Abstract
Background and Purpose
Nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor agonists display a promising analgesic profile in preclinical studies. However, supraspinal N/OFQ produced hyperalgesia in rodents and such effects have not been addressed in primates. Thus, the aim of this study was to investigate the effects of centrally administered ligands on regulating pain and itch in non-human primates. In particular, nociceptive thresholds affected by intracisternal N/OFQ were compared with those of morphine and substance P, known to provide analgesia and mediate hyperalgesia, respectively, in humans.
Experimental Approach
Intrathecal catheters were installed to allow intracisternal and lumbar intrathecal administration in awake and unanaesthetized rhesus monkeys. Nociceptive responses were measured using the warm water tail-withdrawal assay. Itch scratching responses were scored from videotapes recording behavioural activities of monkeys in their home cages. Antagonist studies were conducted to validate the receptor mechanisms underlying intracisternally elicited behavioural responses.
Key Results
Intracisternal morphine (100 nmol) elicited more head scratches than those after intrathecal morphine. Distinct dermatomal scratching locations between the two routes suggest a corresponding activation of supraspinal and spinal μ receptors. Unlike intracisternal substance P, which induced hyperalgesia, intracisternal N/OFQ (100 nmol) produced antinociceptive effects mediated by NOP receptors. Neither peptide increased scratching responses.
Conclusions and Implications
Taken together, these results demonstrated differential actions of ligands in the primate supraspinal region in regulating pain and itch. This study not only improves scientific understanding of the N/OFQ-NOP receptor system in pain processing but also supports the therapeutic potential of NOP-related ligands as analgesics.
Tables of Links
| Targets | |
|---|---|
| GPCRs | |
| NOP receptors | |
| μ receptors |
| Ligands |
|---|
| J-113397 |
| Morphine |
| N/OFQ, nociceptin/orphanin FQ |
| Naltrexone |
| Substance P |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
Pain symptoms derived from various clinical disorders are treated with widely used opioid analgesics (Nuckols et al., 2014). The systemic effects of these analgesics are generally considered to be the integrative outcome of drug actions at peripheral, spinal and supraspinal sites (Sawynok and Liu, 2014). In the past, non-human primates have been used to characterize the systemic effects of opioid analgesics (Negus et al., 1998; Butelman et al., 2004; Sukhtankar et al., 2014), but only in a few studies focusing on the spinal or supraspinal drug effects (Ko et al., 1999; 2006; Hu et al., 2010). Given that the use of non-human primates provides the most phylogenetically appropriate evaluation of the pharmacodynamic and pharmacokinetic actions of drugs (Lin and Ko, 2013; Phillips et al., 2014), it is important to define the drug actions at both spinal and supraspinal levels in non-human primates to enhance the translatability of the preclinical outcomes to humans.
Spinal administration of morphine provides pain relief by activating μ receptors, but it often produces undesirable side effects such as itch and pruritus (Ganesh and Maxwell, 2007; Dominguez and Habib, 2013). Importantly, non-human primate models have been established to simulate the therapeutic profile of spinal morphine in humans. In particular, intrathecal administration of morphine produces long-lasting antinociception and itch scratching responses on the trunk that correspond with the activation of spinal μ-receptors (Ko and Naughton, 2000; Ko et al., 2006). Nonetheless, the effects of morphine at the supraspinal level have not been studied in non-human primates. Given the broad expression of μ-receptors in the CNS (Mansour et al., 1988; Peckys and Landwehrmeyer, 1999; Sim-Selley et al., 1999), it will be valuable to determine whether intracisternal administration of morphine produces antinociception. More importantly, intracisternal versus lumbar intrathecal morphine-elicited dermatomal scratching can be studied to determine whether they correspond with the activation of supraspinal versus spinal μ-receptors.
Nociceptin/orphanin FQ (N/OFQ) is the endogenous peptide agonist of the N/OFQ peptide receptor (NOP) (Meunier et al., 1995; Reinscheid et al., 1995). The N/OFQ-NOP receptor system has been implicated in a wide range of biological actions. In particular, NOP-related ligands show promise to be developed as novel spinal analgesics without morphine-associated side effects (Lambert, 2008; Calo and Guerrini, 2013; Lin and Ko, 2013; Molinari et al., 2013). Spinal administration of N/OFQ produces antinociception in both rodents (Xu et al., 1996; Tian et al., 1997) and non-human primates (Ko et al., 2006; Ko and Naughton, 2009). However, when delivered supraspinally, N/OFQ elicits hyperalgesic effects in rodents (Meunier et al., 1995; Reinscheid et al., 1995; Calo et al., 1998). To date, the effects of supraspinal N/OFQ in non-human primates are still unknown. In line with the opposing effects of spinal and supraspinal N/OFQ in rodents, it is interesting to note that systemic administration of a selective non-peptidic NOP agonist Ro64-6198 did not produce antinociception in rodents (Jenck et al., 2000). In contrast, systemic Ro64-6198 produces full antinociceptive effects in non-human primates (Ko et al., 2009; Cremeans et al., 2012; Sukhtankar et al., 2014). These findings suggest a functional species difference in the systemic effects of ligands of NOP receptors. Nevertheless, considering the possible systemic integration of drug effects at spinal and supraspinal levels, it is crucial to investigate the supraspinal effects of N/OFQ in non-human primates (Lin and Ko, 2013; Schröder et al., 2014). Such studies will not only provide pharmacological evidence of N/OFQ actions at the supraspinal level – a long-standing query in the N/OFQ-NOP receptor system – but also allow scientists to define the role of diverse neuropeptides in modulating somatosensory processes in the supraspinal region of non-human primates.
The aim of this study was to investigate behavioural responses to intracisternal and lumbar intrathecal administration of ligands that differentially regulate pain and itch sensation in non-human primates. Previously, drug delivery through the intracisternal or lumbar intrathecal route in non-human primates required anaesthesia to perform the injection procedure (Lipman et al., 1988; Ko and Naughton, 2000; Broadbear et al., 2004). Anaesthesia prevents the behavioural measurement of immediate drug effects and daily repeated central administration. Therefore, in this study, we first set out to establish a non-human primate model of intrathecal catheterization to enable drug delivery through the catheter tip located in the lumbar region for spinal administration or located in the cisterna magna for supraspinal administration. Second, intracisternal morphine-elicited scratching locations were compared with those of lumbar intrathecal morphine to distinguish supraspinal versus spinal drug actions. Third, nociceptive thresholds affected by intracisternal N/OFQ were compared with morphine and substance P, which are known to provide analgesia and mediate hyperalgesia, respectively, in humans (Wang et al., 1979; Angst et al., 2000; Larson et al., 2000; Seybold, 2009).
