Abstract
Background and Purpose
α-Galactosylceramide (α-GalCer), a pleiotropic immunomodulator with therapeutic potential in neoplastic, autoimmune and allergic diseases, activates invariant natural killer T-cells throughCD1-restricted receptors for α-GalCer on antigen-presenting cells, inducing cytokine secretion. However the haemopoietic effects of α-GalCer remain little explored.
Experimental Approach
α-GalCer-induced modulation of eosinophil production in IL-5-stimulated bone marrow cultures was examined in wild-type (BALB/c, C57BL/6) mice and their mutants lacking CD1, inducible NOS (iNOS), CD95 and IFN-γ, along with the effects of lymphocytes; IFN-γ; caspase and iNOS inhibitors; non-steroidal anti-inflammatory drugs (NSAIDs) and LTD4; and dexamethasone.
Key Results
α-GalCer (10−6–10−8M) suppressed IL-5-stimulated eosinopoiesis by inducing apoptosis. α-GalCer pretreatment in vivo (100 μg·kg−1, i.v.) suppressed colony formation by GM-CSF-stimulated bone marrow progenitors in semi-solid cultures. α-GalCer and dexamethasone synergistically promoted eosinophil maturation. Suppression of eosinophil production by α-GalCer was prevented by aminoguanidine and was undetectable in bone marrow lacking iNOS, CD95, CD28; or CD1d. Separation on Percoll gradients and depletion of CD3+ cells made bone marrow precursors unresponsive to α-GalCer. Responsiveness was restored with splenic lymphocytes. Experiments with (i) IFN-γ-deficient bone marrow, alone or co-cultured with spleen T-cells from wild-type, but not from CD1d-deficient, donors; (ii) IFN-γ neutralization; and (iii) recombinant IFN-γ, showed that these effects of α-GalCer were mediated by IFN-γ. Effects of α-GalCer on eosinophil production were blocked by LTD4 and NSAIDs.
Conclusions and Implications
α-GalCer activation of IFN-γ-secreting, CD1d-restricted lymphocytes induced iNOS-CD95-dependent apoptosis in developing eosinophils. This pathway is initiated by endogenous regulatory lymphocytes, antagonised by LTD4, NSAIDs and aminoguanidine, and modified by dexamethasone.
Tables of Links
| Targets | |
|---|---|
| Enzymes | |
| iNOS |
| Ligands | |
|---|---|
| Aminoguanidine | IFN-γ |
| Aspirin | IL-5 |
| Dexamethasone | Indomethacin |
| GM-CSF | LTD4 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (Alexander et al., 2013).
Introduction
α-Galactosylceramide (α-GalCer), a natural immunomodulator (see Berkers and Ovaa, 2005; Molano and Porcelli, 2006), has therapeutic potential in malignant (Konishi et al., 2004), autoimmune (Molano and Porcelli, 2006; Yamamura et al., 2007) and allergic diseases (Hachem et al., 2005; Matsuda et al., 2005; Benoit et al., 2007; Iwamura and Nakayama, 2007; Kim et al., 2009; Umetsu and De Kruyff, 2010). α-GalCer activates natural killer T (NKT) cells, a specialized subset of innate regulatory/effector lymphocytes bearing α/β T-cell receptors, specific for glycolipid antigens and restricted by CD1 (CD1d in mice) (De Libero and Mori, 2012; Adams, 2014), which exert important immunoregulatory and effector functions, including rapid and intense secretion of cytokines (Yamamura et al., 2007; Matsuda et al., 2008; Bjordahl et al., 2012; Adams, 2014). NKT cells comprise two immunologically distinct, but functionally similar, subpopulations (Matsuda et al., 2008; Bjordahl et al., 2012): ‘semi-invariant’ (iNKT) cells, which recognize α-GalCer through CD1d-restricted α/β receptors bearing an ‘invariant’ rearrangement in their α chains, with a matching assortment of β chains, and type II NKT cells, which lack invariant rearrangements and do not recognize α-GalCer.
Many studies (summarized in Umetsu and De Kruyff, 2010) suggest that the first subset (iNKT cells) promotes the multi-step progression towards an asthma phenotype in humans and mice. Some human (Jyonouchi et al., 2014) and experimental (Kim et al., 2004; Chuang et al., 2011) studies suggest that α-GalCer and/or iNKT cells increase eosinophilic inflammation, an important component of allergic reactions. By contrast, other studies show that iNKT cells suppress asthma through the secretion of IFN-γ, thereby reinforcing immunomodulation therapy (Hachem et al., 2005; Iwamura and Nakayama, 2007; Fujita et al., 2009; Bourgeois et al., 2011). In murine lungs, eosinophil infiltrates are reduced by α-GalCer treatment (Benoit et al., 2007) or by IL-33-activated iNKT cells (Bourgeois et al., 2011).
However, it remains unclear whether bone marrow eosinopoiesis, which underlies chronic eosinophilic infiltrates in allergic disease, provides a separate target for regulation by α-GalCer. In mice, IFN-γ potently induces inducible NOS (iNOS) (Kleinert et al., 2004; Rauch et al., 2013), and NKT cells, producers of IFN-γ, are found in murine bone marrow and spleen (Matsuda et al., 2008). These observations by others, along with our long-term interest on the extrinsic regulation of bone marrow eosinopoiesis by drugs and cytokines (Xavier-Elsas et al., 2008; Queto et al., 2010a,b), prompted us to examine the effects on bone marrow, of α-GalCer, iNKT cells and IFN-γ produced by iNKT cells.
We had previously characterized (Jones et al., 2004; Gaspar-Elsas et al., 2009; Queto et al., 2010a) a proapoptotic mechanism for regulation of eosinophil production, dependent upon iNOS and the ligand for CD95/Fas (CD95L/FasL), which operates in vivo during airway allergen challenge of sensitized mice, and accounts for the therapeutic effect of diethylcarbamazine (DEC), a leukotriene synthesis inhibitor (Matthews and Murphy, 1982), in experimental asthma (Queto et al., 2010a). Although activation of this mechanism by DEC and other drugs (de Luca et al., 2013) prevents the induction of eosinophilia by allergen (Masid-de-Brito et al., 2014b), its blockade by LTD4 and non-steroidal anti-inflammatory drugs (NSAIDs) (Xavier-Elsas et al., 2008) protects developing eosinophils from apoptosis. Furthermore, dexamethasone protects developing eosinophils from apoptosis by preventing expression of iNOS (de Luca et al., 2013).
Here, we describe the suppression of bone marrow eosinophil production by IFN-γ-producing bone marrow and spleen lymphocytes, which are activated by α-GalCer, restricted by CD1d, dependent upon CD28 and counteracted by agonists that block the iNOS-CD95L pathway, including dexamethasone, LTD4 and NSAIDs.
Methods
Animals, ethical aspects, in vivo procedures
All animal care and experimental procedures complied with and were approved by the institutional Animal Ethical Committees (CEUA No. L-010/04 and CEUA No. L-002/09). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 82 animals were used in the experiments described here.
Wild-type mice of the BALB/c and C57BL/6 (B6) strains and their mutants lacking iNOS (Laubach et al., 1995), CD28 (Shahinian et al., 1993), CD1 (Sonoda et al., 1999), or IFN-γ (Dalton et al., 2000), bred at CECAL-FIOCRUZ, Rio de Janeiro, Brazil, or lacking CD95 due to a spontaneous mutation in the BALB/c background (http://jaxmice.jax.org/strain/000485.html), bred at FMRP-USP, Brazil, were used at 6–8 weeks of age as a source of bone marrow and spleen cells. Naïve animals were used, unless otherwise indicated. Where indicated, mice were injected i.p. with 100 μg·kg−1 α-GalCer in a 400 μL volume of PBS containing 2% DMSO (controls received vehicle), once, 24 h before killing and bone marrow collection.
