Abstract
Background and Purpose
Although still used by hundreds of millions of people worldwide, the mechanism of the analgesic action of the pyrazolone derivatives (PDs), dipyrone, propyphenazone and antipyrine remains unknown. The transient receptor potential ankyrin 1 (TRPA1) channel, expressed by nociceptors, is emerging as a major pain transduction pathway. We hypothesized that PDs target the TRPA1 channel and by this mechanism produce their analgesic effect.
Experimental Approach
Calcium responses and currents were studied in cultured TRPA1-expressing rodent dorsal root ganglion neurons and human cells. Acute nociception and mechanical hypersensitivity were investigated in naïve and genetically manipulated mice.
Key Results
Pyrazolone and PDs selectively inhibited calcium responses and currents in TRPA1-expressing cells and acute nocifensor responses in mice evoked by reactive channel agonists (allyl isothiocyanate, acrolein and H2O2). In line with recent results obtained with TRPA1 antagonists and TRPA1 gene deletion, the two most largely used PDs, dipyrone and propyphenazone, attenuated TRPA1-mediated nociception and mechanical allodynia in models of inflammatory and neuropathic pain (formalin, carrageenan, partial sciatic nerve ligation and the chemotherapeutic drug, bortezomib). Notably, dipyrone and propyphenazone attenuated carrageenan-evoked mechanical allodynia, without affecting PGE2 levels. The main metabolites of PDs did not target TRPA1 and did not affect TRPA1-dependent nociception and allodynia.
Conclusions and Implications
Evidence that in rodents the nociceptive/hyperalgesic effect produced by TRPA1 activation is blocked by PDs suggests that a similar pathway is attenuated by PDs in humans and that TRPA1 antagonists could be novel analgesics, devoid of the adverse haematological effects of PDs.
Tables of Links
| Targets | |
|---|---|
| GPCRsa | Ion channelsb |
| PAR2 | TRPA1 |
| Enzymesc | TRPV1 |
| COX-1 | RPV4 |
| COX-2 |
| Ligands | ||
|---|---|---|
| Acrolein | Dibutyryl cAMP | Icilin |
| Allyl isothiocyanate (AITC) | Formalin | Indomethacin |
| Bortezomib | GSH | Menthol |
| Capsaicin | GSK1016790A | PGE2 |
| Capsazepine | H2O2 | Pyrazolone |
| HC-030031 | SLIGKV-NH2 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,cAlexander et al., 2013a,b,c).
Introduction
The pyrazolone (Pyr) derivatives (PDs), antipyrine (AntiP), dipyrone (Dip) and propyphenazone (PPh), introduced in 1883, 1922 and 1951, respectively, have been successfully used for more than a century by hundreds of millions of people worldwide in a series of painful diseases, including migraine, colic and post-surgical and neuropathic pain (Babej-Dolle et al., 1994; Brune, 1997; Ramacciotti et al., 2007; Vallano et al., 2007; Derry et al., 2010). However, despite this long-term therapeutic success, the mechanism of the analgesic action of PDs remains to be understood.
Painkillers, such as non-steroidal antiinflammatory drugs (NSAIDs) or coxibs, relieve pain by inhibiting COX-1 and COX-2 respectively (Simmons et al., 2004). COX inhibition by PDs is, however, weak, resulting in poor anti-inflammatory effects, which do not match their potent analgesic action (Chandrasekharan et al., 2002; Simmons et al., 2004; Hinz et al., 2007; Malvar Ddo et al., 2011). Indeed, while indomethacin comparably inhibited carrageenan-evoked hyperalgesia/oedema, dipyrone was an effective antihyperalgesic and a poor anti-inflammatory agent (Lorenzetti and Ferreira, 1985). Dipyrone failed to reduce PG levels in rat tissues and, accordingly, dipyrone gastrointestinal toxicity is negligible (Weithmann and Alpermann, 1985; Brogden, 1986; Sanchez et al., 2002; Berenguer et al., 2004). Thus, the pharmacological actions of PDs do not replicate the COX-dependent effects of NSAIDs/coxibs and additional mechanisms (Lorenzetti and Ferreira, 1996; Sachs et al., 2004) have not received further support.
The transient receptor potential ankyrin 1 (TRPA1) channel is co-expressed by a subset of primary sensory neurons with cell bodies in dorsal root ganglia (DRG), which express the capsaicin-sensitive transient receptor potential vanilloid 1 (TRPV1) channel, the hypotonic solution-sensitive channel (TRPV4) and other TRP channels (Andrade et al., 2012; Nassini et al., 2014). Both pharmacological and genetic findings indicate that TRPA1 contributes to inflammatory and neuropathic pain models, including those evoked by formalin, spinal nerve ligation and chemotherapeutics (McNamara et al., 2007; Andrade et al., 2012; Trevisan et al., 2013b; Nassini et al., 2014).
Besides allyl isothiocyanate (AITC) and cinnamaldehyde (Bandell et al., 2004; Bautista et al., 2006), reactive/electrophilic by-products of oxidative stress, such as H2O2, 4-hydroxynonenal and acrolein, activate TRPA1 via a Michael addition or oxidation reactions of specific amino acid residues (Hinman et al., 2006; Macpherson et al., 2007; Trevisani et al., 2007; Taylor-Clark et al., 2009). We hypothesized that PDs inhibit TRPA1 and hence produce analgesia. The results indicate that PDs selectively antagonize TRPA1 activation by reactive channel agonists, thereby producing antinociceptive and antihyperalgesic effects.
Methods
Further information can be found in Supporting Information Appendix S1.
Animals
The animal experiments carried out conformed to the European Communities Council (ECC) guidelines for animal care procedures and the Italian legislation (DL 116/92) application of the ECC directive 86/609/EEC. Studies were conducted under the University of Florence research permit number 204/2012-B. Male C57BL/6 (25–30 g) (Harlan Laboratories, Milan, Italy), wild-type, Trpa1+/+ or TRPA1-deleted Trpa1−/− (25–30 g) mice generated by heterozygotes on a C57BL/6 background (B6;129P-Trpa1tm1Kykw/J; Jackson Laboratories, Bar Harbor, ME, USA) (Kwan et al., 2006) or Sprague Dawley rats (35–50 g, male, Harlan Laboratories) were used. For each behavioural experiment, we used groups of six mice. For the in vitro experiments as a whole, we used 30 rats and 28 mice. Animals were housed in a temperature- and humidity-controlled vivarium (12 h dark/light cycle, free access to food and water). Behavioural experiments were performed in a quiet, temperature-controlled (20 to 22°C) room between 0900 and 1700 h. Animals were killed with a high dose of i.p. sodium pentobarbital (200 mg·kg−1). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Reagents
HC-030031 has been synthesized as described previously (Andre et al., 2008). The activating peptide and its reverse peptide for human proteinase-activated receptor 2 (PAR2) (SLIGKV-NH2 and VKGILS-NH2, respectively) were dissolved in distilled water. If not otherwise indicated, all other reagents were from Sigma-Aldrich (Milan, Italy).
Cell culture and isolation of primary sensory neurons
HEK293 naïve cells or HEK293 cells stably transfected with the cDNA of human TRPV1 (hTRPV1-HEK293) or with the cDNA of human TRPV4 (hTRPV4-HEK293) were used and cultured as previously described (Nassini et al., 2012). HEK293 cells were transiently transfected with the cDNAs for wild-type or mutant 3C/K-Q (C619S, C639S, C663S, K708Q) human TRPA1 (Hinman et al., 2006; Trevisani et al., 2007). Human embryonic lung fibroblasts (IMR90; American Type Culture Collection, Manassas, VA, USA), which express the native TRPA1 channel (Nassini et al., 2012), were also used. DRG neurons were isolated from Sprague Dawley rats or C57BL/6 mice and cultured as previously described (Materazzi et al., 2012).
Cellular recordings
Single cell intracellular calcium levels were measured in transfected and untransfected HEK293 cells, IMR90 cells or in rat and mouse DRG neurons as previously reported (Materazzi et al., 2013). Whole-cell patch-clamp recordings were performed as previously reported in rat DRG neurons or in IMR90 cells. TRPA1 or TRPV1 currents were detected as inward currents activated upon cell superfusion with the different stimuli. Peak current was normalized with respect to cell membrane capacitance and expressed as mean of the current density (pA/pF) as previously reported (Nassini et al., 2012).
