6125. [DOI] [PubMed] [Google Scholar]
- Goodwin RJ, Nilsson A, Borg D, Langridge-Smith PR, Harrison DJ, Mackay CL, et al. Conductive carbon tape used for support and mounting of both whole animal and fragile heat-treated tissue sections for MALDI MS imaging and quantitation. J Proteomics. 2012b;75:4912–4920. doi: 10.1016/j.jprot.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Grange AH, Donnelly JR, Sovocool GW, Brumley WC. Determination of elemental compositions from mass peak profiles of the molecular ion (m) and the m + 1 and m + 2 ions. Anal Chem. 1996;68:553–560. doi: 10.1021/ac950867t. [DOI] [PubMed] [Google Scholar]
- Greer T, Sturm R, Li L. Mass spectrometry imaging for drugs and metabolites. J Proteomics. 2011;74:2617–2631. doi: 10.1016/j.jprot.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseclose MR, Massion PP, Chaurand P, Caprioli RM. High-throughput proteomic analysis of formalin-fixed paraffin-embedded tissue microarrays using MALDI imaging mass spectrometry. Proteomics. 2008;8:3715–3724. doi: 10.1002/pmic.200800495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdak E, Green FM, Rakowska PD, Seah MP, Salter TL, Gilmore IS. VAMAS interlaboratory study for desorption electrospray ionization mass spectrometry (DESI MS) intensity repeatability and constancy. Anal Chem. 2014;86:9603–9611. doi: 10.1021/ac502075t. [DOI] [PubMed] [Google Scholar]
- Hahn EJ. Autoradiography of cells double labeled with H-3 and C-14. J Histochem Cytochem. 1979;27:1275. doi: 10.1177/27.9.479570. [DOI] [PubMed] [Google Scholar]
- Hamm G, Bonnel D, Legouffe R, Pamelard F, Delbos J-M, Bouzom F, et al. Quantitative mass spectrometry imaging of propranolol and olanzapine using tissue extinction calculation as normalization factor. J Proteomics. 2012;75:4952–4961. doi: 10.1016/j.jprot.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Hankin JA, Murphy RC. Relationship between MALDI IMS intensity and measured quantity of selected phospholipids in rat brain sections. Anal Chem. 2010;82:8476–8484. doi: 10.1021/ac101079v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren RM, Chughtai K. Mass spectrometric imaging for biomedical tissue analysis. Chem Rev. 2010;110:3237–3277. doi: 10.1021/cr100012c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercules DM. 9-Aminoacridine as a matrix for negative mode matrix-assisted laser desorption/ionization. Rapid Commun Mass Spectrom. 2002;16:1575–1581. [Google Scholar]
- Higashi T, Yamauchi A, Shimada K. 2-Hydrazino-1-methylpyridine: a highly sensitive derivatization reagent for oxosteroids in liquid chromatography-electrospray ionization-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;825:214–222. doi: 10.1016/j.jchromb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Holle A, Haase A, Kayser M, Hohndorf J. Optimizing UV laser focus profiles for improved MALDI performance. J Mass Spectrom. 2006;41:705–716. doi: 10.1002/jms.1041. [DOI] [PubMed] [Google Scholar]
- Hsieh Y, Casale R, Fukuda E, Chen J, Knemeyer I, Wingate J, et al. Matrix-assisted laser desorption/ionization imaging mass spectrometry for direct measurement of clozapine in rat brain tissue. Rapid Commun Mass Spectrom. 2006;20:965–972. doi: 10.1002/rcm.2397. [DOI] [PubMed] [Google Scholar]
- Huwiler KG, Mosher DF, Vestling MM. Optimizing the MALDI-ToF-MS observation of peptides containing disulfide bonds. J Biomol Tech. 2003;14:289–297. [PMC free article] [PubMed] [Google Scholar]
- Ifa DR, Gumaelius LM, Eberlin LS, Manicke NE, Cooks RG. Forensic analysis of inks by imaging desorption electrospray ionization (DESI) mass spectrometry. Analyst. 2007;132:461–467. doi: 10.1039/b700236j. [DOI] [PubMed] [Google Scholar]
- Jaroslav P, Strohalm M, Havlyciek V, Volny M. Molecular mass spectrometry imaging in biomedical and life science research. Histochem Cell Biol. 2010;143:423–443. doi: 10.1007/s00418-010-0753-3. [DOI] [PubMed] [Google Scholar]