Bone marrow studies
Bone marrow cells were collected from both femurs of naïve mice, washed, counted in a haemocytometer, seeded at a density of 106 cells mL−1 of RPMI 1640 medium, 10% FCS and rmIL-5 (1 ng·mL−1; Gaspar-Elsas et al., 1997) in 24-well clusters, and incubated at 37°C, 5% CO2/95% air for 7 days. These conditions allow the addition of agonists and inhibitors at different times, unlike semi-solid culture, where proper mixing and diffusion of substances added after plating is restricted. Eosinopoiesis and its modulation by various agents were strictly dependent upon IL-5 (Gaspar-Elsas et al., 1997; 2000a; 2009). Where indicated, cultures received α-GalCer (10−6–10−12 M), together with indomethacin (10−7 M), aspirin (10−7 M) (Xavier-Elsas et al., 2008; Queto et al., 2010b), the iNOS inhibitor aminoguanidine (10−4 M; Queto et al., 2010a) or the caspase inhibitor zVAD-fmk (20 μM; de Luca et al., 2013). Each agonist or inhibitor was added only once, immediately after IL-5, at the beginning of the culture period. Where indicated, α-GalCer was added at various times after initiation of the culture to define the period during which it must act in order to suppress eosinophil production. Where indicated, cultures received dexamethasone (10−7 M) to enhance eosinophil precursor proliferation while keeping eosinophils cytologically immature and aggregated (Gaspar-Elsas et al., 2009). To these cultures, as well as to IL-5 controls, α-GalCer was added to evaluate its ability to promote dissociation and terminal differentiation of eosinophils (Gaspar-Elsas et al., 2009). Cells present in 7 day cultures were resuspended, collected, counted, cytocentrifuged and stained for eosinophil peroxidase (EPO; Gaspar-Elsas et al., 2009). EPO+ cells were counted and photographed at 400× magnification, under oil. Eosinophil numbers were calculated from total and differential counts.
In another set of experiments, bone marrow from α-GalCer-injected and vehicle-injected control mice was used for semi-solid culture (Gaspar-Elsas et al., 2000a) in 35 mm culture dishes, in triplicate. One mL of Iscove's modifed Dulbecco's medium 20% FCS, containing 2 × 105 bone marrow cells and GM-CSF (2 ng·mL−1 final concentration), was pre-incubated for 2 h with or without α-GalCer before mixing with agar to 0.3% final concentration and plating. Colonies (<50 cells) were scored at day 7 under the inverted microscope at low magnification. In additional controls, the frequency of eosinophil, granulocyte (G), macrophage (M) and granulocyte-macrophage (GM) colonies was determined on agar layers dried (50°C), mounted on microscope slides, stained for EPO and scored under high magnification (Gaspar-Elsas et al., 1997; 2000a; Queto et al., 2010b).
Where indicated, total bone marrow was separated on PercollTM discontinuous (45%-60%-75%; Maximiano et al., 2005) gradients to deplete mature cells, including eosinophils. The immature cells at the 45%-60% interface (the ‘P2 layer’) were collected, washed, counted and adjusted to 106 cells mL−1 medium with 10% FCS for (i) culture as above; (ii) further depletion with anti-CD3 immunobeads; or (iii) staining with anti-CD3 followed by flow cytometric analysis. In control experiments, the mature cells at the 60%-75% interface were collected and cultured as above. Alternatively, P2 layer cells were co-cultured with T lymphocytes from spleens of syngeneic wild-type (BALB/c in Figure 6C–F; B6 in Figure 7) or CD1-knockout (KO) (in Figure 7) donors.
Figure 6.
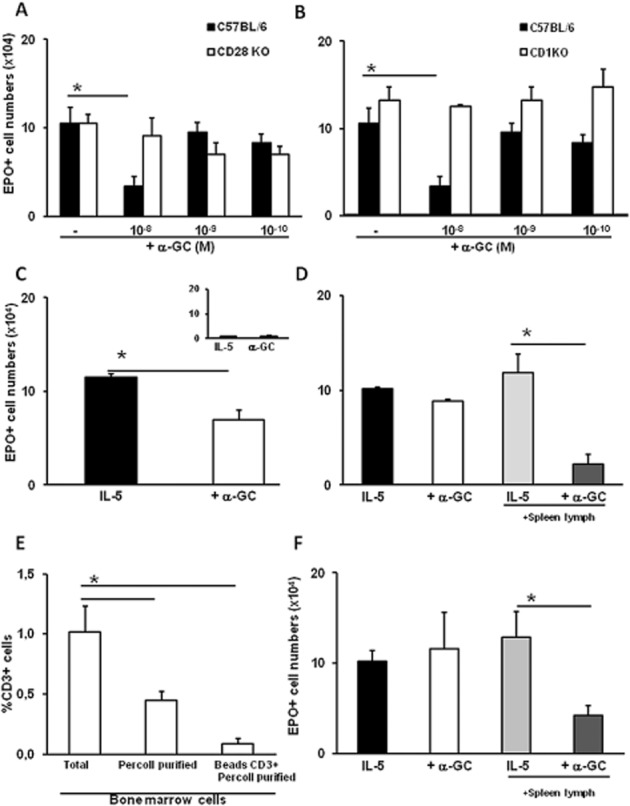
Responsiveness to α-GalCer in bone marrow requires CD1d, CD28 and lymphocytes. Liquid bone marrow cultures were established from C57BL/6 wild-type control (panels A and B) or from CD28-deficient (CD28KO) (panel A) or CD1-deficient (CD1KO) (panel B) mutant mice, or from BALB/c wild-type mice (panels C–F) in the presence of IL-5 alone, or together with α-GalCer (+α−GC), at the indicated concentrations for 7 days. Where indicated, the responses to α-GalCer of unfractionated (total) bone marrow cells were compared with those of Percoll-fractionated subpopulations consisting of mature granulocytes (panel C, inset) or of haemopoietic precursors depleted of mature cells by two different procedures (panels D and F). Where indicated, splenic T lymphocytes (spleen lymph) from syngeneic donors were added to the mature cell-depleted bone marrow cultures up to a 1:10 ratio (panels D and F, as indicated). Panels (A)–(F): Data (mean ± SEM) are numbers of EPO+ cells in 7 days of culture in the indicated conditions. Panel (E): Data (mean ± SEM) are percentage of bone marrow cells bearing CD3, a marker for conventional T and iNKT cells, before fractionation (total), following Percoll centrifugation (P2), and following further immunomagnetic depletion of CD3+ cells (beads CD3+ Percoll purified), as assayed by flow cytometry. *P ≤ 0.01, significantly different as indicated; n = 3 for all panels.
Figure 7.
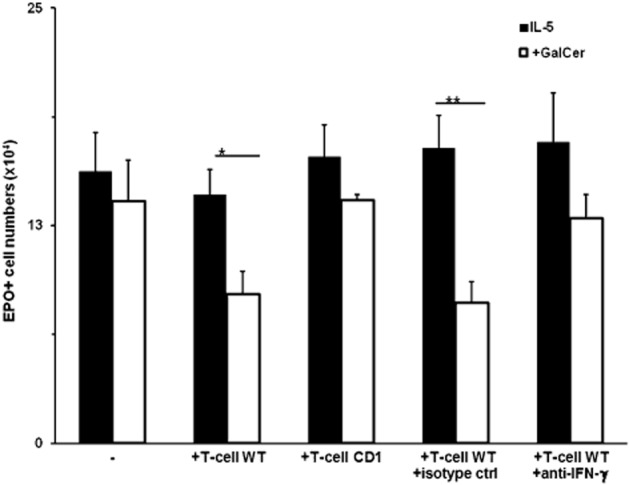
Reconstitution of responses to α-GalCer in IFN-γ-KO bone marrow cultures by CD1-dependent splenic T cells is mediated by IFN-γ. Bone marrow cells from IFN-γ-KO donor mice were collected after fractionation on Percoll gradients from the 60–75% Percoll interface (P2 layer), counted and adjusted to 106·cells mL−1. Spleens were collected from C57BL/6 (WT) or CD1KO (CD1) donor mice, and mononuclear cell suspensions were prepared by Ficoll-Hypaque centrifugation. T lymphocytes (T-cell) were further purified on nylon wool columns. Cultures were established as in Figure 6, panel (F), with 1 ng·mL−1 IL-5, and in the absence or presence of 10−8M α-GalCer (+GalCer) Cultures contained 106 P2 layer bone marrow cells alone (−), or co-cultured with 105 T lymphocytes (+T-cell WT, +T-cell CD1). Where indicated, the co-cultures received anti-IFN-γ neutralizing antibody (100 ng·mL−1; +anti-IFN-γ), or an equivalent amount of isotype-matched irrelevant antibody as a control (+isotype ctrl). Data are mean + SEM of EPO+ cell numbers recovered at day 7. *P ≤ 0.05, **P < 0.01, significant effect of α-GalCer; n = 5 for all means.