Behavioural experiments
For behavioural experiments, after habituation, C57BL/6 and Trpa1+/+ or Trpa1−/− mice were randomized into treatment groups and an investigator blinded to treatments recorded the responses. AITC, acrolein, H2O2, dibutyryl cAMP (Db-cAMP), capsaicin, zinc acetate, icilin and hypotonic solution (0.27% NaCl) were given by intraplantar (i.pl.) injection to provoke acute nociception and hyperalgesia. Formalin (McNamara et al., 2007) and carrageenan (i.pl.) (Bonet et al., 2013) were used as models of inflammatory pain. Partial sciatic nerve ligation (Zhou et al., 2013) and bortezomib (i.p.) (Trevisan et al., 2013b) were used as models of neuropathic pain. pyrazolone, propyphenazone, dipyrone, antipyrine and their metabolites, 4-methylaminoantipyrine (MAA), N-demethylpropyphenazone (dm-propyphenazone) and edaravone (Edar), respectively, HC-030031 or indomethacin were injected i.pl. or i.p. to reduce agonist-evoked responses.
Acute nociceptive behaviour was measured by assessing the total time spent in lifting/licking of the injected hind paw (Trevisani et al., 2007). Mechanical allodynia was measured by the up-and-down paradigm using Von Frey hairs (Chaplan et al., 1994). Cold hypersensitivity was assessed by measuring the acute nocifensive response to acetone (Trevisan et al., 2013b). Paw oedema was measured using an engineer's micrometre (Trevisan et al., 2013a).
PGE2 assay
PGE2 levels were measured by enzyme immunoassay in the paw homogenate as previously reported (Ulmann et al., 2010; Ma et al., 2013).
Molecular modelling
A high resolution structure of the TRPA1 channel has yet to be elucidated. Therefore, in order to explore a possible binding mode of the PDs into the channel, a homology model of the human TRPA1 was developed.
Statistical analysis
Data represent mean ± SEM or 95% confidence interval (CI). Statistical analysis was performed by use of Student's unpaired two-tailed t-test for comparisons between two groups or anova, followed by Bonferroni's post hoc test for comparisons of multiple groups. Potency of antagonists was expressed as IC50, that is, the molar concentration of the antagonist required for 50% inhibition of the maximum effect evoked by the agonist. P < 0.05 was considered statistically significant. GraphPadPrism version 5.00 (GraphPad Software, San Diego, CA, USA) was used.
Results
PDs are selective TRPA1 antagonists
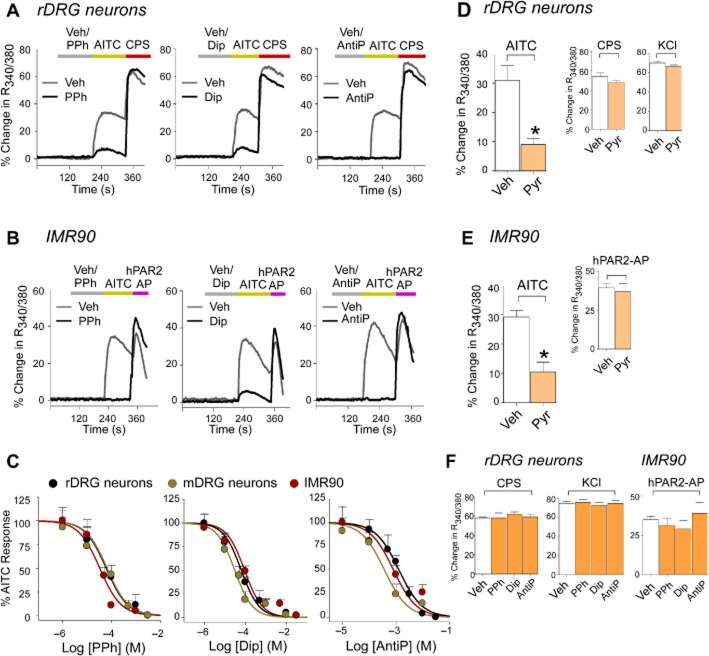
Pyrazolone or PDs, without producing any stimulating effect (Figure 1A and B), inhibited calcium responses evoked by AITC in rat and mouse DRG neurons and in human embryonic lung fibroblasts (IMR90, i.e. the cell type from which TRPA1 was originally cloned) (Jaquemar et al., 1999; Figure 1A–E). They did not affect responses to the selective TRPV1 agonist, capsaicin, high KCl or the PAR2 agonist, SLIGKV-NH2, thus indicating selectivity (Figure 1D–F). IC50s were similar for the two systemically used PDs, propyphenazone and dipyrone (∼60 μM) and higher for antipyrine (∼600 μM) (Figure 1C). Pyrazolone and the PDs did not affect responses to selective TRPV1 and TRPV4 agonists (capsaicin or GSK1016790A, respectively) in channels expressed in recombinant systems (Supporting Information Fig. S1A).
Figure 1.
Pyrazolone (Pyr) and its derivatives selectively inhibit the calcium response evoked by TRPA1 stimulation. (A and B) Typical traces of the inhibitory effect of pre-exposure (10 min) to the PDs, PPh (100 μM), Dip (100 μM) and AntiP (5 mM) on the calcium response evoked by the TRPA1 agonist, AITC (10 μM), in rat cultured DRG (rDRG) neurons and IMR90. (C) Concentration-response curves for the inhibitory effect of PPh (IC50s 33–66 μM), Dip (IC50s 30–91 μM) and AntiP (IC50s 360–1300 μM) on the calcium response evoked by AITC in rDRG neurons, mouse DRG (mDRG) neurons and IMR90 cells (AITC concentrations are 10, 10 and 1 μM respectively). (D and E) Pyr (100 μM) inhibits the calcium response evoked in rDRG neurons and IMR90 cells by AITC (10 μM and 1 μM respectively). (D–F) PPh (100 μM), Dip (100 μM), AntiP (5 mM) and Pyr (100 μM) do not affect the responses evoked by capsaicin (CPS, 0.1 μM) or high KCl (50 mM) in rDRG neurons and by the activating peptide of the human PAR2 receptor (hPAR2-AP, 100 μM) in IMR90 cells. Values are mean ± SEM of n < 25 cells from at least three different experiments for each condition. *P < 0.05 versus vehicle (Veh).
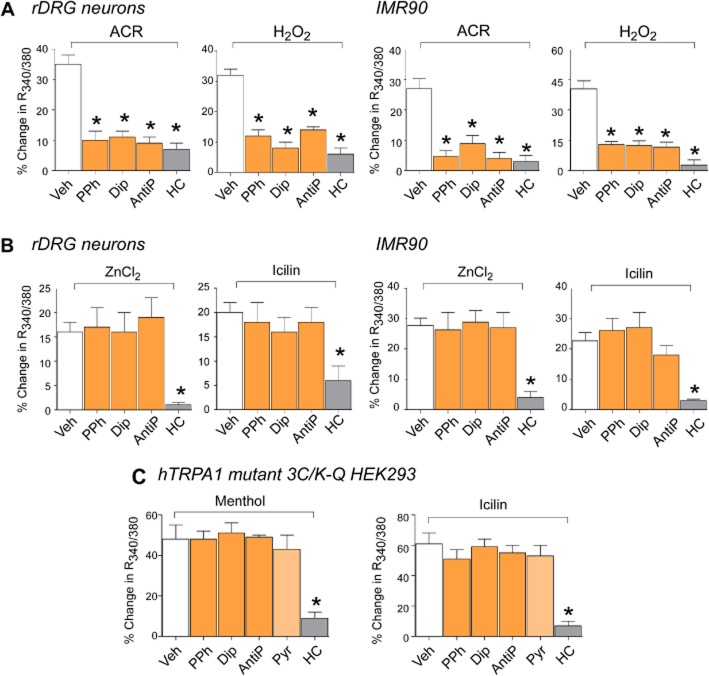
While the selective TRPA1 antagonist, HC-030031 (McNamara et al., 2007), inhibited responses evoked by all TRPA1 agonists, PDs only reduced responses evoked by electrophilic or reactive agonists, such as AITC, acrolein or H2O2 (Figure 2A), with no effect against the non-reactive agonists, ZnCl2 and icilin (Figure 2B), which act independently from binding key cysteine residues of TRPA1 (McKemy et al., 2002; Hu et al., 2009). In a mutated channel, which is without the cysteine and lysine residues, required for channel activation by reactive agonists (3C/K-Q, TRPA1-3C/K-Q) (Hinman et al., 2006; Macpherson et al., 2007; Trevisani et al., 2007), calcium responses to non-reactive stimuli, menthol (100 μM) or icilin (30 μM), were reduced by HC-030031 but unaffected by pyrazolone and PDs (Figure 2C).
Figure 2.
Pyrazolone (Pyr) and its derivatives selectively inhibit the calcium response evoked by reactive TRPA1 agonists. (A and B) PPh (100 μM), Dip (100 μM) and AntiP (5 mM) inhibit the calcium response evoked by acrolein (ACR, 10 μM) or H2O2 (500 μM) and do not affect the response induced by the non-reactive TRPA1 agonists, ZnCl2 (1 μM) and icilin (30 μM) in rat DRG (rDRG) neurons or in IMR90 cells. The selective TRPA1 antagonist, HC-030031 (HC, 30 μM) inhibits responses to both reactive and non-reactive agonists. (C) PPh, Dip, AntiP and Pyr (all, 100 μM) do not affect the calcium response of cells transfected with the cDNA codifying for the mutant human TRPA1 channel (3C/K-Q) responding to the non-electrophilic agonists, menthol (100 μM) and icilin (30 μM). Values are mean ± SEM of n < 25 cells from at least three different experiments for each condition. *P < 0.05 versus vehicle (Veh).