- Jiao J, Zhang Y, Yang P, Lu H. Hydrazinonicotinic acid as a novel matrix for highly sensitive and selective MALDI-MS analysis of oligosaccharides. Analyst. 2014;140:156–161. doi: 10.1039/c4an01659a. [DOI] [PubMed] [Google Scholar]
- Jones EA, Lockyer NP, Kordys J, Vickerman JC. Suppression and enhancement of secondary ion formation due to the chemical environment in static-secondary ion mass spectrometry. J Am Soc Mass Spectrom. 2007;18:1559–1567. doi: 10.1016/j.jasms.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Kaletaş BK, van der Wiel IM, Stauber J, Güzel C, Kros JM, Luider TM, et al. Sample preparation issues for tissue imaging by imaging MS. Proteomics. 2009;9:2622–2633. doi: 10.1002/pmic.200800364. [DOI] [PubMed] [Google Scholar]
- Kallback P, Shariatgorji M, Nilsson A, Andren PE. Novel mass spectrometry imaging software assisting labeled normalization and quantitation of drugs and neuropeptides directly in tissue sections. J Proteomics. 2012;75:4941–4951. doi: 10.1016/j.jprot.2012.07.034. [DOI] [PubMed] [Google Scholar]
- Karas M. Matrix-assisted laser desorption ionization MS: a progress report. Biochem Soc Trans. 1996;24:897–900. doi: 10.1042/bst0240897. [DOI] [PubMed] [Google Scholar]
- Karas M, Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10 000 daltons. Anal Chem. 1988;60:2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- Karas M, Kruger R. Ion formation in MALDI: the cluster ionization mechanism. Chem Rev. 2003;103:427–440. doi: 10.1021/cr010376a. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Humeny A, Peich CC, Becker CM, Pischetsrieder M. Analysis of protein glycation products by MALDI-ToF/MS. Proc Natl Acad Sci U S A. 2005;1043:249–259. doi: 10.1196/annals.1333.030. [DOI] [PubMed] [Google Scholar]
- Knochenmuss R. MALDI mechanisms: wavelength and matrix dependence of the coupled photophysical and chemical dynamics model. Analyst. 2014;139:147–156. doi: 10.1039/c3an01446k. [DOI] [PubMed] [Google Scholar]
- Lalowski M, Magni F, Mainini V, Monogioudi E, Gotsopoulos A, Soliymani R, et al. Imaging mass spectrometry: a new tool for kidney disease investigations. Nephrol Dial Transplant. 2013;28:1648–1656. doi: 10.1093/ndt/gft008. [DOI] [PubMed] [Google Scholar]
- Lanao JM, Fraile MA. Drug tissue distribution: study methods and therapeutic implications. Curr Pharm Des. 2005;11:3829–3845. doi: 10.2174/138161205774580679. [DOI] [PubMed] [Google Scholar]
- Langer O, Muller M. Methods to assess tissue-specific distribution and metabolism of drugs. Curr Drug Metab. 2004;5:463–481. doi: 10.2174/1389200043335379. [DOI] [PubMed] [Google Scholar]
- Laskin J, Heath BS, Roach PJ, Cazares L, Semmes OJ. Tissue imaging using nanospray desorption electrospray ionization mass spectrometry. Anal Chem. 2012;84:141–148. doi: 10.1021/ac2021322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire R, Menguellet SA, Stauber J, Marchaudon V, Lucot JP, Collinet P, et al. Specific MALDI imaging and profiling for biomarker hunting and validation: fragment of the 11S proteasome activator complex, Reg alpha fragment, is a new potential ovary cancer biomarker. J Proteome Res. 2007;6:4127–4134. doi: 10.1021/pr0702722. [DOI] [PubMed] [Google Scholar]
- Lewellen TK. Recent developments in PET detector technology. Phys Med Biol. 2008;53:R287–R317. doi: 10.1088/0031-9155/53/17/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu Y, Gao M, Zhang X. High throughput detection of tetracycline residues in milk using graphene or graphene oxide as MALDI-ToF MS matrix. J Am Soc Mass Spectrom. 2012;23:1424–1427. doi: 10.1007/s13361-012-0400-4. [DOI] [PubMed] [Google Scholar]