Methods
Animals
All animal care and experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health (Bethesda, MD, USA) and approved by the Institutional Animal Care and Use Committee in Wake Forest University School of Medicine (Winston-Salem, NC, USA). All care and procedures were as humane as possible. Studies are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Eight adult male and female rhesus monkeys (Macaca mulatta), 10–17 years, 5.8–14.6 kg, were kept at an indoor facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (Frederick, MD, USA). Animals were individually housed in stainless steel cages in species-specific rooms with environmental controls set to maintain 21–25°C, 40–60% relative humidity and a 12 h light (06:30–18:30)/12 h dark (18:30–06:30) cycle. Their daily diet consisted of approximately 25–30 biscuits (Purina Monkey Chow; Ralston Purina Co., St. Louis, MO, USA), fresh fruit and water ad libitum. Small amounts of primate treats and various cage-enrichment devices were supplied as forms of environmental enrichment. Animals were not exposed to any opioid compound for 1 month prior to the present study. All monkeys used had previously been trained in the warm water tail-withdrawal assay and acclimated to being video-recorded in-cage.
Procedures
Intrathecal catheterization surgery
Prior to the surgery, animals were given atropine (0.04 mg·kg−1, s.c.), buprenorphine (0.01–0.03 mg·kg−1, i.m.), dexamethasone (2 mg·kg−1, i.v.) and cefotaxime (500 mg, i.v.) for pain management and to prevent inflammation and infection. Animals were then anaesthetized with ketamine (10 mg·kg−1, i.m.). A catheter was placed in a saphenous vein for administration of lactated Ringer's solution during the surgery. The animals were intubated and maintained under anaesthesia with inhaled isoflurane (1–2% in 1 L/min O2). The surgical sites were prepared for strict aseptic surgery by cleansing with povidone-iodine followed by 70% isopropyl alcohol. Vital signs including heart rate, respiration rate, indirect blood pressure and body temperature were monitored during the surgery as well as in immediate post-operative recovery. Animals received buprenorphine (0.02 mg·kg−1, i.m.) and meloxicam (0.15 mg·kg−1, s.c.) as post-operative analgesics and ceftiofur (2.2 mg·kg−1, i.m.) as the post-operative antibiotic. Post-operative care and incision site observations were performed daily for 14 days or until fully healed.
A dorsal midline incision centred over the L4-5 vertebral segment was made for lumbar intrathecal catheterization. A 4–5 mm hole (hemilaminectomy) was drilled in the left lateral aspect of the L4 or 5 vertebral body to expose the dura mater. A small incision was then made in the dura mater/arachnoid membrane. The intrathecal catheter (3.0 Fr CBAS®, Instech Solomon catalogue number MIDLOA-CBAS-C30, Plymouth Meeting, PA, USA) was then inserted into the intrathecal space and advanced rostrally so as to place the catheter tip in the lumbar region (approximately L1-2, spinal level) or in the cisterna magna (approximately C1-2, supraspinal level). Eight monkeys were randomly divided into two groups (n = 4 per group). The catheter tip was placed in the lumbar region of one group of animals or in the cisterna magna of the other group. Confirmation of placement of the catheter within the intrathecal space was determined by observing CSF flow by gravity from the tip of the catheter. The skin incision was lengthened so as to expose the lumbodorsal fascia on the opposite side of where the hemilaminectomy was created for placement of a s.c. access port (Instech Solomon MIDLO LOVOL™). The catheter from the lumbar intrathecal space was routed s.c. from the hemilaminectomy site to the vascular access port site, and was attached to the port. All wounds were closed in multiple layers using appropriately sized, long-absorbing suture material.
Intrathecal catheter placement and patency were confirmed by radiography before and after the experiments. The animals were sedated with ketamine (10 mg·kg−1, i.m.); the area around the implanted intrathecal catheter was shaved and prepared aseptically with povidone-iodine and 70% isopropyl alcohol scrubs. A sterile Huber Point needle was used to inject 1 mL of Omnipaque 300 contrast (GE Healthcare Inc., Princeton, NJ, USA); radiographic images were taken in ventral-dorsal projection both before and 30 min after the injection. Upon completion of the radiographic imaging, the catheters were flushed with 0.5 mL saline and the animals were returned to their home cages and monitored during recovery from anaesthesia.
Nociceptive responses
The warm water tail-withdrawal assay was used to evaluate thermal antinociceptive or pronociceptive drug effects (Ko and Naughton, 2009). Monkeys were seated in primate restraint chairs in the designated procedure room, and the lower parts of their shaved tails (approximately 15 cm) were immersed in a thermal flask containing water maintained at either 42, 46 or 50°C. Water at 42 and 46°C was used as non-noxious stimuli, and 50°C water was used as an acute noxious stimulus. Tail-withdrawal latencies were measured at each temperature using a computerized timer by experimenters who were unaware of the experimental conditions. If the monkeys did not remove their tails within 20 s (cut-off), the flask was removed and a maximum time of 20 s was recorded. Test sessions began with baseline measurements at each temperature. Subsequent tail-withdrawal latencies were measured at 15, 30, 45 and 60 min after intracisternal or lumbar intrathecal administration.
Itch scratching responses
Monkeys were recorded in their home cages for scratching behaviour, which has been associated previously with itch sensation (Ko et al., 2004). Each 60 min recording session was conducted at 5 min immediately after intracisternal or lumbar intrathecal drug administration in the designated procedure room. As noted, a maximum of 5 min were needed to transport monkeys from the procedure room to their home cages. A scratch was defined as one brief (<1 s) episode of scraping contact of the forepaw or hind paw on the skin surface of the area above the shoulder (defined as head scratches, i.e. skin dermatomes corresponding with cervical nerves C1-C4 and trigeminal nerves) or the area below the shoulder (defined as body scratches, i.e. skin dermatomes corresponding with cervical nerves C5-C8, all thoracic, lumbar and sacral nerves). Head, body and total scratches were counted and summed for each 15 min time block, as well as the entire 60 min recording session by experimenters who were unaware of the experimental conditions.
Experimental designs
The first part of the study was to measure the behavioural effects of intracisternal morphine sulphate (100 nmol) and to compare these effects with those of lumbar intrathecal morphine at the same dose. This dose was selected based on previous studies (Ko et al., 2006; Hu et al., 2010) showing maximal scratching responses and antinociception. Both nociceptive responses and scratching responses were determined during a 1 h time course after administration. The percentage of head and body scratches was compared, following intracisternal or lumbar intrathecal morphine, to distinguish the magnitudes and temporal changes of dermatomal scratching responses.