Metabolites of PDs are inactive
We also tested whether the metabolites of dipyrone, propyphenazone and antipyrine, MAA, dm-propyphenazone and edaravone, respectively, antagonize TRPA1 or scavenge TRPA1 reactive agonists. Ten minutes of pre-exposure to PD metabolites or the aldehyde and reactive oxygen species scavenger, GSH, failed to affect AITC- acrolein- or H2O2-evoked calcium responses (Supporting Information Fig. S1B). In contrast, 30 min coincubation of AITC, acrolein or H2O2 with GSH, but not with each individual PD or metabolite, reduced calcium responses as compared with responses produced by co-incubation with respective vehicles (Supporting Information Fig. S1C).
Mode of TRPA1 targeting by propyphenazone
To investigate whether PDs interact with specific cysteine residues, we used propyphenazone, given that some of its analogues exhibit moderate electrophilic properties (Li et al., 1998). By means of in silico analysis, we found that propyphenazone could interact with cysteine 608, so that its scaffold orientates in such a way that the oxygen atom of the pyrazolidinone ring forms an H-bond with the hydroxyl group of serine 582. The binding pose was further stabilized by the insertion of a propyphenazone phenyl ring within a lipophilic pocket delimited by hydrophobic residues (Supporting Information Fig. S2).
PDs inhibit currents evoked by AITC
In cultured rat DRG neurons, AITC and capsaicin evoked inward currents, which were reduced by HC-030031 and capsazepine respectively. Responses to AITC, but not to capsaicin, were markedly attenuated by pyrazolone, dipyrone, propyphenazone and antipyrine (Figure 3A). In IMR90 cells, AITC-evoked currents were attenuated by pre-exposure (Figure 3B) and reversed in about 1 min by the subsequent administration of HC-030031, dipyrone and propyphenazone (Figure 3C). Pyrazolone or the PDs did not produce any stimulating effect (Figure 3A,B).
Figure 3.
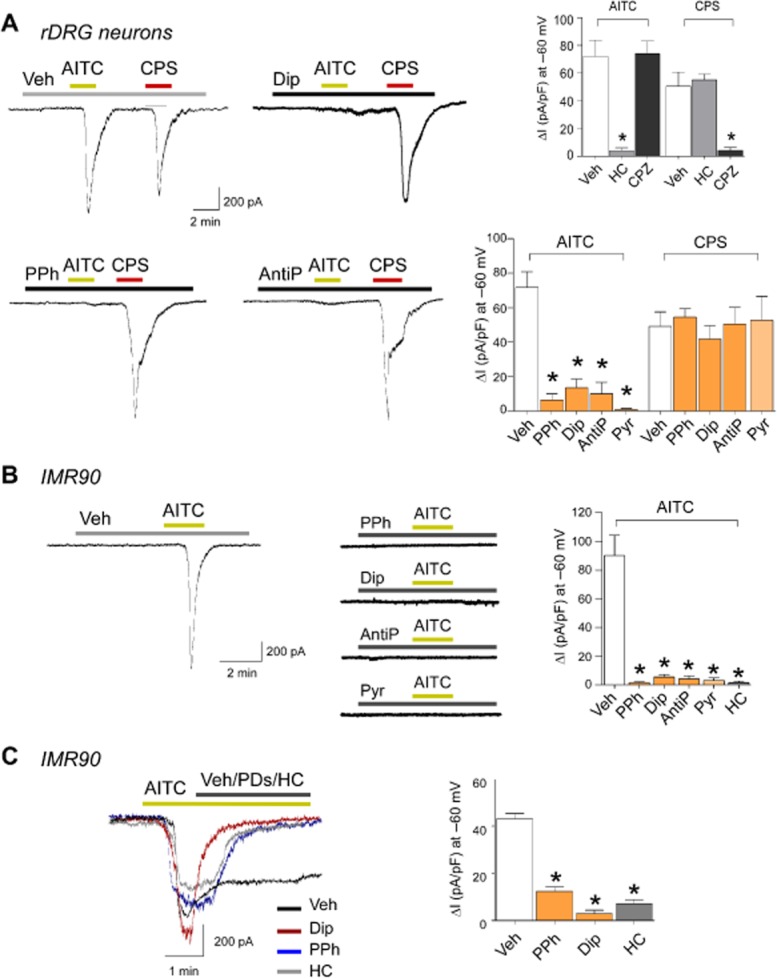
Pyrazolone (Pyr) and its derivatives selectively inhibit ion currents evoked by TRPA1 stimulation. (A) Original current traces and pooled data obtained by whole-cell patch-clamp recordings in rat DRG (rDRG) neurons. Application of AITC (100 μM) or capsaicin (CPS, 1 μM) elicits inward currents at −60 mV, which are blocked by the TRPA1 selective antagonist, HC-030031 (HC, 50 μM) and the TRPV1 selective antagonist capsazepine (CPZ, 10 μM) respectively. PPh (100 μM), Dip (100 μM), AntiP (1 mM) and Pyr (100 μM) prevent AITC-induced currents, but do not affect the currents evoked by CPS. (B) Original current traces and pooled data recorded in IMR90. Under control conditions, AITC (20 μM) activates inward currents at −60 mV, which are completely blocked by PPh (50 μM), Dip (50 μM), AntiP (100 μM), Pyr (50 μM) and HC (50 μM). (C) Original current traces and pooled data of the effect of PPh (50 μM), Dip (50 μM) and HC (50 μM) given after the application of AITC (20 μM) in IMR90 cells. PDs and HC reverse the effect of the agonist. Values are mean ± SEM of at least five cells for each experimental condition. *P < 0.05 versus vehicle (Veh).
PDs selectively block nocifensor responses evoked by reactive TRPA1 agonists
Systemic (i.p.) administration of PDs reduced in a dose-dependent manner acute nociceptive responses evoked by i.pl. AITC injected 30 min after PDs (Figure 4A), for example, when these drugs exerted their maximum antinociceptive effect (Figure 4B). Systemic (i.p.) administration of dm-propyphenazone, MAA and edaravone failed to affect the nociceptive response evoked by i.pl. AITC, injected 30 after the PD metabolites (Figure 4C). The nociceptive effect evoked by i.pl. AITC was markedly reduced by systemic HC-030031, given 60 min before AITC (Figure 4C). PDs did not affect nociception evoked by capsaicin or by a hypo-osmotic stimulus (TRPV1- or TRPV4-mediated responses respectively) (Figure 4D). Nocifensor behaviours by acrolein or H2O2 (i.pl.) were inhibited by HC-030031 (Figure 5A) and reduced in TRPA1-deleted mice (Figure 5A, insets). Responses induced by acrolein and H2O2 were markedly attenuated by PDs (Figure 5A). In contrast, nocifensor responses evoked by the non-reactive agonists, Zn acetate or icilin (i.pl.), which are markedly attenuated in TRPA1-deleted mice and by HC-030031, were completely unaffected by PDs (Figure 5B).
Figure 4.
