Abstract
Background and Purpose
Benzofurans are newly used psychoactive substances, but their pharmacology is unknown. The aim of the present study was to pharmacologically characterize benzofurans in vitro.
Experimental Approach
We assessed the effects of the benzofurans 5-APB, 5-APDB, 6-APB, 6-APDB, 4-APB, 7-APB, 5-EAPB and 5-MAPDB and benzodifuran 2C-B-FLY on the human noradrenaline (NA), dopamine and 5-HT uptake transporters using HEK 293 cells that express the respective transporters. We also investigated the release of NA, dopamine and 5-HT from monoamine-preloaded cells, monoamine receptor-binding affinity and 5-HT2A and 5-HT2B receptor activation.
Key Results
All of the benzofurans inhibited NA and 5-HT uptake more than dopamine uptake, similar to methylenedioxymethamphetamine (MDMA) and unlike methamphetamine. All of the benzofurans also released monoamines and interacted with trace amine-associated receptor 1 (TA1 receptor), similar to classic amphetamines. Most benzofurans were partial 5-HT2A receptor agonists similar to MDMA, but also 5-HT2B receptor agonists, unlike MDMA and methamphetamine. The benzodifuran 2C-B-FLY very potently interacted with 5-HT2 receptors and also bound to TA1 receptors.
Conclusions and Implications
Despite very similar structures, differences were found in the pharmacological profiles of different benzofurans and compared with their amphetamine analogues. Benzofurans acted as indirect monoamine agonists that interact with transporters similarly to MDMA. The benzofurans also interacted with 5-HT receptors. This pharmacological profile probably results in MDMA-like entactogenic psychoactive properties. However, benzofurans induce 5-HT2B receptor activation associated with heart valve fibrosis. The pharmacology of 2C-B-FLY indicates predominant hallucinogenic properties and a risk for vasoconstriction.
Tables of Links
| LIGANDS | ||
|---|---|---|
| 5-HT | Noradrenaline | Pyrilamine |
| Butaclamol | Mazindole | Rauwolscine |
| Citalopram | MDMA | Risperidone |
| Clozapine | Mesulergine | RO5166017 |
| Dopamine | Methamphetamine | SCH23390 |
| Ketanserin | Mianserin | Spiperone |
| LSD | Phentolamine | WIN35428 |
| Nisoxetine | Prazosin |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,bAlexander et al., 2013a,b).
Introduction
Novel psychoactive substances are newly used designer drugs (‘Internet drugs’, ‘research chemicals’, ‘legal highs’) that potentially pose similar health risks to classic illicit substances. In recent years, the number of newly detected psychoactive substances on the illicit drug market has dramatically increased. In the European Union, 41 novel psychoactive substances were identified for the first time in 2010, 49 were identified in 2011, 73 were identified in 2012 and 81 were identified in 2013 within the European Early Warning System (EMCDDA, 2014).
Benzofurans are a group of novel psychoactive substances (King, 2014) of particular interest because they are structurally very similar to the popular recreational drug 3,4-methylenedioxymethamphetamine (MDMA) and its active metabolite 3,4-methylenedioxyamphetamine (MDA; Greene, 2013). 5-(2-Aminopropyl)benzofuran (5-APB) and 6-(2-aminopropyl)benzofuran) (6-APB) are benzofuran analogues of MDA (Figure 1). 5-(2-Aminopropyl)-2,3-dihydrobenzofuran (5-APDB) and 6-(2-aminopropyl)-2,3-dihydrobenzofuran (6-APDB) are dihydrobenzofuran analogues (Figure 1) that were originally synthesized for research purposes (Monte et al., 1993). 4-(2-Aminopropyl)benzofuran (4-APB) and 7-(2-aminopropyl)benzofuran (7-APB) are positional isomers of 5-APB and 6-APB. 1-(2,3-Dihydrobenzofuran-5-yl)-N-methylpropan-2-amine (5-MAPDB) is a dihydrobenzofuran analogue of MDMA, and 5-(2-ethylaminopropyl)benzofuran (5-EAPB) is a benzofuran analogue of MDMA but with an N-ethyl group (Figure 1).
Figure 1.
Chemical structures of benzofurans and related amphetamines.