For flow cytometry, 106 cells in Eppendorf tubes were centrifuged (500× g, 10 min, 20°C) and resuspended in 100 μL of serum-free medium, to which was added antibody to 0.25 μg·mL−1 final concentration, followed by a 30 min incubation in the dark, removal of excess antibody as above, fixation in 400 μL of 2% paraformaldehyde in PBS, and stored at 4°C in the dark before acquisition in a FACScaliburTM (Becton-Dickinson, San Diego, CA, USA), with an argon laser at 488 nm, and analysis by Cell QuestTM (Becton Dickinson). For immunomagnetic depletion of CD3+ cells, including iNKT (Li et al., 2012), we used MidiMACSTM (Cat. No. 130-042-302; Miltenyi Biotec) and biotinylated anti-mouse CD3e antibody followed by streptavidin-coated microbeads. Where indicated, Annexin V staining of IL-5-differentiated eosinophils from 6 day cultures established in the absence and in the presence of α-GalCer was carried out with the TACS Annexin V-FITC Apoptosis Detection Kit from R&D Systems (Ref. No. 4830-01-K).
Preparation of spleen cells
Splenocyte suspensions were prepared by mincing the spleen with scissors and repeated passage through a 5 mL syringe (without needle) in media containing 1% FCS. Spleen mononuclear cells were filtered through nylon-wool columns according to Julius et al. (1973). Recovery was routinely 25–30% of the input mononuclear cells.
Data analysis
Data are presented as mean ± SEM throughout and were analysed with Systat for Windows version 4 software from Systat Inc. (Evanston, IL, USA). Comparisons of more than two means were carried out by factorial anova with Tukey's HSD correction (as recommended in O'Neill and Wetheril, 1971). Where only two groups were compared, we used the two-tailed t-test with separate variances. P < 0.05 was taken to show significant differences between group means.
Materials
Fetal calf serum (FCS) was from HyClone (Logan, UT, USA); culture media RPMI 1640 was from RHyClone, Thermo-Scientific (Waltham, MA, USA); recombinant murine cytokines (GM-CSF, IL-5) was from R&D Systems (Minneapolis, MN, USA); GalCer (Ref. No. ALX-306-027) was from Alexis (Lausen, Switzerland); dexamethasone 21-phosphate (D1159), indomethacin (I7378) and aspirin (A5376) were from Sigma (St. Louis, MO, USA); Z-VAD-fmk (Ref. No. V116) and aminoguanidine hydrochloride, a selective iNOS inhibitor (Ref. No. 396494), were from Sigma-Aldrich (St. Louis, MO, USA); and diaminobenzidine (DAB+) (Ref. No. K3467) solution was from Dako Cytomation (Dako Denmark A/S, Glostrup, Denmark). Purified rat anti-mouse IFN-γ (Ref. No. 18110D), recombinant murine IFN-γ (Ref. No. 193301U) and biotinylated anti-mouse CD3e antibody (Cat. No. 01082J) were from Pharmingen (Franklin Lakes, NJ, USA); streptavidin-coated microbeads (Order No. 481-02) was from Miltenyi Biotec (Bergisch Gladbach, Germany); purified PE-cyanine 5.5-conjugated hamster anti-mouse CD3e antibody (Ref. No. 35-003181, clone: 145-2C11, stock 0.2 mg·mL−1) was from eBioscience (San Diego, CA, USA).
Results
α-GalCer suppresses eosinopoiesis by activating a caspase-dependent mechanism
α-GalCer dose-dependently suppressed eosinophil production in BALB/c bone marrow cultures (Figure 1A), as shown by the significantly decreased numbers of eosinophils recovered from 7 day cultures, established in the presence of IL-5 together with α-GalCer at 10−6–10−8M (but not at 10−10–10−12 M), relative to the values with IL-5 alone. In the presence of α-GalCer alone (Figure 1A, inset), there was no eosinophil production. The effects of α-GalCer (10−8 M) depended upon the timing of its introduction in the culture, as significantly decreased eosinophil recovery, relative to IL-5 controls, was observed following the addition at days 0–1, but not at later times (Figure 1B). To define the kinetics of α-GalCer effects on IL-5-driven eosinophil differentiation, bone marrow cultures were established with rmIL-5 alone, or together with α-GalCer (10−8 M), and the EPO+ cell counts were determined at various times after initiation of the culture. As shown in Figure 1C, EPO+ cell numbers steadily increased from day 1 to day 7 of culture, reflecting eosinophil differentiation from EPO− precursors. At days 5–7, EPO+ cell numbers were significantly reduced in cultures containing α-GalCer. The suppressive effect of α-GalCer on eosinopoiesis was abolished by the caspase inhibitor, zVAD-fmk (20 μM). zVAD-fmk had no effect by itself (Figure 1D). In separate flow cytometry control experiments, we evaluated Annexin V binding to cells in the granulocyte gate, in bone marrow cultures stimulated for 6 days by IL-5 alone (control) or together with α-GalCer (experimental). In these cultures, bone marrow neutrophils are reduced to 1% or less by day 6 (Gaspar-Elsas et al., 2009), and the gated region contains essentially eosinophils. Cells staining positive for Annexin V binding were 3.1% in the controls, as opposed to 38% in α-GalCer-exposed cultures. This greater than 10-fold increase is consistent with an apoptotic mechanism.
Figure 1.
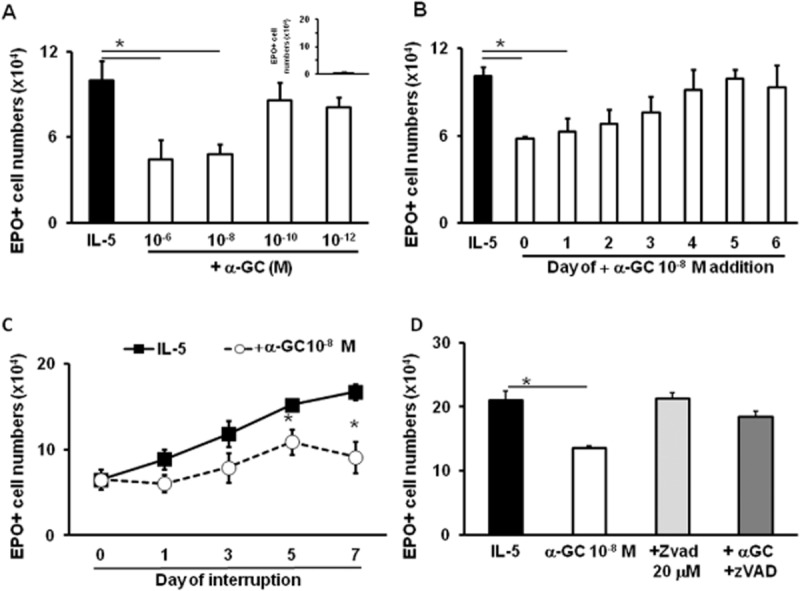
α-GalCer suppresses eosinopoiesis by activating a caspase-dependent mechanism. Bone marrow cultures were established from BALB/c donors in the presence of IL-5 (1 ng·mL−1), alone or together with the indicated concentrations of α-GalCer, caspase inhibitor zVAD-fmk, or both; or α-GalCer alone (10−8 M; panel A, inset). All cultures were maintained for 7 days, except for panel (C). All agents were added at the beginning of the culture, except for panel (B). Data (mean + SEM) are the numbers of EPO+ cells recovered at the end of the culture. *P ≤ 0.05, significantly different from the respective IL-5 controls; n = 4 (panels A and C); n = 3 (panels B and D).