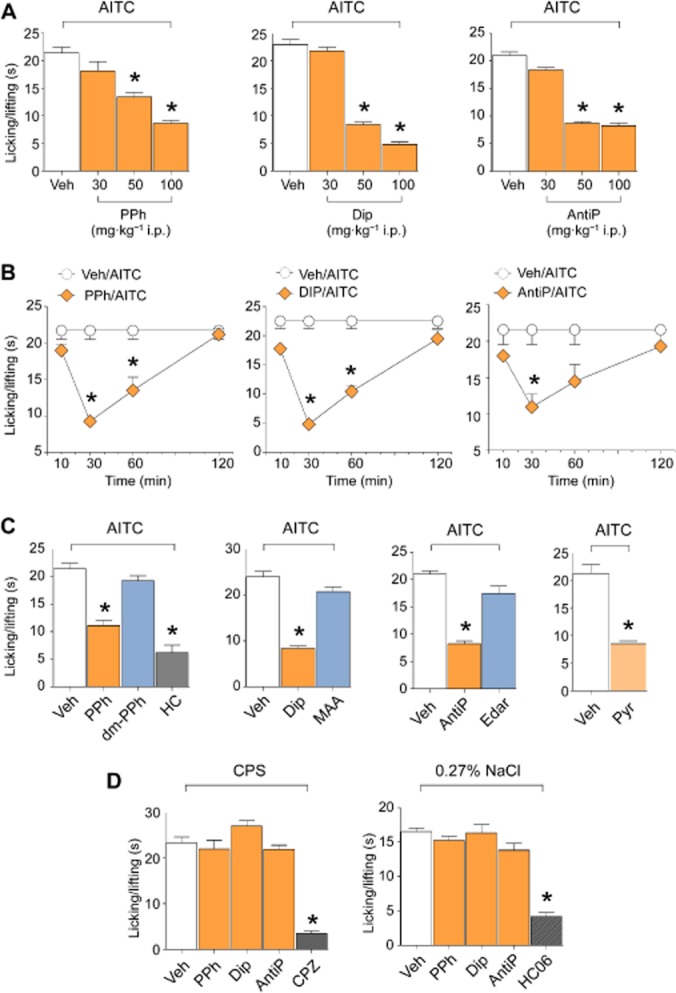
Pyrazolone (Pyr) and its derivatives selectively inhibit nociceptive responses evoked by the TRPA1 agonist, AITC, in mice. (A) Effect of increasing doses (30, 50 and 100 mg·kg−1) of i.p. PPh, Dip and AntiP on the nociceptive response evoked by the i.pl. injection (20 μL, i.pl.) of AITC (10 nmol per paw) in C57BL/6 mice. Measurements were performed 30 min after the i.p. administration of PDs. (B) Time course of the inhibitory effect of PPh, Dip and AntiP (all 50 mg·kg−1, i.p.) on the nociceptive response evoked by AITC (10 nmol per paw, 20 μL i.pl.) measured 10, 30, 60 and 120 min after the i.p. administration of PDs. (C) I.p. administration of Pyr, PPh, Dip, AntiP and the selective TRPA1 antagonist, HC-030031 (HC, 100 mg·kg−1) but not the PD metabolites, dm-PPh, MAA and edaravone (all 50 mg·kg−1), inhibit the nociceptive response evoked by i.pl. injection (20 μL) of AITC (10 nmol per paw) in mice. (D) PPh, Dip and AntiP (all 50 mg·kg−1, i.p.) do not affect nociceptive responses produced by capsaicin (0.1 nmol per paw, i.pl.) or hypotonic solution (0.27% NaCl, i.pl.), which are, however, inhibited by the TRPV1 antagonist, capsazepine (CPZ, 10 mg·kg−1, i.p.) and TRPV4 antagonist, HC-067047 (HC06 10 mg·kg−1, i.p.) respectively. Values are mean ± SEM of at least six mice for each experimental condition. Veh indicates vehicle of PDs, their metabolites, HC, CPZ and HC06. *P < 0.05 versus Veh.
Figure 5.
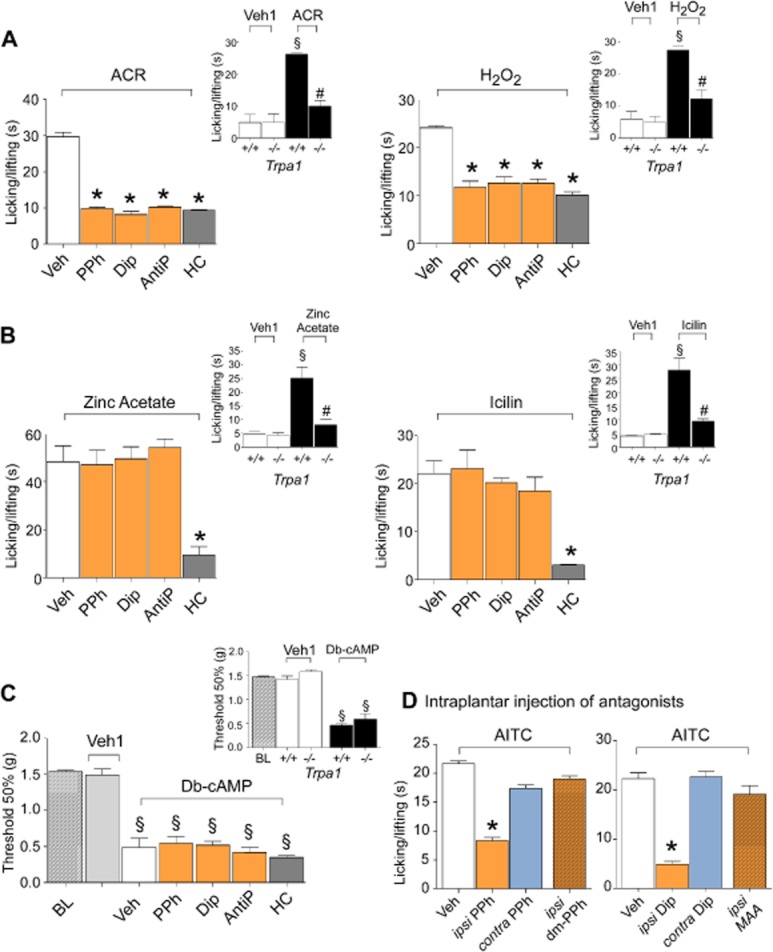
Pyrazolone (Pyr) derivatives selectively inhibit nociceptive responses evoked by reactive TRPA1 agonists in mice. (A and B) PPh, Dip and AntiP (all 50 mg·kg−1, i.p.) inhibit nociceptive responses evoked by the injection (20 μL, i.pl.) of acrolein (ACR, 10 nmol per paw) or H2O2 (1 μmol per paw) but not responses evoked by both zinc acetate and icilin. HC-030031 (HC, 100 mg·kg−1, i.p.) inhibits nociceptive responses evoked by both reactive and non-reactive TRPA1 agonists. (A and B, insets) Responses to both reactive and non-reactive agonists are markedly attenuated in Trpa1−/− mice. (C) Delayed allodynia observed 120 min after (20 μL, i.pl.) Db-cAMP (0.2 μmol per paw), which is similar in Trpa1+/+ and Trpa1−/− mice, is unaffected by PPh, Dip or AntiP (all 50 mg·kg−1, i.p.) (BL, basal level threshold). (D) In mice, ipsilateral (ipsi) injection (20 μL, i.pl.) of a mixture of AITC (10 nmol per paw) with PPh (1 μmol per paw) or Dip (10 μmol per paw), but not with dm-PPh (1 μmol per paw) or MAA (10 μmol·paw−1), reduces the nociceptive response evoked by the ipsilateral injection of the mixture of AITC (10 nmol per paw) with the vehicle (Veh). Nociceptive responses by AITC (10 nmol per paw) are not reduced by (20 μL, i.pl.) PPh (1 μmol per paw) or Dip (10 μmol per paw) injected in the contralateral (contra) paw. Values are mean ± SEM of at least six mice for each experimental condition. Veh indicates vehicle of PDs, their metabolites and HC. Veh1 indicates vehicle of ACR, H2O2, zinc acetate, icilin or Db-cAMP. *P < 0.05 versus Veh; §P < 0.05 versus Veh1; #P < 0.05 versus Trpa1+/+.
In accord with previous findings (Lorenzetti and Ferreira, 1985), the PDs did not reduce Db-cAMP (i.pl.)-evoked mechanical allodynia, a response which is maintained in TRPA1-deleted mice (Figure 5C). To test whether PDs target TRPA1 in peripheral terminals of nociceptors, PDs were injected in the same paw as AITC (ipsi) or in the contralateral paw (contra). Nociception was inhibited only in the ipsi paw (Figure 5D); dm-propyphenazone or MAA failed to attenuate AITC-evoked responses in the ipsi paw (Figure 5D).
PDs selectively reduce TRPA1-dependent nociception and hyperalgesia in models of inflammatory pain
In agreement with earlier reports, HC-030031 (McNamara et al., 2007), but not indomethacin (Malmberg and Yaksh, 1992), inhibited Phase I of the response to formalin, thus confirming and negating the role of TRPA1 and COX metabolites respectively (Figure 6). Propyphenazone and dipyrone reduced the nociception of Phase I, whereas their metabolites were ineffective (Figure 6). As previously reported (Malmberg and Yaksh, 1992; McNamara et al., 2007), the phase II response to formalin was inhibited by a variety of drugs, including, propyphenazone, dipyrone, indomethacin and MAA (Figure 6).
Figure 6.