The second part of the study was to investigate the behavioural effects elicited by intracisternal N/OFQ (0, 10 and 100 nmol). Intracisternal substance P and morphine, within the same dose range (10–100 nmol), were given to show pronociceptive and antinociceptive actions, respectively, as compared with those of N/OFQ in the same animals. Intracisternal saline was given as an experimental control. The tail-withdrawal latencies in 46 and 50°C water were measured to detect potential pronociceptive or antinociceptive effects. Total scratches in each 15 min time block as well as the entire 60 min recording session were also determined. Antagonist studies using the NOP receptor antagonist (J-113397, 0.1 mg·kg−1, s.c.) (Ko et al., 2009) and μ-receptor antagonist (naltrexone, 0.1 mg·kg−1, s.c.) (Ko et al., 2004) with a 15 min pretreatment were conducted to validate the receptor mechanisms underlying behavioural responses of intracisternal N/OFQ (100 nmol) or morphine (100 nmol). The antagonist dose and pretreatment time were chosen based on previous studies showing that J-113397 and naltrexone produced NOP and μ-receptor antagonist effects, respectively, in this species (Ko et al., 2004; 2009). The effects of intracisternal N/OFQ (10–100 nmol) combined with morphine (100 nmol) were also measured in order to determine if N/OFQ produced any anti-morphine action. All dosing conditions were randomized in the same group of animals for this study.
Data analysis
Mean values (mean ± SEM) were calculated from individual values for all behavioural end points. Comparisons were made for the same monkeys across all test sessions in the same experiment. The time course of tail-withdrawal latencies and scratching responses in each 15 min time block were analysed by two-way anova with repeated measures, followed by Bonferroni's multiple comparisons test. Scratching responses in the entire 60 min recording session were analysed by one-way anova with repeated measures, followed by Bonferroni's multiple comparisons test. The criterion for significance for all tests was set at P < 0.05.
Materials
N/OFQ, morphine sulphate, naltrexone HCl (National Institute on Drug Abuse, Bethesda, MD, USA) and substance P (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in sterile water. J-113397 (Tocris Bioscience, Minneapolis, MN, USA) was dissolved in a solution of dimethyl sulfoxide/Tween 80/sterile water in a ratio of 1:1:8. For central administration, a total volume of 1 mL was administered intracisternally or lumbar intrathecally through the s.c. access port followed by 0.35 mL of sterile saline to flush out the dead volume of the port and catheter. For s.c. administration, naltrexone and J-113397 were administered in a volume of 0.1 mL·kg−1. There was a minimum of 1 week interval between drug administrations.
Results
The feasibility and success of surgical lumbar intrathecal catheterization in monkeys is demonstrated in Figure 1. The correct placement of the catheter tip in the cisterna magna was confirmed by comparing radiograph images taken at 0 min before (Figure 1C) and 30 min (Figure 1A and B) after administration of Omnipaque 300 contrast.
Figure 1.
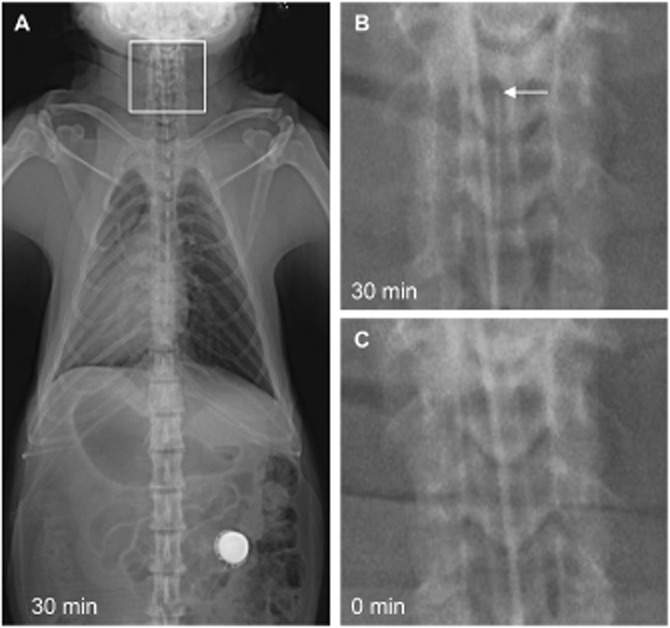
Representative radiographic images of a catheterized monkey with the catheter tip located in the cisterna magna (C1-2). (A) Image in ventral-dorsal projection taken 30 min after administration of Omnipaque 300 contrast. (B) Zoomed-in image of the boxed area in panel A showing the catheter tip with an arrow. (C) Image taken immediately before administration of the contrast as a baseline control.
The behavioural effects of 100 nmol morphine administered intracisternally were compared with those of the same dose administered lumbar intrathecally, in monkeys. Both intracisternal and lumbar intrathecal morphine produced significant antinociceptive effects as manifested by increased tail-withdrawal latencies in 50°C water in a time-dependent manner [F(4,24) = 150.6; P < 0.05] (Figure 2A). Morphine administered by either route also evoked profound scratching responses in a time-dependent manner [F(3,18) = 13.64; P < 0.05] that peaked 20–35 min after administration and were sustained at a similar level throughout the remaining observation period (Figure 2B). There was no statistical difference in the magnitudes of antinociceptive effects and the total scratching numbers between intracisternal and intrathecal morphine (Figure 2A and B).
Figure 2.