Similarity of α-GalCer effects to those of PGE2
The effects of α-GalCer in bone marrow culture resemble those previously described for PGE2 in the same system (Gaspar-Elsas et al., 2000b). PGE2 acts through NO production by iNOS, followed by CD95L-mediated apoptosis (Jones et al., 2004), and its effectiveness is abolished by NSAIDs (indomethacin and aspirin) and by the cysteinyl-leukotrienes (LTD4, LTC4 and LTE4). Cysteinyl-leukotrienes are thought to mediate the actions of NSAIDs in this system because montelukast and MK571 (type I CysLT receptor antagonists) block NSAID effects (Xavier-Elsas et al., 2008). For these reasons, we evaluated the possible role of NO, iNOS and CD95 in the actions of α-GalCer, as well as the ability of NSAIDs and LTD4 to block these actions. Initially, we confirmed a requirement for iNOS in the effects of α-GalCer, as these were prevented by aminoguanidine, which had no effect in the absence of α-GalCer (Figure 2A). This is consistent (Figure 2B) with the difference in effectiveness of α-GalCer in cultures of iNOS-deficient bone marrow (which did not respond) and wild-type B6 controls (which responded as expected). Furthermore, bone marrow from CD95-deficient lpr mutants of the BALB/c background did not show a significant response (P = 0.72) to α-GalCer (Figure 2C), unlike that of wild-type controls of this background (Figure 2A). Finally, indomethacin, aspirin (Lintomen et al., 2002) and LTD4 (Xavier-Elsas et al., 2008; Queto et al., 2010b) showed comparable effectiveness in preventing the suppression of eosinopoiesis by α-GalCer (Figure 2D).
Figure 2.
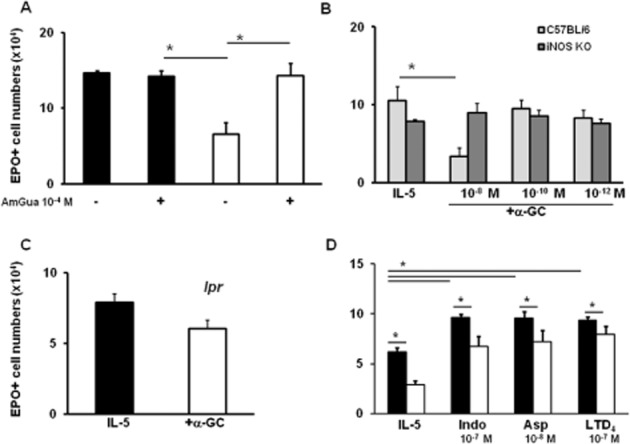
The effectiveness of α-GalCer depends on both iNOS and CD95. Bone marrow was collected from BALB/c (panels A and D) or from C57BL/6 wild-type controls and mutant iNOS-deficient mice (panel B) or from BALB/c lpr mutant mice, lacking CD95 (panel C). Cultures were established for 7 days in the presence of IL-5 (1 ng·mL−1), alone or together with α-GalCer (10−8 M). Panel (A), where indicated (+), aminoguanidine (AmGua; 10−4 M); panel (D), as indicated, indomethacin (Indo, 10−7 M), aspirin (Asp, 10−8 M) or LTD4 (10−7 M). **P ≤ 0.05, significantly different as indicated; n = 3 for all panels.
PGE2 is able to synergically interact with dexamethasone to induce terminal differentiation of immature eosinophils, even though it does not suppress eosinopoiesis in the presence of dexamethasone (Gaspar-Elsas et al., 2009). To determine whether the parallels between α-GalCer and PGE2 would extend to this maturation-promoting effect, we established liquid bone marrow cultures in the presence of IL-5 together with dexamethasone (10−7 M), with or without α-GalCer (10−8 M). α-GalCer significantly decreased the numbers of eosinophils recovered from 7 day cultures, relative to IL-5 controls (Figure 3A). Dexamethasone increased significantly the total yield of EPO+ cells (Gaspar-Elsas et al., 2009). α-GalCer lost its suppressive effect in the presence of dexamethasone. Figure 3B illustrates the representative aspects of the cultures: with IL-5 alone, eosinophils were predominantly single cells with strong brown cytoplasmic staining, dispersed among EPO− cells (stromal cells and macrophages, which are IL-5-independent; Gaspar-Elsas et al., 1997), visible after haematoxylin counterstaining (Figure 3B, upper left). Their morphology includes uniform size, doughnut-shaped nucleus and coarse, tightly packed, cytoplasmic granules (Gaspar-Elsas et al., 2000a; 2009). Cultures established with α-GalCer have fewer EPO+ cells, with no change in cytological features (upper right), nor in the number of EPO− cells. By contrast, EPO+ cells in cultures stimulated by IL-5 and dexamethasone were found both as single cells and as large clusters of rather uniform appearance, containing EPO+ cells of various sizes, irregular shapes, incomplete nuclear segmentation and large cytoplasm with less densely packed brown granules (lower left). When present together, α-GalCer and dexamethasone interacted, so that apoptosis was prevented and eosinophil production brought back to the IL-5 control levels. This was accompanied by the absence of large clusters and presence of mature morphology (lower right). The effects of dexamethasone alone on eosinophil numbers could be accounted for by a significant increase in morphologically immature eosinophils (Figure 3C); those of α-GalCer alone by significant reduction in mature eosinophils. When both were present, there was a striking reduction in immature eosinophil numbers, accompanied by an equally significant increase in mature eosinophil numbers, relative to IL-5 plus dexamethasone controls. Overall, total eosinophil numbers were significantly increased by this synergic combination, relative to the IL-5 controls, but yielding mostly mature cells.
Figure 3.
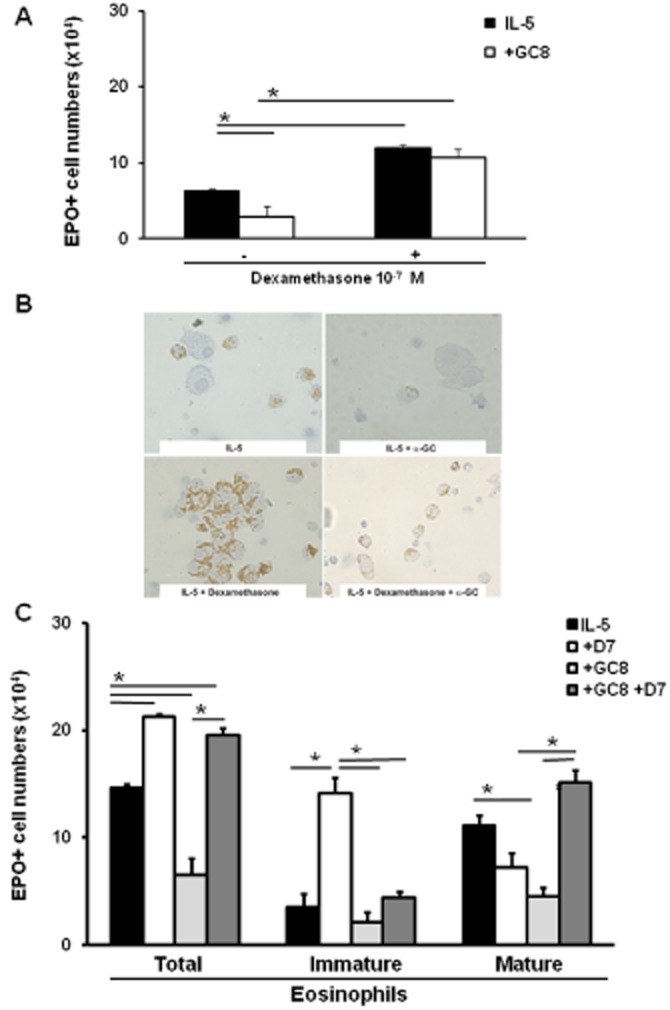
α-GalCer synergizes with dexamethasone to promote eosinophil maturation. Bone marrow cultures were established from BALB/c bone marrow for 7 days with IL-5 (1 ng·mL−1), alone or together with dexamethasone (10−7 M, D7), α-GalCer (10−8 M, GC8) or both. Panels (A) and (C): Effects of dexamethasone, α-GalCer or both on total and differential EPO+ cell counts. Data are mean + SEM of the counts (panel A, total; panel C, differential) of EPO+ cells in 7 day cultures established in the indicated conditions. Panel (A): Cultures established with IL-5 alone or together with α-GalCer (10−8 M), were maintained without (−) or with (+) dexamethasone (10−7 M). Panel (B): Representative images of eosinophils (recognizable by EPO+, brown-stained, coarse cytoplasmic granules) in the indicated culture conditions. Immature eosinophils, a prominent feature of dexamethasone-exposed cultures, show variable size and nucleocytoplasmic ratios, with incomplete nuclear segmentation, and often form homotypic aggregates. Mature eosinophils are smaller, with regular size and donut-shaped nuclei, usually found as single cells. Apoptotic eosinophils, more frequent in α-GalCer-exposed cultures, are pyknotic and occasionally found attached to the surface of macrophages. Panel (C): Cultures established with IL-5 alone or together with dexamethasone (10−7 M) (+D7), α-GalCer 10−8 M (+GC8) or both (+GC8 + D7) were examined for the proportions of mature and immature eosinophils in the total EPO+ cell numbers. *P ≤ 0.01, significantlydifferent as indicated; n = 3.