- Liu X, Ide JL, Norton I, Marchionni MA, Ebling MC, Liu LJ, et al. Molecular imaging of drug transit through the blood–brain barrier with MALDI mass spectrometry imaging. Sci Rep. 2013;3:2859. doi: 10.1038/srep02859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mange A, Chaurand H, Perrochia H, Roger P, Caprioli RM, Solassol J. Liquid chromatography-tandem and MALDI imaging mass spectrometry analyses of RCL2/CS100-fixed, paraffin-embedded tissues: proteomics evaluation of an alternate fixative for biomarker discovery. J Proteome Res. 2009;8:5619–5628. doi: 10.1021/pr9007128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manier ML, Reyzer ML, Goh A, Dartois V, Via LE, Barry CE, et al. Reagent precoated targets for rapid in-tissue derivatization of the anti-tuberculosis drug isoniazid followed by MALDI imaging mass spectrometry. J Am Soc Mass Spectrom. 2011;22:1409–1419. doi: 10.1007/s13361-011-0150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko DC, Saffert RT, Cunningham SA, Hyman J, Walsh J, Arbefeville S, et al. Evaluation of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization-time of flight mass spectrometry systems for identification of nonfermenting gram-negative bacilli isolated from cultures from cystic fibrosis patients. J Clin Microbiol. 2012;50:2034–2039. doi: 10.1128/JCM.00330-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meetani MA, Voorhees KJ. MALDI mass spectrometry analysis of high molecular weight proteins from whole bacterial cells: pretreatment of samples with surfactants. J Am Soc Mass Spectrom. 2005;16:1422–1426. doi: 10.1016/j.jasms.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Monro AM. Interspecies comparisons in toxicology: the utility and futility of plasma concentrations of the test substance. Regul Toxicol Pharmacol. 1990;12:137–160. doi: 10.1016/s0273-2300(05)80055-8. [DOI] [PubMed] [Google Scholar]
- Monroe EB, Annangudi SP, Hatcher NG, Gutstein HB, Rubakhin SS, Sweedler JV. SIMS and MALDI MS imaging of the spinal cord. Proteomics. 2008;8:3746–3754. doi: 10.1002/pmic.200800127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. Tissue concentrations: do we ever learn? J Antimicrob Chemother. 2008;61:235–237. doi: 10.1093/jac/dkm476. [DOI] [PubMed] [Google Scholar]
- Nemes P, Vertes A. Laser ablation electrospray ionization for atmospheric pressure molecular imaging mass spectrometry. Methods Mol Biol. 2010;656:159–171. doi: 10.1007/978-1-60761-746-4_9. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Fehniger TE, Gustavsson L, Andersson M, Kenne K, Marko-Varga G, et al. Fine mapping the spatial distribution and concentration of unlabeled drugs within tissue micro-compartments using imaging mass spectrometry. PLoS ONE. 2010;5:e11411. doi: 10.1371/journal.pone.0011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A, Forngren B, Bjurström S, Goodwin RJA, Basmaci E, Gustafsson I, et al. In situ mass spectrometry imaging and ex vivo characterization of renal crystalline deposits induced in multiple preclinical drug toxicology studies. PLoS ONE. 2012;7:e47353. doi: 10.1371/journal.pone.0047353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren H, Hagenhoff B, Malmberg P, Nilsson M, Richter K. Bioimaging ToF-SIMS: high resolution 3D imaging of single cells. Microsc Res Tech. 2007;70:969–974. doi: 10.1002/jemt.20502. [DOI] [PubMed] [Google Scholar]
- Parson WB, Koeniger SL, Johnson RW, Erickson J, Tian Y, Stedman C, et al. Analysis of chloroquine and metabolites directly from whole-body animal tissue sections by liquid extraction surface analysis (LESA) and tandem mass spectrometry. J Mass Spectrom. 2012;47:1420–1428. doi: 10.1002/jms.3068. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, Davenport AP, McGrath JC, Peters JA, Southan C, Spedding M, Yu W, Harmar AJ NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl. Acids Res. 2014;42:D1098–1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson J, Norris JL, Aerni HR, Svenningsson P, Caprioli RM, Andren PE. Molecular profiling of experimental Parkinson's disease: direct analysis of peptides and proteins on brain tissue sections by MALDI mass spectrometry. J Proteome Res. 2004;3:289–295. doi: 10.1021/pr0499747. [DOI] [PubMed] [Google Scholar]