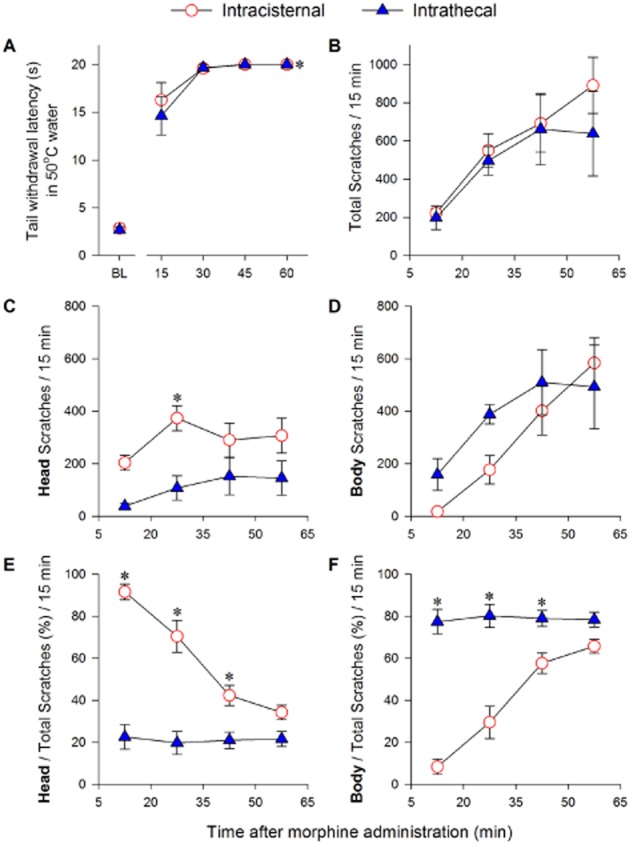
Comparison of behavioural responses elicited by intracisternal and lumbar intrathecal morphine (100 nmol). (A) Time course of thermal nociceptive responses evaluated by measuring tail-withdrawal latency in 50°C water at baseline (BL) before and 15, 30, 45 and 60 min after administration. *P < 0.05, significantly different from the baseline for all time points for both intracisternal and lumbar intrathecal morphine. (B–F) Time course of scratching responses in each 15 min time block during a 60 min observation period. Behavioural responses were recorded 5 min after morphine administration. The number of total scratches (B), head and body scratches (C and D), were counted and the percentage of head and body scratches (E and F) were calculated. Each data point represents mean ± SEM (n = 4). *P < 0.05, significant difference between intracisternal and lumbar intrathecal morphine at the corresponding time point.
To define a potential difference in itch scratching patterns elicited by intracisternal versus lumbar intrathecal morphine, scratches above the shoulder (head scratches) and scratches below the shoulder (body scratches) were counted separately. Figure 2C and D shows that intracisternal and lumbar intrathecal morphine time-dependently evoked scratches on both head [F(3,18) = 6.14; P < 0.05] and body [F(3,18) = 14.31; P < 0.05]. Intracisternal morphine elicited more head scratches and less body scratches than lumbar intrathecal morphine starting at 5–20 min after drug administration. Moreover, the percentage of head and body scratches were compared to illustrate distinct scratching patterns between intracisternal and lumbar intrathecal morphine [F(1,6) = 48.79; P < 0.05] (Figure 2E and F). Lumbar intrathecal morphine elicited about 80% body scratches and 20% head scratches. This pattern stayed the same throughout the entire 60 min observation session. In contrast, during 5–20 min after administration, intracisternal morphine elicited about 90% head scratches and only 10% body scratches. This scratching pattern changed significantly in a time-dependent manner [F(3,18) = 27.19; P < 0.05]. As time progressed, the percentage of head scratches decreased whereas the percentage of body scratches increased. Specifically, at each of the first three 15 min observation sessions (i.e. 5–20, 20–35 and 35–50 min after administration), intracisternal morphine elicited a significantly higher percentage of head scratches than did lumbar intrathecal morphine (Figure 2E) whereas lumbar intrathecal morphine elicited a significantly higher percentage of body scratches than did intracisternal morphine (Figure 2F). At the last 15 min observation session (i.e. 50–65 min after administration), there was no significant difference between intracisternal and lumbar intrathecal morphine in terms of their scratching locations (Figure 2E and F).
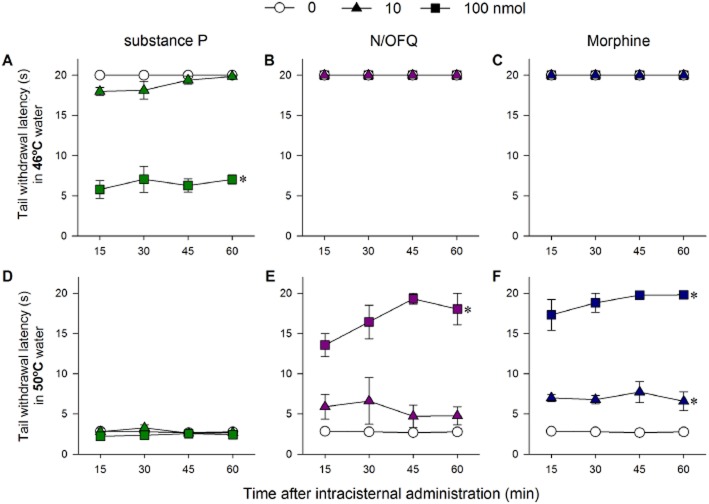
The distinct changes in the thermal nociceptive thresholds of monkeys to intracisternal N/OFQ, substance P and morphine, used in doses of 10 and 100 nmol are summarised in Figure 3. In 46°C water, a non-noxious stimulus, intracisternal substance P dose-dependently produced hyperalgesic effects [F(2,6) = 204.3; P < 0.05], whereas both N/OFQ and morphine had no effects (Figure 3A, B and C). In 50°C water, a noxious stimulus, intracisternal substance P at either 10 or 100 nmol did not produce antinociceptive responses (Figure 3D). In contrast, both N/OFQ [F(2,6) = 50.3; P < 0.05] and morphine [F(2,6) = 278.4; P < 0.05] produced antinociceptive responses in 50°C water in a dose-dependent manner (Figure 3E and F). Intracisternal N/OFQ-induced antinociception was reversed by the NOP receptor antagonist J-113397 (Supporting Information Fig. S1). In addition, intracisternal N/OFQ (10 or 100 nmol), when given together with morphine (100 nmol) did not affect intracisternal morphine-induced antinociception against 50°C water [F(2,6) = 0.6; P > 0.05] (Supporting Information Fig. S2).
Figure 3.
Comparison of thermal nociceptive responses of intracisternal N/OFQ, substance P and morphine at the dose of 0, 10 and 100 nmol. (A–C) Time course of tail-withdrawal latency in 46°C water. (D–F) Time course of tail-withdrawal latency in 50°C water. Behavioural responses were measured 15, 30, 45 and 60 min after intracisternal administration. Each data point represents mean ± SEM (n = 4). *P < 0.05, significantly different from vehicle, for all time points.