α-GalCer suppresses GM-CSF-stimulated progenitors in vivo and in vitro
To define whether the suppressive effects of α-GalCer were restricted to the eosinophil lineage, we further evaluated α-GalCer effects on the formation of myeloid colonies by bone marrow progenitors cultured in the presence of the multi-lineage haemopoietic factor, GM-CSF. In preliminary experiments with BALB/c mice (not shown), α-GalCer significantly decreased the total numbers of myeloid colonies in the same concentration range (10−6–10−8M), in which it suppressed eosinophil production in the liquid culture. This effect was accounted for by a rather uniform reduction of colony types of all lineages, not by exclusive or selective inhibition of eosinophil-containing colonies. We subsequently examined whether exposure to α-GalCer in vivo would affect subsequent colony formation ex vivo, by injecting B6 mice with α-GalCer or vehicle (see the Methods section) and collecting bone marrow 24 h later for the semi-solid culture. The ability to respond to GM-CSF by forming myeloid colonies, and the ability to respond to α-GalCer in vitro, in the absence or presence of α-GalCer pre-exposure in vivo, were evaluated. As shown in Figure 4, α-GalCer exposure in vivo significantly reduced colony formation ex vivo (P = 0.03 relative to the vehicle-injected control). Bone marrow exposure to α-GalCer in vivo did not prevent subsequent in vitro responses to α-GalCer in vitro, which were significant in both vehicle and α-GalCer-injected mice. The response to α-GalCer in vitro was blocked by aminoguanidine (not shown).
Figure 4.
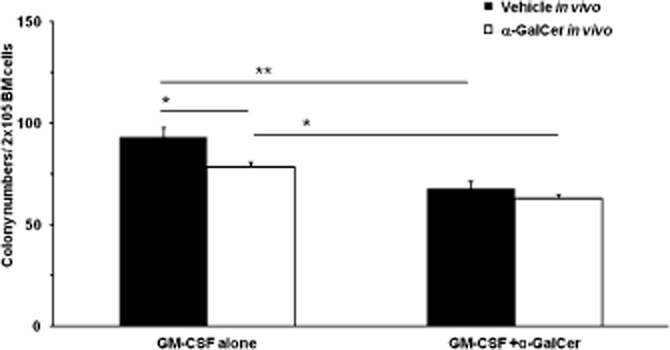
α-GalCer acts in vivo and in vitro to suppress colony formation. C57BL/6 mice were injected i.p. with α-GalCer 100 μg·kg−1 in a 400 μL volume of PBS containing 2% DMSO (vehicle), once, 24 h before killing and bone marrow collection Controls received vehicle in the same conditions Bone marrow cells (2 × 105 cells mL−1, in Iscove's modifed Dulbecco's medium with 20% FCS) were pre-incubated for 2 h in the presence of GM-CSF (2 ng·mL−1 final concentration), alone (GM-CSF) or together with α-GalCer 10−8M (GM-CSF+α-GalCer) before mixing with agar (0.3% final concentration) and plating in triplicate. Total myeloid colonies were scored at day 7 under an inverted microscope. Data are mean ± SEM (n = 4 for all groups). *P ≤ 0.05, **P < 0.01, significantly different as indicated.
The effectiveness of α-GalCer requires IFN-γ production and signalling
The evidence for a critical involvement of iNOS in the suppression of eosinopoiesis prompted the evaluation of a role for IFN-γ in mediating this effect of α-GalCer because IFN-γ is both a product of activated iNKT cells and a strong inducer of iNOS. As shown in Figure 5A, α-GalCer had no significant effect in bone marrow cultures from IFN-γ-deficient mice. By contrast, a dose-dependent suppression of eosinophil production by α-GalCer (10−8M, but not 10−10–10−12M) was observed in wild-type controls of the same background (B6) in identical conditions. This strongly suggests that the ability to produce IFN-γ underlies the effectiveness of α-GalCer in these conditions. We further examined whether α-GalCer (10−8M) achieved maximal suppression of eosinophil production, or, alternatively, whether its effectiveness would be further increased by exogenously added IFN-γ. Addition of recombinant murine IFN-γ suppressed eosinophil production directly (Figure 5B) at 5 ng·mL−1 but not at a lower concentration (2 ng·mL−1). At this ineffective concentration (2 ng·mL−1), however, IFN-γ retained the ability to interact synergically with α-GalCer and significantly increased the suppressive activity of the latter (Figure 5B). Both sets of observations (IFN-γ alone vs. IFN-γ plus α-GalCer) suggest that the suppressive effect of α-GalCer is limited by the amount of IFN-γ it induces. Finally, independent evidence for mediation of α-GalCer effects by secondary induction of IFN-γ was obtained with the use of neutralizing IFN-γ-specific antibodies (Figure 5C). In bone marrow exposed to α-GalCer, we observed significant suppression of eosinophil production, relative to the controls, both without and with an irrelevant isotype control antibody. By contrast, no such effect was observed with α-GalCer if neutralizing antibody to IFN-γ was present, while the neutralizing antibody had no significant effect in the absence of α-GalCer.
Figure 5.
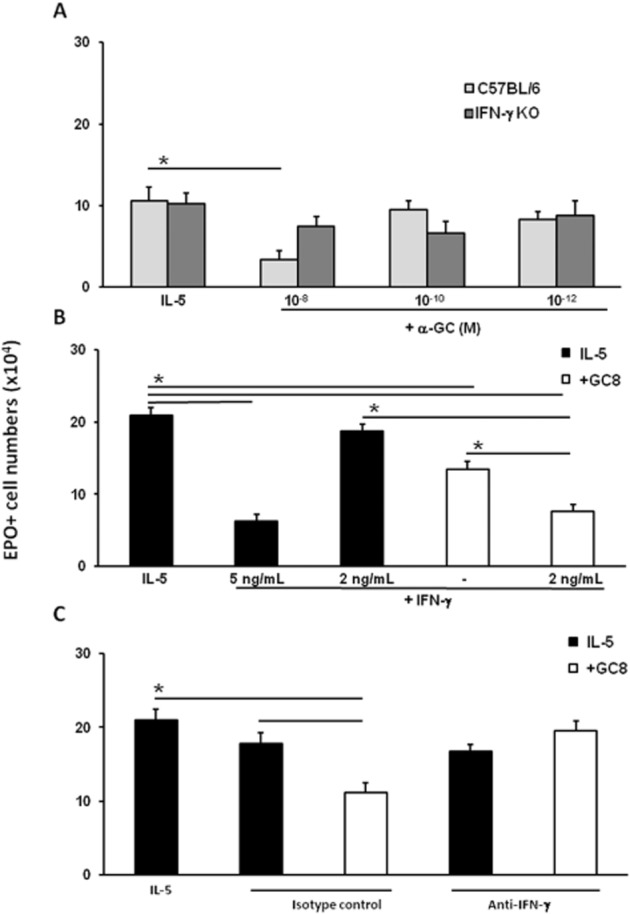
Contribution of IFN-γ to the effects of α-GalCer. Liquid bone marrow cultures were established from C57BL/6 wild-type controls and from IFN-γ-deficient mutants (IFN-γ-KO) (panel A), or from BALB/c mice (panels B and C), in the presence of IL-5 alone, or together with α-GalCer (+αGC), at the indicated concentrations. Where indicated, cultures also received: panel (B) (+IFN-γ): rmIFN-γ at the indicated concentrations or medium (−) as a control; panel (C): monoclonal neutralizing antibodies to an irrelevant antigen (isotype control) or to mIFN-γ. Data are mean ± SEM of the numbers of EPO+ recovered from cultures at day 7. *P ≤ 0.01, significantly different as indicated; n = 4 (panel A); n = 3 (panels B and C).