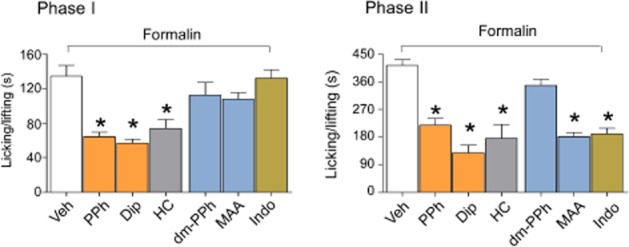
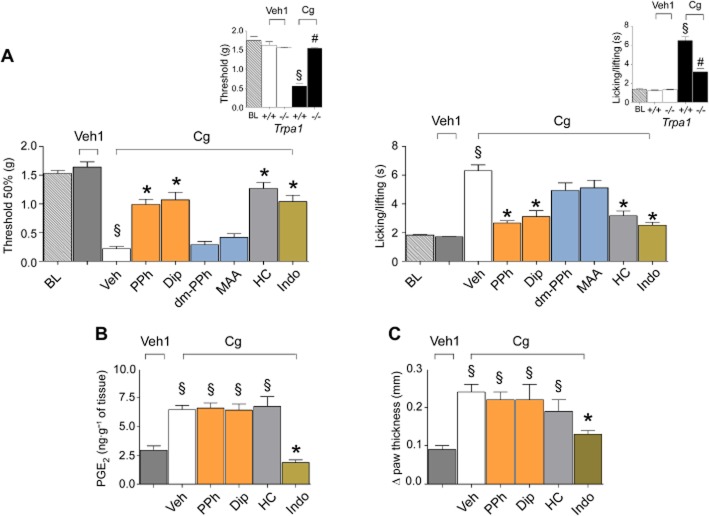
Pyrazolone (Pyr) derivatives produce antinociception and antihyperalgesia in the formalin model of inflammatory pain. I.p. administration of PPh or Dip (both 50 mg·kg−1) and the selective TRPA1 antagonist, HC-030031 (HC, 100 mg·kg−1, i.p.), inhibit phase I and phase II of the formalin test. The metabolites of PPh and Dip, dm-PPh and MAA (both 50 mg·kg−1) do not affect phase I. MAA, but not dm-PPh, inhibits phase II of the formalin test. Indomethacin (Indo, 30 mg·kg−1, i.p.) inhibits phase II, but does not affect phase I. Values are mean ± SEM of at least six mice for each experimental condition. Veh indicates the vehicle of PDs, their metabolites, HC and Indo. *P < 0.05 versus Veh.
Carrageenan-evoked pain-like symptoms were markedly reduced in TRPA1-deleted mice (Figure 7A, insets). HC-030031, propyphenazone and dipyrone, but not MAA and dm-propyphenazone, attenuated both mechanical and cold hyperalgesia induced by i.pl. carrageenan (Figure 7A). While the antihyperalgesic and anti-inflammatory actions of indomethacin were associated with abolition of the carrageenan-evoked increase in PGE2 tissue levels, the antihyperalgesic effect of PDs and HC-030031 was associated with no inhibition of the increased oedema and PGE2 levels (Figure 7B,C).
Figure 7.
PDs produce antinociception and antihyperalgesia in the carrageenan model of inflammatory pain. (A) Mechanical allodynia and cold hypersensitivity evoked by i.pl. (20 μL) injection of carrageenan (Cg, 300 μg per paw) in Trpa1+/+ are markedly attenuated in Trpa1−/− mice and inhibited by PPh or Dip (both 50 mg·kg−1), the selective TRPA1 antagonist, HC-030031 (HC, 100 mg·kg−1, i.p.), and indomethacin (Indo, 30 mg·kg−1, i.p.) but not by the PD metabolites, dm-PPh and MAA (both 50 mg·kg−1, i.p.) (BL, basal level threshold). (B) PGE2 levels in paw homogenate are increased 180 min after Cg injection (300 μg·20 μL−1, i.pl.). PPh, Dip (both 50 mg·kg−1, i.p.) and HC (100 mg·kg−1, i.p.) do not affect the increase in PGE2, which is, however, prevented by Indo (30 mg·kg−1, i.p.). (C) Indo (30 mg·kg−1, i.p.), but not PPh, Dip (both 50 mg·kg−1, i.p.) and HC (100 mg·kg−1, i.p.), inhibits paw oedema induced by i.pl. (20 μL) injection of Cg (300 μg per paw). Values are mean ± SEM of at least six mice for each experimental condition. Veh indicates the vehicle of PDs, their metabolites, HC and Indo. Veh1 indicates the vehicle of Cg. *P < 0.05 versus Veh; §P < 0.05 versus Veh1; #P < 0.05 versus Trpa1+/+.
PDs selectively reduce TRPA1-dependent nociception and hyperalgesia in models of neuropathic pain
A single administration in mice of the chemotherapeutic agent, bortezomib, has been previously shown to produce mechanical and cold hypersensitivity, which was completely TRPA1-dependent (Trevisan et al., 2013b). Here, we confirmed that bortezomib-evoked mechanical and cold hypersensitivity was HC-030031-dependent and we found that it was completely indomethacin-insensitive (Supporting Information Fig. S3) and markedly attenuated by propyphenazone and dipyrone, but not by dm-propyphenazone and MAA (Figure 8A). Mechanical allodynia evoked by spinal nerve ligation, a response attenuated in TRPA1-deleted mice (Figure 8B), was reduced by PDs and HC-030031, but not by PD metabolites or indomethacin (Figure 8C). Notably, PDs and HC-030031 were unable to further affect the reduced allodynia observed in TRPA1-deleted mice (Figure 8C). Identical results were obtained when cold hypersensitivity was assessed (Figure 8C).
Figure 8.
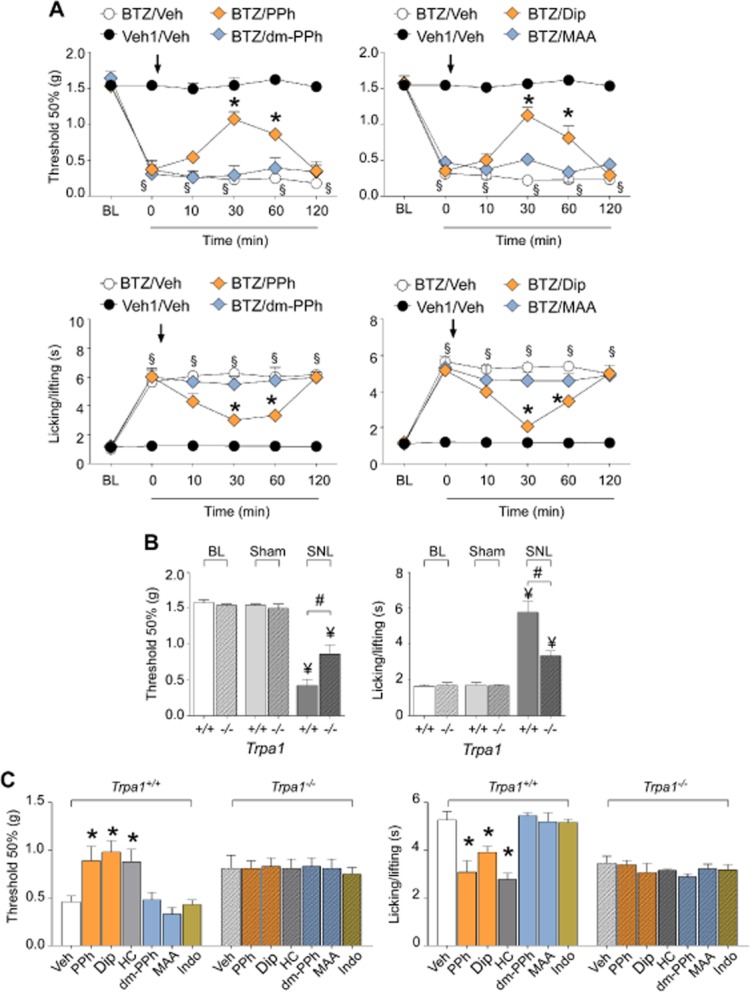
PDs produce antihyperalgesic effects in models of neuropathic pain. (A) At day 7 after treatment with bortezomib (BTZ, 1 mg·kg−1, i.p.) mechanical allodynia and cold hypersensitivity are increased (BL, basal level threshold at day 0 before BTZ). At day 7, injection (arrow) of PPh or Dip (both 50 mg·kg−1, i.p.), but not of their metabolites, dm-PPh and MAA (both 50 mg·kg−1, i.p.), inhibits mechanical allodynia and cold hypersensitivity induced by BTZ. (B) At day 10 after partial sciatic nerve ligation (SNL), Trpa1+/+ mice develop mechanical allodynia and cold hypersensitivity, which are partially reduced in Trpa1−/− mice and not present in mice that underwent the sham procedure (Sham). (C) At day 10 after SNL, injection of PPh, Dip (both 50 mg·kg−1, i.p.) and HC (100 mg·kg−1, i.p.) reverses the mechanical allodynia and cold hypersensitivity in Trpa1+/+ mice, but does not provide further protection in Trpa1−/− mice. dm-PPh and MAA (both 50 mg·kg−1, i.p.) or indomethacin (Indo, 30 mg·kg−1, i.p.) do not affect mechanical allodynia and cold hypersensitivity in either Trpa1+/+ or Trpa1−/− mice. Values are mean ± SEM of at least six mice for each experimental condition. Veh indicates the vehicle of PDs, their metabolites, HC and Indo. Veh1 indicates the vehicle of BTZ. §P < 0.05 versus Veh1/Veh; *P < 0.05 versus BTZ/Veh (A) or Veh (C); ¥P < 0.05 versus respective BL and Sham; #P < 0.05 versus Trpa1+/+.