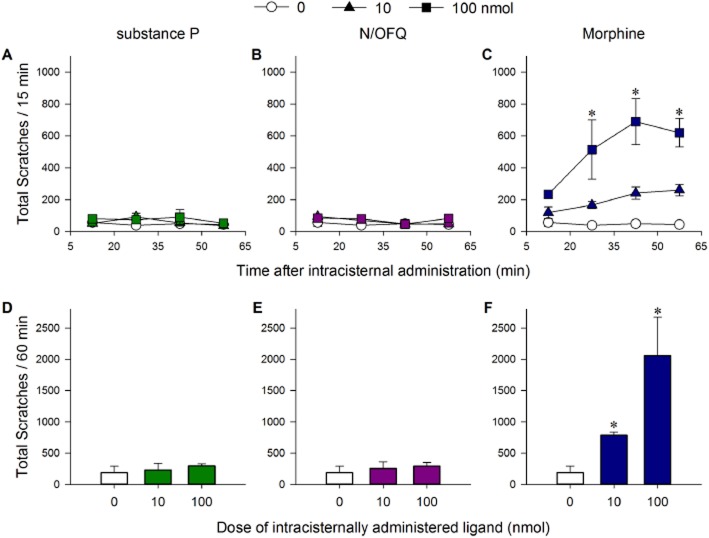
The distinct scratching responses of monkeys receiving intracisternal N/OFQ, substance P or morphine are shown in Figure 4. Although 100 nmol intracisternal N/OFQ and substance P produced antinociceptive and hyperalgesic effects, respectively, as shown in Figure 3, they did not elicit scratching responses (Figure 4A, B, D and E). In contrast, intracisternal morphine evoked significant scratching responses in a dose- [F(2,6) = 33.43; P < 0.05] and time-dependent [F(3,9) = 4.36; P < 0.05] manner (Figure 4C and F). Intracisternal morphine-elicited scratching was blocked by the μ-receptor antagonist naltrexone (Supporting Information Fig. S3). It is worth noting that intracisternal administration of N/OFQ, substance P and morphine at any of the doses tested here did not cause any observable side effects, including sedation and motor impairment.
Figure 4.
Comparison of scratching responses of intracisternal N/OFQ, substance P and morphine at the dose of 0, 10 and 100 nmol. (A–C) Time course of total scratches in each 15 min time block. (D–F) Total scratches in the entire 60 min observation period. Behavioural responses were recorded for a total of 60 min starting at 5 min after intracisternal administration. Each data point represents mean ± SEM (n = 4). *P < 0.05, significantly different from vehicle, at the corresponding time point (C) or as total responses (F).
Discussion
To our knowledge, this is the first study to establish the lumbar intrathecal catheterization surgery in non-human primates to study drug actions at both supraspinal and spinal levels. There are two major novel findings reported here. First, intracisternal morphine elicited much more head scratching than did lumbar intrathecal morphine. Dermatomal scratching locations elicited by intracisternal and lumbar intrathecal morphine revealed a corresponding activation of supraspinal and spinal μ-receptors. Second, intracisternal N/OFQ produced antinociceptive effects in non-human primates, which advances our knowledge of the N/OFQ-NOP receptor system in the supraspinal region.
Conventionally, anaesthesia is required in non-human primates to deliver drugs directly to the CNS (Ko et al., 1999; Ko and Naughton, 2000; Broadbear et al., 2004) or collect CSF (Yu et al., 1997; Clingerman et al., 2010). Anaesthesia makes it easier to perform acute puncture in the lumbar or cisterna magna, but it prevents the measurement of early drug actions. With proficient surgical techniques, we can install and maintain lumbar intrathecal catheterization in non-human primates. All animals in this study recovered well from the surgery without complications. As indicated by radiographic imaging (Figure 1), the catheter tip was successfully placed and maintained in the cisterna magna for supraspinal drug delivery. The catheterization and tip placement is surgically challenging and extreme caution needs to be taken to avoid nerve damage. Nonetheless, with successful surgical outcomes, animals with implanted catheters allow easy drug delivery lumbar intrathecally or intracisternally. This study represents an improvement for studying drug actions in the CNS of awake, unanaesthetized non-human primates.
Similar to lumbar intrathecal morphine (Ko and Naughton, 2000; Ko et al., 2004), intracisternal morphine produced full antinociceptive effects and elicited robust itch scratching responses (Figure 2A and B). Approximately during the first 30 min after administration, intracisternal morphine elicited most head and facial scratches. In contrast, lumbar intrathecal morphine elicited the most body (trunk and limbs) scratches. Distinct scratching locations between the two routes are consistent with skin dermatomes that correspond with trigeminal and spinal nerves (Lee et al., 2008). Interestingly, the percentage of intracisternal morphine-induced head scratches decreased gradually whereas the percentage of body scratches increased over a 60 min observation session (Figure 2E and F). This phenomenon may be due to a caudal distribution of intracisternal morphine along with CSF and reflects the temporal and spatial changes in the sites of action for intracisternal morphine-induced scratching. More importantly, these scratching responses were naltrexone-reversible (Supporting Information Fig. S3), indicating a critical role of supraspinal μ-receptors in eliciting itch sensation. The medullary dorsal horn has been demonstrated to be one central site for μ-receptors to produce facial scratching in non-human primates (Thomas et al., 1993). In addition, two populations of trigeminothalamic tract neurons were identified in rats to explain opioid-induced itch and analgesia (Moser and Giesler, 2013). These findings together indicate that itch can be elicited centrally, i.e. robust head and body scratches are derived from activation of supraspinal and spinal μ-receptors respectively. Further studies using non-human primates can define the role of endogenous neuropeptides from both supraspinal and spinal regions in regulating itch sensation.
Another intriguing finding is that in non-human primates, intracisternal N/OFQ and morphine similarly produced antinociceptive effects (Figure E and F). In contrast, intracisternal substance P produced hyperalgesic effects (Figure 3A). Thus, by testing N/OFQ, morphine and substance P in the same subjects, the present study reveals distinct functions of ligands centrally modulating nociceptive thresholds in non-human primates. Intracerebroventricular administration of N/OFQ in rodents caused either hyperalgesia (Meunier et al., 1995; Reinscheid et al., 1995; Calo et al., 1998) or no effects (Tian et al., 1997). Clearly, there are species differences in the behavioural effects of the supraspinal N/OFQ-NOP receptor system. Because antinociceptive effects of intracisternal N/OFQ could be blocked by the NOP receptor antagonist J-113397 (Supporting Information Fig. S1), it is reasonable to suppose that these functional differences are derived from the nociceptive neurons expressing NOP receptors. The nucleotide sequence and amino acid sequence of the NOP receptors from rhesus monkeys are 96–98% identical to human NOP receptors (Koga et al., 2009). Anatomical studies have indicated that the distribution of NOP receptors in the CNS of non-human primates is largely similar to those observed in humans (Peluso et al., 1998; Berthele et al., 2003; Bridge et al., 2003). Notable areas of species differences include lower expression levels in raphe nuclei in non-human primates, compared with rodents (Neal et al., 1999a,b; Berthele et al., 2003; Bridge et al., 2003), and higher expression levels in caudate nucleus and putamen in non-human primates and humans, but not in rodents (Peluso et al., 1998; Berthele et al., 2003; Bridge et al., 2003). However, there has been no neurobiological study of the functions of NOP receptors on nociceptive processes, particularly in the supraspinal neural circuits (Schröder et al., 2014).