α-GalCer requires bone marrow lymphocytes, CD1d and CD28
An indirect mode of action for α-GalCer in regulating bone marrow eosinophil production from naïve mice would require an endogenous lymphocyte population to be present in freshly harvested bone marrow, and to be activated in vitro by α-GalCer through CD1d (the restriction element) with an essential contribution of CD28 (the co-stimulation receptor). We evaluated these requirements with the use of CD1d- and CD28-deficient mice and depletion/reconstitution strategies (Figure 6). In the initial experiments, we examined the responses to α-GalCer in bone marrow from wild-type controls (B6) and from CD28-deficient (Figure 6A) or CD1d-deficient (Figure 6B) mutant mice. Neither mutant bone marrow responded to α-GalCer, in contrast to the wild-type controls, which showed a significant suppressive response to 10−8M (but not to 10−9–10−10M) α-GalCer, as expected. We next used discontinuous Percoll gradients to deplete bone marrow of mature granulocytes and mononuclear cells, while retaining eosinophil precursors (Maximiano et al., 2005). As illustrated in Figure 6C and D, unfractionated bone marrow responded to α-GalCer, while the fraction containing mature cells did not produce eosinophils (Figure 6C, inset); by contrast, the fraction depleted of mature cells produced eosinophils, but no longer responded to α-GalCer (compare two left columns in Figure 6D to each other and to Figure 6C). The response to α-GalCer could be reconstituted by exogenously added splenic lymphocytes (compare two right columns in Figure 6D to each other and to Figure 6C). The reconstituting lymphocyte population in Figure 6D, which amounts to only 10% of the cells in culture, does not produce eosinophils (not shown). This is consistent with the assumption that lymphocytes absent from the depleted culture can be replaced by splenic lymphocytes, a known source of iNKT cells. The separation of mature cells by the Percoll gradient fractionation effectively removes CD3+ cells from the bone marrow cells, as shown by flow cytometry (Figure 6E). A significant removal was achieved by gradient centrifugation alone, and a further step of immunomagnetic depletion with anti-CD3 beads resulted in no further removal. Use of these two-step purified cells for reconstituting bone marrow depleted of mature cells (Figure 6F) yielded results entirely consistent with those obtained without the supplementary step of anti-CD3 depletion (Figure 6D).
The combined results from Figures 5 and 6 prompted us to examine the role of IFN-γ in reconstitution of α-GalCer responses by splenic lymphocytes dependent upon CD1. As shown in Figure 7, this was done by co-culturing bone marrow cells from IFN-γ-deficient mice, depleted of mature cells as above, with spleen lymphocytes (purified using nylon wool) from wild-type donors or from CD1-KO negative controls. As expected, IFN-γ-KO bone marrow precursors produced eosinophils, but did not respond to α-GalCer. Addition of 10% lymphocytes from a wild-type donor did not significantly affect eosinophil production, but restored response to α-GalCer. By contrast, 10% lymphocytes from a CD1-KO donor failed to do so, even though mutant and wild-type mice differ only in the presence of iNKT cells. Further evidence that IFN-γ production underlies the ability of iNKT cells to suppress eosinophil production was provided by neutralization of their effect by a specific anti-IFN-γ antibody, but not by isotype-matched control antibody.
Discussion
This study was prompted by reports of the participation of iNKT cells in normal (Liu et al., 2005) and pathological (Umetsu and De Kruyff, 2010; Bjordahl et al., 2012) immune responses, along with evidence that α-GalCer or iNKT cells may suppress eosinophilic inflammation in experimental models (Benoit et al., 2007; Bourgeois et al., 2011). Here, we report that α-GalCer suppressed eosinophil differentiation and colony formation by activating bone marrow-resident iNKT cells. The underlying mechanism required iNOS and CD95, was mediated by IFN-γ, induces apoptosis and was blocked by dexamethasone and by the pro-allergic lipid mediator, LTD4, as well as by NSAIDs (which induce cysteinyl-leukotrienes). Together, these observations: (i) show, for the first time, a regulatory role for iNKT cells in eosinophil production, which may be relevant to their reportedly beneficial effects in asthma models; (ii) establish the iNOS-CD95L pathway as inducible by innate cellular immunity; and (iii) substantially increase understanding of this pathway by highlighting its relationship to the pro-allergic effects of dexamethasone and cysteinyl-leukotrienes, and to recent observations on the control of eosinopoiesis by regulatory lymphocytes (Xavier-Elsas et al., 2013) and glucocorticoids (Masid-de-Brito et al., 2014a).
The following specific points deserve detailed discussion and further experimental analyses.
Lineage-specific and non-specific effects of α-GalCer in bone marrow
The effects of α-GalCer on eosinophil production were characterized here to the point of establishing its critical requirements, as has been previously carried out for PGE2 (de Luca et al., 2013). α-GalCer is a powerful activator of the iNOS-CD95L pathway, initially described in bone marrow culture (Jones et al., 2004) and has been shown to underlie the therapeutic actions of DEC in vivo in an asthma model (Queto et al., 2010a). Importantly, α-GalCer is only known to act through activation of regulatory lymphocytes, unlike DEC, which has cellular and molecular targets both heterogeneous and ill-defined (Matthews and Murphy, 1982). The present study of α-GalCer actions demonstrated indirect activation of the pathway through a regulatory lymphocyte population, distinct from the ultimate target (eosinophil precursors). It further provided a well-characterized mechanism (IFN-γ-induced NOS), which has been extensively addressed in molecular studies (Rauch et al., 2013). By contrast, there is less information on the molecular pathways linking PGE2 (and especially DEC) to the expression and activation of iNOS. In addition, myeloid progenitors were suppressed in clonogenic assays. This strongly resembled the effects of PGE2 on murine bone marrow (Gaspar-Elsas et al., 2000b) and raises the question of whether the similarity extends to mechanism (activation of a specific iNOS-dependent pathway), as suggested by the dependence of α-GalCer on caspases and its ability to induce Annexin V staining of granulocytes in IL-5-stimulated 6 day cultures. The possible contribution of IFN-γ to multilineage suppression, as well as the possible neutropenia-inducing effect of α-GalCer in vivo, deserves further exploration, as multilineage suppression and neutropenia are more likely to be harmful than suppression of eosinopoiesis alone.
Modification of α-GalCer effects by dexamethasone
One important similarity between α-GalCer and PGE2 in our study is the radical modification of their effects by dexamethasone present in the culture. Dexamethasone suppresses iNOS expression in these conditions (de Luca et al., 2013) and hence, apoptosis is blocked, as it is by aminoguanidine or iNOS gene inactivation. However, α-GalCer remains able to interact with dexamethasone, promoting terminal maturation, as shown for PGE2 (Gaspar-Elsas et al., 2009). It is unclear whether activation of iNKT cells in the presence of dexamethasone will lead to IFN-γ production, but a possible role of IFN-γ in the maturation effect must be evaluated in future experiments. A further issue is suggested by the similarity between the eosinophilia-promoting effects of dexamethasone in this study and those of endogenous glucocorticoids, which signal through the same receptors, in allergic inflammation models (Masid-de-Brito et al., 2014a). One should consider the possibility that signalling by endogenous glucocorticoids might interfere with the ability of exogenous immunomodulators to suppress eosinophilia, another issue for future investigation.
Relationship to allergy
These results increase our understanding of the benefits of α-GalCer in allergy, about which consensus is lacking. Eosinophils are undisputably an important part of allergy and asthma pathogenesis (Rosenberg et al., 2007), and the iNOS-CD95L pathway suppresses eosinophilia in inflammatory sites and in bone marrow when activated by DEC (Queto et al., 2010a). Therefore, future studies of the benefits of α-GalCer in asthma or allergy models should consider whether suppressed eosinophilia and eosinopoiesis contribute to the observed benefit. The evidence that cysteinyl-leukotrienes antagonize the suppressive effects of α-GalCer raises the issue of whether part of their eosinophilia-promoting activity depends upon blocking the inhibitory effects of IFN-γ from various sources (including iNKT, which may be activated by self-antigens in the absence of α-GalCer; Molano and Porcelli, 2006). Another important finding of this study is that the effect of α-GalCer was converted from suppressive (anti-allergic) to stimulatory (pro-allergic) by interaction with dexamethasone. This agrees with previous studies on another activator of the iNOS-CD95L pathway (PGE2) but is also consistent with the accumulating evidence that glucocorticoids, endogenous or synthetic, may interact with cytokines to enhance eosinophil production (Masid-de-Brito et al., 2014a). It is unclear at present whether IFN-γ would interact with dexamethasone, or, alternatively, whether iNKT cells, in the presence of dexamethasone, would produce, instead of IFN-γ, another cytokine that enhances eosinophil maturation. This issue will certainly be addressed in future studies of this model.