- Prideaux B, Stoeckli M. Mass spectrometry imaging for drug distribution studies. J Proteomics. 2012;75:4999–5013. doi: 10.1016/j.jprot.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Quirke JM, Van Berkel GJ. Electrospray tandem mass spectrometric study of alkyl 1-methylpyridinium ether derivatives of alcohols. J Mass Spectrom. 2001;36:1294–1300. doi: 10.1002/jms.233. [DOI] [PubMed] [Google Scholar]
- Reyzer ML, Hsieh Y, Ng K, Korfmacher WA, Caprioli RM. Direct analysis of drug candidates in tissue by matrix-assisted laser desorption/ionization mass spectrometry. J Mass Spectrom. 2003;38:1081–1092. doi: 10.1002/jms.525. [DOI] [PubMed] [Google Scholar]
- Rohner TC, Staab D, Stoeckli M. MALDI mass spectrometric imaging of biological tissue sections. Mech Ageing Dev. 2005;126:177–185. doi: 10.1016/j.mad.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Rubakhin SS, Jurchen JC, Monroe EB, Sweedler JV. Imaging mass spectrometry: fundamentals and applications to drug discovery. Drug Discov Today. 2005;10:823–837. doi: 10.1016/S1359-6446(05)03458-6. [DOI] [PubMed] [Google Scholar]
- Scannell JW, Blanckley A, Boldon H, Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nat Rev Drug Discov. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- Schafer KC, Denes J, Albrecht K, Szaniszlo T, Balog J, Skoumal R, et al. In vivoin situ tissue analysis using rapid evaporative ionization mass spectrometry. Angew Chem Int Ed Engl. 2009;48:8240–8242. doi: 10.1002/anie.200902546. [DOI] [PubMed] [Google Scholar]
- Schober Y, Guenther S, Spengler B, Rompp A. Single cell matrix-assisted laser desorption/ionization mass spectrometry imaging. Anal Chem. 2012;84:6293–6297. doi: 10.1021/ac301337h. [DOI] [PubMed] [Google Scholar]
- Schwartz SA, Reyzer ML, Caprioli RM. Direct tissue analysis using matrix-assisted laser desorption/ionization mass spectrometry: practical aspects of sample preparation. J Mass Spectrom. 2003;38:699–708. doi: 10.1002/jms.505. [DOI] [PubMed] [Google Scholar]
- Scott AJ, Jones JW, Orschell CM, MacVittie TJ, Kane MA, Ernst RK. Mass spectrometry imaging enriches biomarker discovery approaches with candidate mapping. Health Phys. 2014;106:120–128. doi: 10.1097/HP.0b013e3182a4ec2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariatgorji M, Nilsson A, Goodwin RJA, Svenningsson P, Schintu N, Banka Z, et al. Deuterated matrix-assisted laser desorption ionization matrix uncovers masked mass spectrometry imaging signals of small molecules. Anal Chem. 2012;84:7152–7157. doi: 10.1021/ac301498m. [DOI] [PubMed] [Google Scholar]
- Shroff R, Svatos A. 1,8-Bis(dimethylamino)naphthalene: a novel superbasic matrix for matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis of fatty acids. Rapid Commun Mass Spectrom. 2009;23:2380–2382. doi: 10.1002/rcm.4143. [DOI] [PubMed] [Google Scholar]
- Skold K, Svensson M, Nilsson A, Zhang X, Nydahl K, Caprioli RM, et al. Decreased striatal levels of PEP-19 following MPTP lesion in the mouse. J Proteome Res. 2006;5:262–269. doi: 10.1021/pr050281f. [DOI] [PubMed] [Google Scholar]
- Smith NF, Raynaud FI, Workman P. The application of cassette dosing for pharmacokinetic screening in small-molecule cancer drug discovery. Mol Cancer Ther. 2007;6:428–440. doi: 10.1158/1535-7163.MCT-06-0324. [DOI] [PubMed] [Google Scholar]
- Solon EG. Autoradiography: high-resolution molecular imaging in pharmaceutical discovery and development. Expert Opin Drug Discov. 2007;2:503–514. doi: 10.1517/17460441.2.4.503. [DOI] [PubMed] [Google Scholar]
- Solon EG, Schweitzer A, Stoeckli M, Prideaux B. Autoradiography, MALDI-MS, and SIMS-MS imaging in pharmaceutical discovery and development. AAPS J. 2010;12:11–26. doi: 10.1208/s12248-009-9158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooy K, Webster SP, Noble J, Binnie M, Walker BR, Seckl JR, et al. Partial deficiency or short-term inhibition of 11beta-hydroxysteroid dehydrogenase type 1 improves cognitive function in aging mice. J Neurosci. 2010;30:13867–13872. doi: 10.1523/JNEUROSCI.2783-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli M, Staab D, Schweitzer A, Gardiner J, Seebach D. Imaging of a beta-peptide distribution in whole-body mice sections by MALDI mass spectrometry. J Am Soc Mass Spectrom. 2007;18:1921–1924. doi: 10.1016/j.jasms.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Streletskii AV, Kozlova A, Esipov DS, Kaiushin AL, Korosteleva MD, Esipov SE. Determination of oligonucleotide molecular masses by MS-MALDI. Bioorg Khim. 2005;31:151–158. doi: 10.1007/s11171-005-0019-3. [DOI] [PubMed] [Google Scholar]