Unlike rodent studies showing opposing nociceptive responses between supraspinal and spinal N/OFQ, antinociceptive effects of intracisternal N/OFQ in non-human primates may offer a hypothetical explanation to a profound species difference in the antinociceptive efficacy and tolerability of systemically administered NOP-related ligands, as pointed out in a recent review (Schröder et al., 2014). It is possible that the general ineffectiveness of systemic administration of NOP-related ligands in rodents is an integrated outcome of the opposite nociceptive effects at spinal and supraspinal levels (Jenck et al., 2000; Schröder et al., 2014). In non-human primates, the potent antinociception produced by systemic NOP-related ligands may result from the synergistic effects of spinal and supraspinal antinociceptive actions (Ko et al., 2009; Cremeans et al., 2012). As intracisternal N/OFQ did not produce anti-morphine actions (Supporting Information Fig. S2), it would be very interesting to further investigate whether intracisternal N/OFQ was additive or synergistic with morphine by testing a range of dosing combinations, with the isobologram analysis (Cremeans et al., 2012). Collectively, effects of intracisternal NOP-related ligands on nociception can be further studied in non-human primates under different pain modalities (Butelman et al., 2004; Hu et al., 2010; Sukhtankar et al., 2014) in order to establish a translational profile of their therapeutic potential as strong analgesics against diverse pain symptoms.
Despite its hyperalgesic effect, intracisternal substance P, at either 10 or 100 nmol, did not elicit scratching responses, similar to intracisternal N/OFQ, but in contrast to the robust scratching responses elicited by intracisternal morphine (Figure 4). Lumbar intrathecal administration of substance P did not elicit a significant scratching response (Ko and Naughton, 2009). Given that scratching responses are indicative of itch sensation in non-human primates (Ko and Naughton, 2000; Ko et al., 2004; Hu et al., 2010; Sukhtankar et al., 2014), these findings suggest that substance P at both spinal and supraspinal levels of the CNS does not play a significant role in eliciting itch sensation in non-human primates. Scratching behaviour in rodents can be interpreted as a sign of itch or pain (De Castro-Costa et al., 1987; Kuraishi et al., 1995; Lee et al., 2003). Some rodent studies suggest centrally administered substance P elicited pain-like behaviour (Hylden and Wilcox, 1981; Mishra and Hoon, 2013), but other rodent studies indicate that central substance P plays a role in mediating itch and scratching behaviour (Bossut et al., 1988; Akiyama et al., 2013). Our findings from non-human primates seem to be in agreement with human studies, indicating that central substance P is involved in nociceptive processes (Larson et al., 2000; Seybold, 2009). Previously, intracisternal drug delivery was used to study the function of receptors located in the hypothalamus (Ko et al., 2003; Broadbear et al., 2004). With an intrathecal catheter located in the cisterna magna, these non-human primates will provide a valuable foundation for a variety of future research studies. They will not only allow the collection of CSF of animals under different conditions but also enable scientists to investigate the effects of endogenous ligands on both behavioural and physiological functions in conscious, unanaesthetized non-human primates.
In summary, this study provides the first functional evidence of the different supraspinal actions of N/OFQ, morphine and substance P in regulating pain and itch in non-human primates. With this advance in surgical techniques, pharmacological studies can be correspondingly advanced to define the functional role of neuropeptides and their receptors located in the supraspinal and spinal regions for regulating somatosensory function. It certainly offers a potentially very useful translational model to study pain and analgesics, and itch and antipruritics, in terms of their efficacy and sites of action. More importantly, these pharmacological findings address a long-standing fundamental question in the N/OFQ-NOP receptor system, namely to determine the antinociceptive effects of supraspinal N/OFQ in non-human primates. Medicinal chemists have developed several novel analgesics with varied efficacies on both NOP and μ-receptors (Husbands, 2013; Molinari et al., 2013; Zaveri et al., 2013; Linz et al., 2014). This study not only improves our understanding of N/OFQ-NOP receptor pharmacology in pain processing but also facilitates future studies of identifying novel ligands with optimal efficacies on NOP and μ-receptors as analgesics and their potential exploration in clinical studies.
Acknowledgments
The authors thank Dr. James Eisenach for his assistance to initiate this study. In addition, the authors thank Ms. Colette Cremeans, Erin Gruley, Jillian Odom and Shannon Wittenauer for excellent technical assistance. Research reported in this publication was supported by the US National Institutes of Health, NIAMS (R01-AR059193 and R21-AR064456) and NIDA (R01-DA032568 and R21-DA035359) and the US Department of Defense (W81XWH-13-2-0045). The content is solely the responsibility of the authors and does not necessarily represent the official views of the US federal agencies.
Glossary
- N/OFQ
nociceptin/orphanin FQ
- NOP receptor
N/OFQ peptide receptor
Author contributions
All authors performed the research. H. D., K. H., D. D. S. and M. C. K. designed the research study. H. D., D. D. S. and M. C. K. analysed the data. H. D., K. H., T. S. , V. M. and M. C. K. wrote the paper.
Conflict of interest
All authors declare that there are no conflicts of interest. M. C. K. has received research contracts from Grünenthal GmbH and Purdue Pharma LP.
Supporting Information
Figure S1 Effects of the NOP receptor antagonist J-113397 on intracisternal N/OFQ-induced antinociception in rhesus monkeys. J-113397 (0.1 mg·kg−1) or vehicle (0.1 mL·kg−1) was administered s.c. 15 min before administration of intracisternal N/OFQ 100 nmol. Tail-withdrawal latencies in 50°C water were measured 15, 30, 45 and 60 min after intracisternal administration. Each data point represents mean ± SEM (n = 4). Symbols represent different dosing conditions for the same monkeys. *P < 0.05, significantly different from vehicle, for all time points.
Figure S2 Comparison of thermal nociceptive responses of intracisternal morphine (100 nmol) given alone or in combination of N/OFQ, at doses of 10 or 100 nmol. Tail-withdrawal latencies in 50°C water were measured 15, 30, 45 and 60 min after intracisternal administration. Each data point represents mean ± SEM (n = 4).