Discussion
Since the seminal discovery of antipyrine by Ludwig Knorr in 1883, PDs have been one of the most successful classes of drugs in pain pharmacotherapy. However, the question of the mechanism of their analgesic action has remained unresolved. Here, we demonstrated that pyrazolone and its derivatives, dipyrone, propyphenazone and antipyrine, are selective antagonists of the TRPA1 channel, with no agonistic effects on TRPA1. PDs, such as NSAIDs/coxibs, have been proposed to act through inhibition of PG synthesis (Simmons et al., 2004; Hinz et al., 2007; Malvar Ddo et al., 2011). However, this hypothesis was soon challenged by a series of preclinical and clinical findings. Pain models responsive to dipyrone are clearly distinct from those inhibited by classical COX inhibitors (Brune and Alpermann, 1983; Lorenzetti and Ferreira, 1985). IC50s of dipyrone to inhibit COX-1 and COX-2 (0.35 and 1 mM, respectively) (Chandrasekharan et al., 2002) markedly exceed the plasma concentrations attainable with clinical doses (Volz and Kellner, 1980). The higher efficiacy of dipyrone versus NSAIDs in reducing pain in neuropathic pain conditions, such as sciatic pain or post-surgical pain (Babej-Dolle et al., 1994; Derry et al., 2010) as well as poor gastrointestinal, cardiovascular and renal toxicity (Pogatzki-Zahn et al., 2014), further differentiate PDs from NSAIDs with regard to their pharmacological mechanism of action. However, it should be noted that while the role of PDs in inflammatory pain is established over more than a century by a number of studies, little evidence has been gathered regarding the efficacy of PDs in neuropathic pain. This discrepancy may be due to the safety issues (agranulocytosis), which arose years before (Edwards and McQuay, 2002) the scientific community had reached a common definition and identification of neuropathic pain (Merskey and Bogduk, 1994). Thus, the ability to explore the effect of PDs in neuropathic pain has most likely been hampered by the recently acquired awareness of their poor safety profile. Nevertheless, the efficacy of dipyrone has been shown in acute lumbago or sciatic pain (Babej-Dolle et al., 1994) and in conditions of mixed pain, such as post-operative pain (Grundmann et al., 2006; Pogatzki-Zahn et al., 2014).
In this study, we provide robust evidence against the role of PG inhibition in the analgesic action of PDs. Although PDs and HC-030031 reduced carrageenan-evoked hyperalgesia similarly to indomethacin, the associated increase in oedema and PGE2 levels was attenuated only by indomethacin and not affected by PDs and HC-030031. Thus, the marked lack of association between the antihyperalgesic and anti-inflammatory action and of PDs contrasts with the entirely COX-dependent effects of indomethacin. The present observations, consistent with the poor ability of PDs to inhibit COXs (Chandrasekharan et al., 2002; Simmons et al., 2004), casts further doubts on COX inhibition as the main mechanism responsible for the analgesic action of PDs. In addition, the remarkable selectivity of PDs at inhibiting TRPA1 activation should be outlined. Notably, PDs only attenuated nociception evoked by the injection in the paw of the selective TRPA1 agonist, AITC, without affecting the action of the selective TRPV1 agonist, capsaicin, or a hypoosmotic stimulus which activates TRPV4.
Dipyrone, through the action of its main metabolite, MAA, has been reported to possess COX-inibitory, antioxidant and scavenging properties (Costa et al., 2006; Hinz et al., 2007; Pierre et al., 2007; Aldini et al., 2010), which early on were claimed to contribute to PD-evoked analgesia (Weithmann and Alpermann, 1985). MAA, through an iron-dependent mechanism, may sequestrate radicals that are necessary to initiate COX catalytic cycle (Pierre et al., 2007). Therapeutic effects of edaravone have also been associated with its scavenging activity (Mao et al., 2009). The finding that PDs selectively inhibit TRPA1 channel activation by reactive agonists raises the plausible hypothesis that PD metabolites may contribute to the analgesic action of dipyrone, propyphenazone and antipyrine by sequestering reactive electrophilic agonists. However, PD metabolites were unable to reduce nociception or hyperalgesia in all the TRPA1-dependent pain-like responses investigated in the present study. In addition, only prolonged incubation with the classical scavenger molecule, GSH, abolished TRPA1 activation by eletrophifilic or reactive agonists, AITC, acrolein or H2O2, while MAA, dm-propyphenazone and edaravone were ineffective. Finally, although propyphenazone shares all the antinociceptive and antihyepralgesic effects of dipyrone (Costa et al., 2006), propyphenazone and dm-propyphenazone did not show any antioxidant properties (Costa et al., 2006). Thus, the potential antioxidant and scavenging properties of MAA and edaravone do not seem to be responsible for the TRPA1-dependent analgesic action of their precursors.
In summary, our findings showed that: (i) PDs, but not their metabolites, are selective TRPA1 antagonists; (ii) the TRPA1-dependent analgesic profile of PDs is not shared by their metabolites; (iii) attenuation by PDs of pain-like effects in the carrageenan test is completely unrelated to PG inhibition; and (iv) in all pain models, the ability of dipyrone and propyphenazone to produce antinociceptive or antihyperalgesic effects in vivo is exquisitely TRPA1-dependent. Thus, we propose that the analgesic activity of PDs resides substantially in their ability to directly target the TRPA1 channel. This hypothesis is corroborated by the observation that the IC50s required to inhibit the rodent and human native TRPA1 (∼60 μM) are close to those found in humans after therapeutic doses of dipyrone or propyphenazone, the two PDs applied by a systemic route of administration (Volz and Kellner, 1980).
Emerging evidence indicates that oxidative and nitrative stress and the ensuing lipid peroxidation by-products produce nociception and hyperalgesia by targeting TRPA1 (Bautista et al., 2006; Trevisani et al., 2007; Taylor-Clark et al., 2009). This novel pathway has been reported to contribute to models of both inflammatory and neuropathic pain (McNamara et al., 2007; Andrade et al., 2012; Trevisan et al., 2013b; Nassini et al., 2014). The selective antagonism against potentially reactive endogenous agonists (acrolein and H2O2), corroborated by mutagenesis studies and in silico analysis, strengthens the proposal that PDs target TRPA1 by binding to cysteine residues required for TRPA1 channel activation by reactive endogenous agonists. A consequence of such a hypothesis is that, in models of inflammatory and neuropathic pain, PDs block the signalling pathway activated by oxidative stress by-products, via stimulation of the TRPA1 channel, expressed in primary sensory neurons (Andrade et al., 2012; Nassini et al., 2014). Attenuation of the pain-producing TRPA1-dependent pathway activated by oxidative stress by-products might also contribute to the analgesic action of PDs in various types of pain in humans (Babej-Dolle et al., 1994; Ramacciotti et al., 2007; Derry et al., 2010).
In spite of their generally good tolerability, the use and further development of PDs has been hampered by their adverse haematological effects, as indicated by the reported increased incidence of blood dyscrasias and agranulocytosis (Hedenmalm and Spigset, 2002), which has led to the withdrawal of dipyrone and propyphenazone in several countries. More recently, pharmacovigilance reports failed to detect a strong association between dipyrone use and agranulocytosis (Ibanez et al., 2005). Nevertheless, a substantial portion of the world population can no longer use a group of effective and otherwise well-tolerated pain killers because of this severe adverse haematological reaction. The present findings strongly support the rationale for the development of TRPA1 antagonists as new analgesics for the treatment of inflammatory and neuropathic pain. The new chemical entities with TRPA1 antagonistic properties, while maintaining good efficacy in pain treatment and general safety profile of dipyrone or propyphenazone, should be devoid of the life-threatening adverse haematological reactions, presumably associated with the chemical structure of PDs.
Acknowledgments
We thank M.J. Gunthorpe (GlaxoSmithKline, UK) for the hTRPV1-HEK293 cells and N.W. Bunnett (Monash Institute of Pharmaceutical Sciences, Australia) for the hTRPV4-HEK293 cells, D. Julius (University of California, San Francisco, CA, USA) for the human TRPA1 wild type and human TRPA1 mutant (C619S, C639S, C663S, K708Q) cDNAs and G. Cirino (University of Naples, Italy) for SLIGKV-NH2 and VKGILS-NH2. This work was supported by Progetto Impatto-Ministero Dello Sviluppo Economico (2012–2013) (P. G.); Associazione Italiana per la Ricerca sul Cancro (AIRC) (R. N.); Ministero dell'Istruzione, dell'Università e della Ricerca, PRIN-2010Y4WMCR-007 (S. M.) and Ente Cassa di Risparmio di Firenze-2010 (S. M.).