- Stumpf WE. Whole-body and microscopic autoradiography to determine tissue distribution of biopharmaceuticals – target discoveries with receptor micro-autoradiography engendered new concepts and therapies for vitamin D. Adv Drug Deliv Rev. 2013;65:1086–1097. doi: 10.1016/j.addr.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Takats Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004;306:471–473. doi: 10.1126/science.1104404. [DOI] [PubMed] [Google Scholar]
- Todd PJ, Schaaff TG, Chaurand P, Caprioli RM. Organic ion imaging of biological tissue with secondary ion mass spectrometry and matrix-assisted laser desorption/ionization. J Mass Spectrom. 2001;36:355–369. doi: 10.1002/jms.153. [DOI] [PubMed] [Google Scholar]
- Tolmachev AV, Monroe ME, Jaitly N, Petyuk VA, Adkins JN, Smith RD. Mass measurement accuracy in analyses of highly complex mixtures based upon multidimensional recalibration. Anal Chem. 2006;78:8374–8385. doi: 10.1021/ac0606251. [DOI] [PubMed] [Google Scholar]
- Troendle FJ, Reddick CD, Yost RA. Detection of pharmaceutical compounds in tissue by matrix-assisted laser desorption/ionization (MALDI) and laser desorption/chemical ionization (LD/CI) MS/MS with a quadrupole ion trap. J Am Soc Mass Spectrom. 1999;10:1315–1321. [Google Scholar]
- Vickerman JC. Molecular imaging and depth profiling by mass spectrometry – SIMS, MALDI or DESI? Analyst. 2011;136:2199–2217. doi: 10.1039/c1an00008j. [DOI] [PubMed] [Google Scholar]
- Weaver EM, Hummon AB. Imaging mass spectrometry: from tissue sections to cell cultures. Adv Drug Deliv Rev. 2013;65:1039–1055. doi: 10.1016/j.addr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Wheeler OH, Rosado-Lojo O. Kinetics of formation and hydrolysis of steroid Girard-T hydrazones. Tetrahedron. 1962;18:477–482. [Google Scholar]
- White RE, Manitpisitkul P. Pharmacokinetic theory of cassette dosing in drug discovery screening. Drug Metab Dispos. 2001;29:957–966. [PubMed] [Google Scholar]
- Wiseman JM, Ifa DR, Song Q, Cooks RG. Tissue imaging at atmospheric pressure using desorption electrospray ionization (DESI) mass spectrometry. Angew Chem Int Ed Engl. 2006;45:7188–7192. doi: 10.1002/anie.200602449. [DOI] [PubMed] [Google Scholar]
- Wiseman JM, Ifa DR, Zhu Y, Kissinger CB, Manicke NE, Kissinger PT, et al. Desorption electrospray ionization mass spectrometry: Imaging drugs and metabolites in tissues. Proc Natl Acad Sci U S A. 2008;105:18120–18125. doi: 10.1073/pnas.0801066105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KJ, Steding A, Becker CH. Matrix-assisted laser desorption time-of-flight mass spectrometry of oligonucleotides using 3-hydroxypicolinic acid as an ultraviolet-sensitive matrix. Rapid Commun Mass Spectrom. 1993;7:142–146. doi: 10.1002/rcm.1290070206. [DOI] [PubMed] [Google Scholar]
- Yanes O, Woo HK, Northen TR, Oppenheimer SR, Shriver L, Apon J, et al. Nanostructure initiator mass spectrometry: tissue imaging and direct biofluid analysis. Anal Chem. 2009;81:2969–2975. doi: 10.1021/ac802576q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Altman E, Perry MB, Li J. Study of matrix additives for sensitive analysis of lipid A by matrix-assisted laser desorption ionization mass spectrometry. Appl Environ Microbiol. 2010;76:3437–3443. doi: 10.1128/AEM.03082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierhut ML, Yen YF, Chen AP, Bok R, Albers MJ, Zhang V, et al. Kinetic modeling of hyperpolarized 13C1-pyruvate metabolism in normal rats and TRAMP mice. J Magn Reson. 2010;202:85–92. doi: 10.1016/j.jmr.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]