Figure S3 Effects of the μ-receptor antagonist naltrexone on intracisternal morphine-elicited itch scratching responses in rhesus monkeys. Naltrexone (0.1 mg·kg−1) or vehicle (0.1 mL·kg−1) was administered s.c. 15 min before administration of intracisternal morphine 100 nmol. (A) Time course of total scratches in each 15 min time block. (B) Total scratches in the entire 60 min observation period. Behavioural responses were recorded for a total of 60 min starting at 5 min after intracisternal administration. Each data point represents mean ± SEM (n = 4). *P < 0.05, significantly different from vehicle, for all time points (A) or as total responses (B).
References
- Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, et al. Roles for substance P and gastrin-releasing peptide as neurotransmitters released by primary afferent pruriceptors. J Neurophysiol. 2013;109:742–748. doi: 10.1152/jn.00539.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst MS, Ramaswamy B, Riley ET, Stanski DR. Lumbar epidural morphine in humans and supraspinal analgesia to experimental heat pain. Anesthesiology. 2000;92:312–324. doi: 10.1097/00000542-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Berthele A, Platzer S, Dworzak D, Schadrack J, Mahal B, Buttner A, et al. [3H]-nociceptin ligand-binding and nociceptin opioid receptor mrna expression in the human brain. Neuroscience. 2003;121:629–640. doi: 10.1016/s0306-4522(03)00484-6. [DOI] [PubMed] [Google Scholar]
- Bossut D, Frenk H, Mayer DJ. Is substance P a primary afferent neurotransmitter for nociceptive input? II. Spinalization does not reduce and intrathecal morphine potentiates behavioral responses to substance P. Brain Res. 1988;455:232–239. doi: 10.1016/0006-8993(88)90081-9. [DOI] [PubMed] [Google Scholar]
- Bridge KE, Wainwright A, Reilly K, Oliver KR. Autoradiographic localization of (125)i[Tyr(14)] nociceptin/orphanin FQ binding sites in macaque primate CNS. Neuroscience. 2003;118:513–523. doi: 10.1016/s0306-4522(02)00927-2. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rivier JE, Rice KC, Woods JH. Corticotropin-releasing hormone antagonists, astressin B and antalarmin: differing profiles of activity in rhesus monkeys. Neuropsychopharmacology. 2004;29:1112–1121. doi: 10.1038/sj.npp.1300410. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, Kreek MJ. Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther. 2004;311:155–163. doi: 10.1124/jpet.104.068411. [DOI] [PubMed] [Google Scholar]
- Calo G, Guerrini R. Medicinal chemistry, pharmacology, and biological actions of peptide ligands selective for the nociceptin/orphanin FQ receptor. In: Ko MC, Husbands SM, editors. Research and Development of Opioid-Related Ligands. ACS Symposium Series 1131. Washington DC: American Chemical Society; 2013. pp. 275–325. In: (eds). [Google Scholar]
- Calo G, Rizzi A, Marzola G, Guerrini R, Salvadori S, Beani L, et al. Pharmacological characterization of the nociceptin receptor mediating hyperalgesia in the mouse tail withdrawal assay. Br J Pharmacol. 1998;125:373–378. doi: 10.1038/sj.bjp.0702087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clingerman KJ, Spray S, Flynn C, Fox HS. A technique for intracisternal collection and administration in a rhesus macaque. Lab Anim (NY) 2010;39:307–311. doi: 10.1038/laban1010-307. [DOI] [PubMed] [Google Scholar]
- Cremeans CM, Gruley E, Kyle DJ, Ko MC. Roles of mu-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J Pharmacol Exp Ther. 2012;343:72–81. doi: 10.1124/jpet.112.194308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro-Costa M, Gybels J, Kupers R, Van Hees J. Scratching behaviour in arthritic rats: a sign of chronic pain or itch? Pain. 1987;29:123–131. doi: 10.1016/0304-3959(87)90186-2. [DOI] [PubMed] [Google Scholar]
- Dominguez JE, Habib AS. Prophylaxis and treatment of the side-effects of neuraxial morphine analgesia following cesarean delivery. Curr Opin Anaesthesiol. 2013;26:288–295. doi: 10.1097/ACO.0b013e328360b086. [DOI] [PubMed] [Google Scholar]
- Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333. doi: 10.2165/00003495-200767160-00003. [DOI] [PubMed] [Google Scholar]
- Hu E, Calo G, Guerrini R, Ko MC. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain. 2010;148:107–113. doi: 10.1016/j.pain.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husbands SM. Buprenorphine and related orvinols. In: Ko MC, Husbands SM, editors. Research and Development of Opioid-Related Ligands. ACS Symposium Series 1131. Washington DC: American Chemical Society; 2013. pp. 275–325. In: (eds). [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981;217:212–215. doi: 10.1016/0006-8993(81)90203-1. [DOI] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, et al. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci U S A. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology. 2000;92:795–805. doi: 10.1097/00000542-200003000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Naughton NN. Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys. J Pain. 2009;10:509–516. doi: 10.1016/j.jpain.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, Woods JH. Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther. 1999;291:1113–1120. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Willmont KJ, Lee H, Flory GS, Woods JH. Ultra-long antagonism of kappa opioid agonist-induced diuresis by intracisternal nor-binaltorphimine in monkeys. Brain Res. 2003;982:38–44. doi: 10.1016/s0006-8993(03)02938-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Song MS, Edwards T, Lee H, Naughton NN. The role of central mu opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther. 2004;310:169–176. doi: 10.1124/jpet.103.061101. [DOI] [PubMed] [Google Scholar]
- Ko MC, Wei H, Woods JH, Kennedy RT. Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies. J Pharmacol Exp Ther. 2006;318:1257–1264. doi: 10.1124/jpet.106.106120. [DOI] [PubMed] [Google Scholar]
- Ko MC, Woods JH, Fantegrossi WE, Galuska CM, Wichmann J, Prinssen EP. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34:2088–2096. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Ichikawa D, Nambu H, Azuma-Kanoh T, Sakai N, Takaki-Kawagoe H, et al. Cloning and characterization of the rhesus monkey nociceptin/orphanin FQ receptor. Genes Genet Syst. 2009;84:319–325. doi: 10.1266/ggs.84.319. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Lambert DG. The nociceptin/orphanin FQ receptor: a target with broad therapeutic potential. Nat Rev Drug Discov. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- Larson AA, Giovengo SL, Russell IJ, Michalek JE. Changes in the concentrations of amino acids in the cerebrospinal fluid that correlate with pain in patients with fibromyalgia: implications for nitric oxide pathways. Pain. 2000;87:201–211. doi: 10.1016/S0304-3959(00)00284-0. [DOI] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH, Ko MC. Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol. 2003;14:501–508. doi: 10.1097/01.fbp.0000095082.80017.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MW, McPhee RW, Stringer MD. An evidence-based approach to human dermatomes. Clin Anat. 2008;21:363–373. doi: 10.1002/ca.20636. [DOI] [PubMed] [Google Scholar]