Mediation of α-GalCer effects by IFN-γ
Some studies have reported beneficial effects in allergy and asthma models with α-GalCer, which were attributed to IFN-γ (Hachem et al., 2005; Fujita et al., 2009). Other studies showed exacerbation of experimental allergic disease models, not necessarily attributable to IFN-γ, which illustrates the risks of equating the effector cell (iNKT) with the mediator of one of its specific effects (IFN-γ). Our conclusion relies on the inability of α-GalCer to induce suppression of eosinopoiesis in bone marrow from IFN-γ-deficient mice, as well as on the ability of neutralizing anti-IFN-γ antibody to prevent this effect of α-GalCer in bone marrow from wild-type controls. Both approaches are reliable and, most importantly, independent of sensitivity of the assay, because by definition, IFN-γ-KO mice will have no IFN-γ, and in excess of neutralizing antibody, there should be full neutralization. Direct evidence that exogenous IFN-γ, as would be expected, duplicated the effects of α-GalCer, was also provided. Although some indication of the effective concentration range for exogenous IFN-γ was obtained, one should be cautious in assuming that α-GalCer-stimulated cells that reduce eosinopoiesis by a certain amount are secreting enough IFN-γ to achieve the same concentration as in the culture in which addition of exogenous IFN-γ elicited a comparable effect. Because iNKT cells may secrete IFN-γ in close proximity to their targets, they may achieve higher local concentrations than those reached in the total culture volume. Maintenance of a higher local concentration may also account for the ability of exogenous IFN-γ to boost the effect of α-GalCer. Of course, issues related to local concentration, diffusibility and turnover of IFN-γ would affect the interpretation of cytokine measurements, which is not the case with the intervention approaches (KO mice and neutralization) we have adopted.
Characterization of the regulatory/effector cell
One of the most important issues concerning this study is the nature of the regulatory/effector cell behind the effect of α-GalCer. Based upon previous studies (see the Introduction section), it is clear that α-GalCer is not recognized by conventional T lymphocytes, nor by type II NKT cells. Furthermore, iNKT cells are found in significant numbers in murine bone marrow and spleen and should be interchangeable between these sites. iNKT cells should be absent in bone marrow from CD1-deficient mice and, in wild-type mice, require not only ligand (α-GalCer) but co-stimulation as well, which was shown to be inadequate in CD28-deficient mice. We have provided experimental evidence for all of the above. Therefore, the criterion used was a combination of antigen specificity (α-GalCer), reliance on a specific type of antigen-presenting molecule (CD1d), dependence on CD28, residence in both bone marrow and spleen with comparable phenotype, expression of TCR (CD3+) production of IFN-γ. This view is supported by the evidence summarized in Figures 6. The IFN-γ-producing T-cell, as shown in co-culture experiments, is only found in spleen T-cells from CD1d+ wild-type donors. This approach allowed us to define unambiguously iNKT cell function on the basis of α-GalCer reactivity and restriction by CD1d, just as they can be defined by flow cytometry on the basis of binding of α-GalCer/CD1d fluorescent complexes (Matsuda et al., 2008). We conclude that α-GalCer-activated iNKT cells are one of the regulatory lymphocyte subsets that can suppress eosinopoiesis in bone marrow, which differs from those previously described in an oral tolerization model (Xavier-Elsas et al., 2013) because it is CD1d-restricted.
Acknowledgments
This work was supported by CNPq fellowships (Research Productivity Fellowships to P. X.-E. and M. I. G.-E.); CAPES PhD fellowships to T. Q. and B. L.; and by FAPERJ fellowship (Doutorado Nota Dez) from FAPERJ to T. Q.; funding for the experimental work came from CNPq Grant 470377/2011-9 to P. X.-E., including funding to B. M. V. for technical assistance; grants from FAPERJ (E-26/111.555/2010 and E-26/103.138/2011) to P. X.-E. The authors acknowledge the technical support provided by Monica Barradas, Daniela Masid-de-Brito and Cássio Luiz C. Almeida da Silva in the early phases of this study.
Glossary
- DEC
diethylcarbamazine
- α-GalCer
α-galactosylceramide
- iNKT cells
invariant natural killer T lymphocytes
Author contributions
M. I. G.-E. made the initial observations and carried out experiments with the help of T. Q., D. M.-B., B. M. V. and B. L. F. Q. C. provided indispensable reagents and animals, and insightful discussion of the data. P. X.-E. reviewed the experimental data and the literature on the subject and wrote the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- Adams EJ. Lipid presentation by human CD1 molecules and the diverse T cell populations that respond to them. Curr Opin Immunol. 2014;26:1–6. doi: 10.1016/j.coi.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit AC, Huang Y, Maneewatchararangsri S, Tapchaisri P, Anderson R. Regulation of airway eosinophil and neutrophil infiltration by α-galactosylceramide in a mouse model for respiratory syncytial virus (RSV) vaccine-augmented disease. Vaccine. 2007;25:7754–7762. doi: 10.1016/j.vaccine.2007.08.062. [DOI] [PubMed] [Google Scholar]
- Berkers CR, Ovaa H. Immunotherapeutic potential for ceramide-based activators of iNKT cells. Trends Pharmacol Sci. 2005;26:252–257. doi: 10.1016/j.tips.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bjordahl RL, Gapin L, Marrack P, Refaeli Y. iNKT cells suppress the CD8+ T cell response to a murine Burkitt's-like B cell lymphoma. PLoS ONE. 2012;7:e42635. doi: 10.1371/journal.pone.0042635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois EA, Levesco A, Diem S, Chauvineau A, Bergès H, Milpied P, et al. A natural protective function of invariant NKT cells in a mouse model of innate-cell-driven lung inflammation. Eur J Immunol. 2011;41:299–305. doi: 10.1002/eji.201040647. [DOI] [PubMed] [Google Scholar]
- Chuang YH, Wang TC, Jen HY, Yu AL, Chiang BL. α-Galactosylceramide-induced airway eosinophilia is mediated through the activation of NKT cells. J Immunol. 2011;186:4687–4692. doi: 10.4049/jimmunol.1003659. [DOI] [PubMed] [Google Scholar]
- Dalton DK, Haynes L, Chu CQ, Swain SL, Wittmer S. Interferon gamma eliminates responding CD4 T cells during mycobacterial infection by inducing apoptosis of activated CD4 T cells. J Exp Med. 2000;192:117–122. doi: 10.1084/jem.192.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Libero G, Mori L. Novel insights into lipid antigen presentation. Trends Immunol. 2012;33:103–111. doi: 10.1016/j.it.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Fujita H, Teng A, Nozawa R, Takamoto-Matsui Y, Katagiri-Matsumura H, Ikezawa Z, et al. Production of both IL-27 and IFN-gamma after the treatment with a ligand for invariant NK T cells is responsible for the suppression of Th2 response and allergic inflammation in a mouse experimental asthma model. J Immunol. 2009;183:254–260. doi: 10.4049/jimmunol.0800520. [DOI] [PubMed] [Google Scholar]
- Gaspar-Elsas MI, Queto T, Vasconcelos Z, Jones CP, Lannes-Vieira J, Xavier-Elsas P. Evidence for a regulatory role alpha4 integrins in the maturation of eosinophils generated from the bone-marrow in the presence of dexamethasone. Clin Exp Allergy. 2009;39:1187–1198. doi: 10.1111/j.1365-2222.2009.03289.x. [DOI] [PubMed] [Google Scholar]
- Gaspar-Elsas MIC, Joseph D, Xavier-Elsas P, Vargaftig BB. Rapid increase in bone-marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am J Resp Cell Mol Biol. 1997;17:404–413. doi: 10.1165/ajrcmb.17.4.2691. [DOI] [PubMed] [Google Scholar]