Glossary
- AITC
allyl isothiocyanate
- AntiP
antipyrine
- CI
confidence interval
- Db-cAMP
dibutyryl cAMP
- Dip
dipyrone
- dm-PPh
N-demethylpropyphenazone
- DRG
dorsal root ganglia
- Edar
edaravone
- EEC
European Communities Council
- IMR90
human embryonic lung fibroblasts
- MAA
4-methylaminoantipyrine
- NSAIDs
non-steroidal anti-inflammatory drugs
- PAR2
proteinase activated receptor 2
- PDs
pyrazolone derivatives
- PPh
propyphenazone
- Pyr
pyrazolone
- TRPA1
transient receptor potential ankyrin 1
- TRPV1
transient receptor potential vanilloid 1
Author contributions
R. N., S. M., P. G., R. P., S. B. and A. C. designed the experiments and interpreted the results. C. F., S.M. and F. D. L. performed the calcium experiments. E. C. performed the electrophysiological experiments. R. N., I. M. M., R. T. and D. P. performed the in vivo experiments and T. T. performed the docking studies.
Conflict of interest
R. P. is full-time employee at Chiesi Farmaceutici S.p.A. Other authors state no conflict of interest.
Supporting Information
Appendix S1 Supplemental methods.
Figure S1 (A) PPh, Dip, AntiP and Pyr (all, 100 μM) do not affect the calcium response evoked by capsaicin (CPS, 0.1 μM) in HEK293 cells transfected with the cDNA of the human TRPV1 (hTRPV1-HEK), a response blocked by capsazepine (CPZ, 10 μM) and the calcium response evoked by the TRPV4 agonist, GSK1016790A (GSK, 0.1 μM), in HEK293 cells transfected with the cDNA of the human TRPV4 (hTRPV4-HEK), a response blocked by the TRPV4 antagonist, HC-067047 (HC06, 10 μM). (B) Pre-exposure (10 min before) to metabolites of PPh, Dip and AntiP, dm-PPh (500 μM), MAA (1 mM) and Edar (1 mM), respectively, and to the aldehyde and reactive oxygen species scavenger, GSH (1 mM) do not affect the calcium response to AITC in rat DRG neurons (10 μM) and IMR90 cells (1 μM). (C) In another series of experiments, acrolein (ACR, 3 μM) or H2O2 (500 μM) were co-incubated for 30 min with PPh (100 μM), Dip (1 mM), AntiP (5 mM), Pyr (100 μM), GSH (1 or 5 mM against ACR or H2O2, respectively), dm-PPh (500 μM), MAA (1 mM), edaravone (1 mM) or their vehicles (Veh). IMR90 cells were then challenged with the mixtures. Responses produced by the mixture of ACR or H2O2 and Vehs are not affected by Pyr, PDs or their metabolites, but are abated by GSH. Pre-exposure (10 min prior stimuli) of IMR90 cells to GSH (1 or 5 mM against ACR or H2O2, respectively) does not affect the calcium response evoked by ACR (3 μM) or H2O2 (500 μM). Values are mean ± SEM of at least n < 25 cells from at least three different experiments for each experimental condition. Veh indicates the combination of the vehicles of the various compounds. *P < 0.05 versus Veh.
Figure S2 Hypothetical interaction of PPh with TRPA1. Receptor (left) and binding site (right) visualization. The TRPA1 electron density map is also reported in the receptor visualization. The interaction between PPh and C608 allows the formation of an H-bond between the hydroxyl group of S582 and the oxygen of the pyrazolidinone ring of PPh. The isopropyl substituent appears to be solvent exposed and the methyl group interacts with L609. Finally, the phenyl ring is inserted into a lipophilic pocket mainly delimited by L584, L588, V596 and I600.
Figure S3 Pharmacological interventions in mouse models of neuropathic pain. At day 7 after treatment with bortezomib (BTZ, 1 mg·kg−1, i.p.), mechanical allodynia and cold hypersensitivity are increased (BL, baseline at day 0 before BTZ). At day 7, injection (arrow) of HC-030031 (HC, 100 mg·kg−1, i.p.), but not indomethacin (Indo, 30 mg·kg−1, i.p.), inhibits mechanical allodynia and cold hypersensitivity induced by BTZ. Values are mean ± SEM of at least six mice for each experimental condition. Veh indicates the vehicle of HC or Indo. Veh1 indicates the vehicle of BTZ. §P < 0.05 versus Veh1/Veh; *P < 0.05 versus BTZ/Veh.
References
- Aldini G, Vistoli G, Regazzoni L, Benfatto MC, Bettinelli I, Carini M. Edaravone inhibits protein carbonylation by a direct carbonyl-scavenging mechanism: focus on reactivity, selectivity, and reaction mechanisms. Antioxid Redox Signal. 2010;12:381–392. doi: 10.1089/ars.2009.2814. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: enzymes. Br J Pharmacol. 2013c;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade EL, Meotti FC, Calixto JB. TRPA1 antagonists as potential analgesic drugs. Pharmacol Ther. 2012;133:189–204. doi: 10.1016/j.pharmthera.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–2582. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babej-Dolle R, Freytag S, Eckmeyer J, Zerle G, Schinzel S, Schmeider G, et al. Parenteral dipyrone versus diclofenac and placebo in patients with acute lumbago or sciatic pain: randomized observer-blind multicenter study. Int J Clin Pharmacol Ther. 1994;32:204–209. [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Berenguer B, Alarcon De La Lastra C, Motilva V, La Casa C, Herrerias JM, Pozo D, et al. Effects of celecoxib on acid-challenged gastric mucosa of rats: comparison with metamizol and piroxicam. Dig Dis Sci. 2004;49:937–947. doi: 10.1023/b:ddas.0000034552.20917.5e. [DOI] [PubMed] [Google Scholar]
- Bonet IJ, Fischer L, Parada CA, Tambeli CH. The role of transient receptor potential A 1 (TRPA1) in the development and maintenance of carrageenan-induced hyperalgesia. Neuropharmacology. 2013;65:206–212. doi: 10.1016/j.neuropharm.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Brogden RN. Pyrazolone derivatives. Drugs. 1986;32(Suppl. 4):60–70. doi: 10.2165/00003495-198600324-00006. [DOI] [PubMed] [Google Scholar]
- Brune K. The early history of non-opioid analgesics. Acute Pain. 1997;I:33–40. [Google Scholar]
- Brune K, Alpermann H. Non-acidic pyrazoles: inhibition of prostaglandin production, carrageenan oedema and yeast fever. Agents Actions. 1983;13:360–363. doi: 10.1007/BF01971489. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Costa D, Marques AP, Reis RL, Lima JL, Fernandes E. Inhibition of human neutrophil oxidative burst by pyrazolone derivatives. Free Radic Biol Med. 2006;40:632–640. doi: 10.1016/j.freeradbiomed.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Derry S, Faura C, Edwards J, McQuay HJ, Moore RA. Single dose dipyrone for acute postoperative pain. Cochrane Database Syst Rev. 2010;(8) doi: 10.1002/14651858.CD003227.pub2. CD003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JE, McQuay HJ. Dipyrone and agranulocytosis: what is the risk? Lancet. 2002;360:1438. doi: 10.1016/S0140-6736(02)11489-9. [DOI] [PubMed] [Google Scholar]
- Grundmann U, Wornle C, Biedler A, Kreuer S, Wrobel M, Wilhelm W. The efficacy of the non-opioid analgesics parecoxib, paracetamol and metamizol for postoperative pain relief after lumbar microdiscectomy. Anesth Analg. 2006;103:217–222. doi: 10.1213/01.ane.0000221438.08990.06. Table of contents. [DOI] [PubMed] [Google Scholar]
- Hedenmalm K, Spigset O. Agranulocytosis and other blood dyscrasias associated with dipyrone (metamizole) Eur J Clin Pharmacol. 2002;58:265–274. doi: 10.1007/s00228-002-0465-2. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Cheremina O, Bachmakov J, Renner B, Zolk O, Fromm MF, et al. Dipyrone elicits substantial inhibition of peripheral cyclooxygenases in humans: new insights into the pharmacology of an old analgesic. FASEB J. 2007;21:2343–2351. doi: 10.1096/fj.06-8061com. [DOI] [PubMed] [Google Scholar]
- Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol. 2009;5:183–190. doi: 10.1038/nchembio.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez L, Vidal X, Ballarin E, Laporte JR. Agranulocytosis associated with dipyrone (metamizol) Eur J Clin Pharmacol. 2005;60:821–829. doi: 10.1007/s00228-004-0836-y. [DOI] [PubMed] [Google Scholar]
- Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem. 1999;274:7325–7333. doi: 10.1074/jbc.274.11.7325. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Li XL, Wang YM, Tian B, Matsuura T, Meng JB. The solid-state Michael addition of 3-methyl-1-phenyl-5-pyrazolone. J Heterocycl Chem. 1998;35:129–134. [Google Scholar]