- Lin AP, Ko MC. The therapeutic potential of nociceptin/orphanin FQ receptor agonists as analgesics without abuse liability. ACS Chem Neurosci. 2013;4:214–224. doi: 10.1021/cn300124f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz K, Christoph T, Tzschentke TM, Koch T, Schiene K, Gautrois M, et al. Cebranopadol: a novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J Pharmacol Exp Ther. 2014;349:535–548. doi: 10.1124/jpet.114.213694. [DOI] [PubMed] [Google Scholar]
- Lipman B, Palmer D, Noble J, Haughton V, Collier D. Effect of lumbar puncture on flow of cerebrospinal fluid. Invest Radiol. 1988;23:359–360. doi: 10.1097/00004424-198805000-00005. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Anatomy of CNS opioid receptors. Trends Neurosci. 1988;11:308–314. doi: 10.1016/0166-2236(88)90093-8. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari S, Camarda V, Rizzi A, Marzola G, Salvadori S, Marzola E, et al. [Dmt1]N/OFQ(1–13)-NH2: a potent nociceptin/orphanin FQ and opioid receptor universal agonist. Br J Pharmacol. 2013;168:151–162. doi: 10.1111/j.1476-5381.2012.02115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser HR, Giesler GJ., Jr Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci. 2013;33:6093–6101. doi: 10.1523/JNEUROSCI.0216-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999a;406:503–547. [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. J Comp Neurol. 1999b;412:563–605. [PubMed] [Google Scholar]
- Negus SS, Gatch MB, Mello NK, Zhang X, Rice K. Behavioral effects of the delta-selective opioid agonist SNC80 and related compounds in rhesus monkeys. J Pharmacol Exp Ther. 1998;286:362–375. [PubMed] [Google Scholar]
- Nuckols TK, Anderson L, Popescu I, Diamant AL, Doyle B, Di Capua P, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience. 1999;88:1093–1135. doi: 10.1016/s0306-4522(98)00251-6. [DOI] [PubMed] [Google Scholar]
- Peluso J, LaForge KS, Matthes HW, Kreek MJ, Kieffer BL, Gaveriaux-Ruff C. Distribution of nociceptin/orphanin FQ receptor transcript in human central nervous system and immune cells. J Neuroimmunol. 1998;81:184–192. doi: 10.1016/s0165-5728(97)00178-1. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, 't Hart BA, et al. Why primate models matter. Am J Primatol. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Liu J. Contributions of peripheral, spinal, and supraspinal actions to analgesia. Eur J Pharmacol. 2014;734:114–121. doi: 10.1016/j.ejphar.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Schröder W, Lambert DG, Ko MC, Koch T. Functional plasticity of the N/OFQ-NOP receptor system determines analgesic properties of NOP receptor agonists. Br J Pharmacol. 2014;171:3777–3800. doi: 10.1111/bph.12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009;194:451–491. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Daunais JB, Porrino LJ, Childers SR. Mu and kappa1 opioid-stimulated [35S]guanylyl-5′-O-(gamma-thio)-triphosphate binding in cynomolgus monkey brain. Neuroscience. 1999;94:651–662. doi: 10.1016/s0306-4522(99)00344-9. [DOI] [PubMed] [Google Scholar]
- Sukhtankar DD, Lee H, Rice KC, Ko MC. Differential effects of opioid-related ligands and NSAIDs in nonhuman primate models of acute and inflammatory pain. Psychopharmacology (Berl) 2014;231:1377–1387. doi: 10.1007/s00213-013-3341-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DA, Williams GM, Iwata K, Kenshalo DR, Jr, Dubner R. The medullary dorsal horn. A site of action of morphine in producing facial scratching in monkeys. Anesthesiology. 1993;79:548–554. [PubMed] [Google Scholar]
- Tian JH, Xu W, Fang Y, Mogil JS, Grisel JE, Grandy DK, et al. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br J Pharmacol. 1997;120:676–680. doi: 10.1038/sj.bjp.0700942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149–151. doi: 10.1097/00000542-197902000-00013. [DOI] [PubMed] [Google Scholar]
- Xu XJ, Hao JX, Wiesenfeld-Hallin Z. Nociceptin or antinociceptin: potent spinal antinociceptive effect of orphanin FQ/nociceptin in the rat. Neuroreport. 1996;7:2092–2094. [PubMed] [Google Scholar]
- Yu J, Butelman ER, Woods JH, Chait BT, Kreek MJ. Dynorphin A (1–8) analog, E-2078, crosses the blood-brain barrier in rhesus monkeys. J Pharmacol Exp Ther. 1997;282:633–638. [PubMed] [Google Scholar]
- Zaveri NT, Jiang F, Olsen C, Polgar WE, Toll L. Designing bifunctional NOP receptor-mu opioid receptor ligands from NOP receptor-selective scaffolds. Part I. Bioorg Med Chem Lett. 2013;23:3308–3313. doi: 10.1016/j.bmcl.2013.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effects of the NOP receptor antagonist J-113397 on intracisternal N/OFQ-induced antinociception in rhesus monkeys. J-113397 (0.1 mg·kg−1) or vehicle (0.1 mL·kg−1) was administered s.c. 15 min before administration of intracisternal N/OFQ 100 nmol. Tail-withdrawal latencies in 50°C water were measured 15, 30, 45 and 60 min after intracisternal administration. Each data point represents mean ± SEM (n = 4). Symbols represent different dosing conditions for the same monkeys. *P < 0.05, significantly different from vehicle, for all time points.
Figure S2 Comparison of thermal nociceptive responses of intracisternal morphine (100 nmol) given alone or in combination of N/OFQ, at doses of 10 or 100 nmol. Tail-withdrawal latencies in 50°C water were measured 15, 30, 45 and 60 min after intracisternal administration. Each data point represents mean ± SEM (n = 4).
Figure S3 Effects of the μ-receptor antagonist naltrexone on intracisternal morphine-elicited itch scratching responses in rhesus monkeys. Naltrexone (0.1 mg·kg−1) or vehicle (0.1 mL·kg−1) was administered s.c. 15 min before administration of intracisternal morphine 100 nmol. (A) Time course of total scratches in each 15 min time block. (B) Total scratches in the entire 60 min observation period. Behavioural responses were recorded for a total of 60 min starting at 5 min after intracisternal administration. Each data point represents mean ± SEM (n = 4). *P < 0.05, significantly different from vehicle, for all time points (A) or as total responses (B).