- Gaspar-Elsas MIC, Maximiano ES, Joseph D, Alves L, Topilko A, Vargaftig BB, et al. Upregulation by glucocorticoids of responses to eosinopoietic cytokines in bone-marrow from normal and allergic mice. Br J Pharmacol. 2000a;129:1453–1552. doi: 10.1038/sj.bjp.0703145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Elsas MIC, Joseph D, Lintomen L, Maximiano ES, Bodstein MM, Xavier-Elsas P, et al. Murine myeloid progenitor responses to GM-CSF and eosinophil precursor responses to IL-5 represent distinct targets for downmodulation by prostaglandin E2. Br J Pharmacol. 2000b;130:1362–1368. doi: 10.1038/sj.bjp.0703403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem P, Lisbonne M, Michel ML, Diem S, Roongapinun S, Lefort J, et al. Alpha-galactosylceramide-induced iNKT cells suppress experimental allergic asthma in sensitized mice: role of IFN-gamma. Eur J Immunol. 2005;35:2793–2802. doi: 10.1002/eji.200535268. [DOI] [PubMed] [Google Scholar]
- Iwamura C, Nakayama T. Role of alpha-galactosylceramide-activated Valpha14 natural killer T cells in the regulation of allergic diseases. Allergol Int. 2007;56:1–6. doi: 10.2332/allergolint.R-06-136. [DOI] [PubMed] [Google Scholar]
- Jones C, Paula Neto HA, Assreuy J, Vargaftig BB, Elsas MIG, Xavier Elsas P. Prostaglandin E2 and dexamethasone regulate eosinophil differentiation and survival through nitric oxide-CD95-dependent pathway. Nitric Oxide. 2004;111:184–193. doi: 10.1016/j.niox.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Julius MH, Simpson E, Herzenberg LA. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973;3:645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Jyonouchi S, Smith CL, Saretta F, Abraham V, Ruymann KR, Modayur-Chandramouleeswaran P, et al. Invariant natural killer T cells in children with eosinophilic esophagitis. Clin Exp Allergy. 2014;44:58–68. doi: 10.1111/cea.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Pichavant M, Matangkasombut P, Koh YI, Savage PB, DeKruyff RH, et al. The development of airway hyperreactivity in T-bet-deficient mice requires CD1d-restricted NKT cells. J Immunol. 2009;182:3252–3261. doi: 10.4049/jimmunol.0803339. [DOI] [PubMed] [Google Scholar]
- Kim JO, Kim DH, Chang WS, Hong C, Park SH, Kim S, et al. Asthma is induced by intranasal coadministration of allergen and natural killer T-cell ligand in a mouse model. J Allergy Clin Immunol. 2004;114:1332–1338. doi: 10.1016/j.jaci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Konishi J, Yamazaki K, Yokouchi H, Shinagawa N, Iwabuchi K, Nishimura M. The characteristics of human NKT cells in lung cancer – CD1d independent cytotoxicity against lung cancer cells by NKT cells and decreased human NKT cell response in lung cancer patients. Human Immunol. 2004;65:1377–1388. doi: 10.1016/j.humimm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci U S A. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LP, Fang YC, Dong GF, Lin Y, Saito S. Depletion of invariant NKT cells reduces inflammation-induced preterm delivery in mice. J Immunol. 2012;188:4681–4689. doi: 10.4049/jimmunol.1102628. [DOI] [PubMed] [Google Scholar]
- Lintomen L, Elsas MICG, Maximiano ES, Paula Neto HA, Joseph D, Vargaftig BB, et al. Allergenic sensitization prevents upregulation of haemopoiesis by cyclooxygenase inhibitors in mice. Br J Pharmacol. 2002;135:1315–1323. doi: 10.1038/sj.bjp.0704580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Idoyaga J, Charalambous A, Fujii S, Bonito A, Mordoh J, et al. Innate NKT lymphocytes confer superior adaptive immunity via tumor-capturing dendritic cells. J Exp Med. 2005;202:1507–1516. doi: 10.1084/jem.20050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Luca B, Xavier-Elsas P, Barradas M, Luz RA, Queto T, Jones C, et al. Essential roles of PKA, iNOS, CD95/CD95L, and terminal caspases in suppression of eosinopoiesis by PGE2 and other cAMP-elevating agents. ScientificWorldJournal. 2013;2013:208705. doi: 10.1155/2013/208705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masid-de-Brito D, Xavier-Elsas P, Luz RA, Queto T, Silva CLCA, Lopes RS, et al. Essential roles of endogenous glucocorticoids and TNF/TNFR1 in promoting bone-marrow eosinopoiesis in ovalbumin-sensitized, airway-challenged mice. Life Sci. 2014a;94:74–82. doi: 10.1016/j.lfs.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Masid-de-Brito D, Queto T, Gaspar-Elsas MI, Xavier-Elsas P. Roles of 5-lipoxygenase and cysteinyl-leukotriene type 1 receptors in the hematological response to allergen challenge and its prevention by diethylcarbamazine in a murine model of asthma. 2014b;2014 doi: 10.1155/2014/403970. Mediators Inflamm : 403970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Suda T, Sato J, Nagata T, Koide Y, Chida K, et al. alpha-Galactosylceramide, a ligand of natural killer T cells, inhibits allergic airway inflammation. Am J Respir Cell Mol Biol. 2005;33:22–31. doi: 10.1165/rcmb.2004-0010OC. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews W, Murphy RC. Inhibition of leukotriene biosynthesis in mastocytoma cells by diethylcarbamazine. Biochem Pharmacol. 1982;31:2129–2132. doi: 10.1016/0006-2952(82)90435-x. [DOI] [PubMed] [Google Scholar]
- Maximiano ES, Xavier Elsas P, Sales SCM, Jones C, Joseph D, Vargaftig BB, et al. Cells isolated from bone-marrow and lungs of allergic BALB/C mice and cultured in the presence of IL-5 are respectively resistant and susceptible to apoptosis induced by dexamethasone. Intl Immunopharmacol. 2005;5:857–870. doi: 10.1016/j.intimp.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Molano A, Porcelli SA. Invariant NKT cell regulation of autoimmunity. Drug Discovery Today Dis Mechan. 2006;3:193–198. [Google Scholar]
- O'Neill R, Wetheril GB. The present state of multiple comparison. J R Stat Soc. 1971;33:218–250. [Google Scholar]
- Queto T, Xavier-Elsas P, Gardel MA, De Luca B, Barradas M, Masid D, et al. Inducible nitric oxide synthase/CD95L-dependent suppression of pulmonary and bone marrow eosinophilia by diethylcarbamazine. Am J Resp Crit Care Med. 2010a;181:429–437. doi: 10.1164/rccm.200905-0800OC. [DOI] [PubMed] [Google Scholar]
- Queto T, Gaspar Elsas MI, Masid de Brito D, Vasconcelos ZFM, Ferraris FK, Penido C, et al. Cysteinyl-leukotriene type 1 receptors transduce a critical signal for the up-regulation of eosinophilopoiesis by interleukin-13 and eotaxin in murine bone marrow. J Leukoc Biol. 2010b;87:885–889. doi: 10.1189/jlb.1108709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledge base of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–D1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch I, Müller M, Decker D. The regulation of inflammation by interferons and their STATs. JAK-STAT. 2013;2:e23820. doi: 10.4161/jkst.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Shahinian A, Pfeffer K, Lee KP, Kundig T, Kishihara K, Wakeham A, et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- Sonoda K, Exley M, Snapper S, Balk SP, Stein-Streilein J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J Exp Med. 1999;190:1215–1226. doi: 10.1084/jem.190.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu D, De Kruyff RH. Natural killer T cells are important in the pathogenesis of asthma: the many pathways to asthma. J Allergy Clin Immunol. 2010;125:975–979. doi: 10.1016/j.jaci.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier-Elsas P, Queto T, Sales SCM, Elsas MICG, Kanaoka Y, Lam B. Cysteinyl-leukotrienes mediate the enhancing effects of indomethacin and aspirin on murine bone-marrow culture. Br J Pharmacol. 2008;153:528–535. doi: 10.1038/sj.bjp.0707586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier-Elsas P, Silva CLCA, Pinto L, Queto T, Vieira B, Aranha M, et al. Modulation of the effects of lung immune response on bone-marrow by oral antigen exposure. Biomed Res Int. 2013;2013:474132. doi: 10.1155/2013/474132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura T, Sakuishi K, Illés Z, Miyake S. Understanding the behavior of invariant NKT cells in autoimmune diseases. J Neuroimmunol. 2007;191:8–15. doi: 10.1016/j.jneuroim.2007.09.014. [DOI] [PubMed] [Google Scholar]