- Lorenzetti BB, Ferreira SH. Mode of analgesic action of dipyrone: direct antagonism of inflammatory hyperalgesia. Eur J Pharmacol. 1985;114:375–381. doi: 10.1016/0014-2999(85)90383-8. [DOI] [PubMed] [Google Scholar]
- Lorenzetti BB, Ferreira SH. Activation of the arginine-nitric oxide pathway in primary sensory neurons contributes to dipyrone-induced spinal and peripheral analgesia. Inflamm Res. 1996;45:308–311. doi: 10.1007/BF02280997. [DOI] [PubMed] [Google Scholar]
- Ma Y, Li Y, Li X, Wu Y. Anti-inflammatory effects of 4-methylcyclopentadecanone on edema models in mice. Int J Mol Sci. 2013;14:23980–23992. doi: 10.3390/ijms141223980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992;263:136–146. [PubMed] [Google Scholar]
- Malvar Ddo C, Soares DM, Fabricio AS, Kanashiro A, Machado RR, Figueiredo MJ, et al. The antipyretic effect of dipyrone is unrelated to inhibition of PGE(2) synthesis in the hypothalamus. Br J Pharmacol. 2011;162:1401–1409. doi: 10.1111/j.1476-5381.2010.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao YF, Yan N, Xu H, Sun JH, Xiong YC, Deng XM. Edaravone, a free radical scavenger, is effective on neuropathic pain in rats. Brain Res. 2009;1248:68–75. doi: 10.1016/j.brainres.2008.10.073. [DOI] [PubMed] [Google Scholar]
- Materazzi S, Fusi C, Benemei S, Pedretti P, Patacchini R, Nilius B, et al. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. 2012;463:561–569. doi: 10.1007/s00424-011-1071-x. [DOI] [PubMed] [Google Scholar]
- Materazzi S, Benemei S, Fusi C, Gualdani R, De Siena G, Vastani N, et al. Parthenolide inhibits nociception and neurogenic vasodilatation in the trigeminovascular system by targeting the TRPA1 channel. Pain. 2013;154:2750–2758. doi: 10.1016/j.pain.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey H, Bogduk N, editors. Classification of Chronic Pain. Seattle, WA: International Association for the Study of Pain Press; 1994. (eds) [Google Scholar]
- Nassini R, Pedretti P, Moretto N, Fusi C, Carnini C, Facchinetti F, et al. Transient Receptor Potential Ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One. 2012;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Benemei S, Geppetti P. The TRPA1 Channel in Inflammatory and Neuropathic Pain and Migraine. Rev Physiol Biochem Pharmacol. 2014;176:1–43. doi: 10.1007/112_2014_18. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42(Database Issue):D1098–1106. doi: 10.1093/nar/gkt1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre SC, Schmidt R, Brenneis C, Michaelis M, Geisslinger G, Scholich K. Inhibition of cyclooxygenases by dipyrone. Br J Pharmacol. 2007;151:494–503. doi: 10.1038/sj.bjp.0707239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogatzki-Zahn E, Chandrasena C, Schug SA. Nonopioid analgesics for postoperative pain management. Curr Opin Anaesthesiol. 2014;27:513–519. doi: 10.1097/ACO.0000000000000113. [DOI] [PubMed] [Google Scholar]
- Ramacciotti AS, Soares BG, Atallah AN. Dipyrone for acute primary headaches. Cochrane Database Syst Rev. 2007;(18) doi: 10.1002/14651858.CD004842.pub2. CD004842. [DOI] [PubMed] [Google Scholar]
- Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hypernociception: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K + channel pathway. Proc Natl Acad Sci U S A. 2004;101:3680–3685. doi: 10.1073/pnas.0308382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez S, Alarcon de la Lastra C, Ortiz P, Motilva V, Martin MJ. Gastrointestinal tolerability of metamizol, acetaminophen, and diclofenac in subchronic treatment in rats. Dig Dis Sci. 2002;47:2791–2798. doi: 10.1023/a:1021077810548. [DOI] [PubMed] [Google Scholar]
- Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Ghatta S, Bettner W, Undem BJ. Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol. 2009;75:820–829. doi: 10.1124/mol.108.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan G, Hoffmeister C, Rossato MF, Oliveira SM, Silva MA, Ineu RP, et al. Transient receptor potential ankyrin 1 receptor stimulation by hydrogen peroxide is critical to trigger pain during monosodium urate-induced inflammation in rodents. Arthritis Rheum. 2013a;65:2984–2995. doi: 10.1002/art.38112. [DOI] [PubMed] [Google Scholar]
- Trevisan G, Materazzi S, Fusi C, Altomare A, Aldini G, Lodovici M, et al. Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 2013b;73:3120–3131. doi: 10.1158/0008-5472.CAN-12-4370. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29:2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallano A, Malouf J, Payrulet P, Banos JE. Analgesic use and pain in the hospital settings. Eur J Clin Pharmacol. 2007;63:619–626. doi: 10.1007/s00228-007-0303-7. [DOI] [PubMed] [Google Scholar]
- Volz M, Kellner HM. Kinetics and metabolism of pyrazolones (propyphenazone, aminopyrine and dipyrone) Br J Clin Pharmacol. 1980;10(Suppl. 2):299S–308S. doi: 10.1111/j.1365-2125.1980.tb01813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weithmann KU, Alpermann HG. Biochemical and pharmacological effects of dipyrone and its metabolites in model systems related to arachidonic acid cascade. Arzneimittelforschung. 1985;35:947–952. [PubMed] [Google Scholar]
- Zhou Y, Suzuki Y, Uchida K, Tominaga M. Identification of a splice variant of mouse TRPA1 that regulates TRPA1 activity. Nat Commun. 2013;4:2399. doi: 10.1038/ncomms3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplemental methods.
Figure S1 (A) PPh, Dip, AntiP and Pyr (all, 100 μM) do not affect the calcium response evoked by capsaicin (CPS, 0.1 μM) in HEK293 cells transfected with the cDNA of the human TRPV1 (hTRPV1-HEK), a response blocked by capsazepine (CPZ, 10 μM) and the calcium response evoked by the TRPV4 agonist, GSK1016790A (GSK, 0.1 μM), in HEK293 cells transfected with the cDNA of the human TRPV4 (hTRPV4-HEK), a response blocked by the TRPV4 antagonist, HC-067047 (HC06, 10 μM). (B) Pre-exposure (10 min before) to metabolites of PPh, Dip and AntiP, dm-PPh (500 μM), MAA (1 mM) and Edar (1 mM), respectively, and to the aldehyde and reactive oxygen species scavenger, GSH (1 mM) do not affect the calcium response to AITC in rat DRG neurons (10 μM) and IMR90 cells (1 μM). (C) In another series of experiments, acrolein (ACR, 3 μM) or H2O2 (500 μM) were co-incubated for 30 min with PPh (100 μM), Dip (1 mM), AntiP (5 mM), Pyr (100 μM), GSH (1 or 5 mM against ACR or H2O2, respectively), dm-PPh (500 μM), MAA (1 mM), edaravone (1 mM) or their vehicles (Veh). IMR90 cells were then challenged with the mixtures. Responses produced by the mixture of ACR or H2O2 and Vehs are not affected by Pyr, PDs or their metabolites, but are abated by GSH. Pre-exposure (10 min prior stimuli) of IMR90 cells to GSH (1 or 5 mM against ACR or H2O2, respectively) does not affect the calcium response evoked by ACR (3 μM) or H2O2 (500 μM). Values are mean ± SEM of at least n < 25 cells from at least three different experiments for each experimental condition. Veh indicates the combination of the vehicles of the various compounds. *P < 0.05 versus Veh.
Figure S2 Hypothetical interaction of PPh with TRPA1. Receptor (left) and binding site (right) visualization. The TRPA1 electron density map is also reported in the receptor visualization. The interaction between PPh and C608 allows the formation of an H-bond between the hydroxyl group of S582 and the oxygen of the pyrazolidinone ring of PPh. The isopropyl substituent appears to be solvent exposed and the methyl group interacts with L609. Finally, the phenyl ring is inserted into a lipophilic pocket mainly delimited by L584, L588, V596 and I600.
Figure S3 Pharmacological interventions in mouse models of neuropathic pain. At day 7 after treatment with bortezomib (BTZ, 1 mg·kg−1, i.p.), mechanical allodynia and cold hypersensitivity are increased (BL, baseline at day 0 before BTZ). At day 7, injection (arrow) of HC-030031 (HC, 100 mg·kg−1, i.p.), but not indomethacin (Indo, 30 mg·kg−1, i.p.), inhibits mechanical allodynia and cold hypersensitivity induced by BTZ. Values are mean ± SEM of at least six mice for each experimental condition. Veh indicates the vehicle of HC or Indo. Veh1 indicates the vehicle of BTZ. §P < 0.05 versus Veh1/Veh; *P < 0.05 versus BTZ/Veh.