5-APB and 6-APB appeared on the drug market in 2010–2011 (Chan et al., 2013; Jebadurai et al., 2013; Stanczuk et al., 2013; Archer et al., 2014; Elliott and Evans, 2014; King, 2014), with reports of intoxication (Chan et al., 2013; Greene, 2013; Jebadurai et al., 2013; Seetohul and Pounder, 2013). 4-APB was first reported to the EMCDDA in 2010 (King, 2014) and is typically detected in products that are sold as 6-APB as a by-product (Stanczuk et al., 2013; Strano Rossi et al., 2014). Users report that the effects of 5-APB and 6-APB are comparable with MDMA but more intense (Greene, 2013; Jebadurai et al., 2013). Adverse effects include nausea, sympathomimetic stimulation and agitation (Chan et al., 2013; Greene, 2013). 5-APDB and 6-APDB were first reported to the EMCDDA in 2012, and another three benzofurans, including 5-EAPB, were first reported in 2013 (King, 2014). Presently, no published studies have reported the psychotropic and toxic effects of these benzofurans, but 5-APDB, 6-APDB and 5-EAPB are being discussed in drug user forums (Bluelight, 2013a,b; Drugs-Forum, 2013). Little is known about the pharmacology of benzofurans. 5-APB and 6-APB have been shown to inhibit the human dopamine, noradrenaline and 5-HT transporters (DAT, NET and SERT, respectively; Iversen et al., 2013) and are agonists at the rat 5-HT2A receptor (Dawson et al., 2014) and human and rat 5-HT2B receptor (Iversen et al., 2013; Dawson et al., 2014). Additionally, fast cyclic voltammetry experiments in rat brain slices indicated that 5-APB releases dopamine at high concentrations (Dawson et al., 2014). 5-APDB and 6-APDB also inhibited the monoamine transporters with greater affinity for the SERT over the DAT compared with MDA in crude rat synaptosome preparations (Monte et al., 1993).
The benzodifurans 8-bromo-2,3,6,7-benzo-dihydro-difuran-ethylamine (2C-B-FLY) and 1-(8-bromobenzo[1,2-b;4,5-b′]difuran-4-yl)-2-aminopropane) (bromo-dragonFLY) are known as ‘fly’ drugs because of their chemical structures (Figure 1). A series of benzodifurans were originally synthesized to study 5-HT2A receptor function (Monte et al., 1997; Parker et al., 1998; Chambers et al., 2001). The recreational use of 2C-B-FLY and bromo-dragonFLY began to be reported in 2007 (Andreasen et al., 2009; Greene, 2013; King, 2014), and there are case reports of severe agitation, hallucinations, seizures and fatalities associated with bromo-dragonFLY (Andreasen et al., 2009; Wood et al., 2009; Nielsen et al., 2010). 2C-B-FLY and bromo-dragonFLY are potent 5-HT2A receptor agonists (Monte et al., 1996; Chambers et al., 2001), but interactions with other monoamine receptors and their transporters have not been tested.
Systematic evaluations of the pharmacological profiles of benzofurans are lacking. We determined the potencies of a series of benzofurans and the benzodifuran 2C-B-FLY to inhibit the DAT, NET and SERT and tested transporter-mediated monoamine release in vitro. We also characterized the binding profiles at monoamine receptors and assessed 5-HT2A and 5-HT2B receptor activation. The 5-HT2A receptor mediates hallucinogenic effects (Nichols, 2004), and the 5-HT2B receptor has been implicated in drug-associated endocardial fibrosis (Roth, 2007). MDMA, MDA, β-keto-MDA and methamphetamine were included as comparator substances.
Methods
Monoamine uptake transport inhibition
Inhibition of the human NET, DAT and SERT was assessed in HEK 293 cells that were stably transfected with the transporters as specified previously (Hysek et al., 2012c). Briefly, the cells were suspended in uptake buffer. We incubated the cells for 10 min with different concentrations of the test compounds and then added the corresponding [3H] monoamine (5 nM final concentration) at room temperature. After 10 min, we stopped uptake by separating the cells from the buffer using centrifugation through silicone oil (Hysek et al., 2012c). The centrifugation tubes were frozen in liquid nitrogen and cut to separate the cell pellet from the silicone oil and assay buffer layers. The cell pellet was then lysed. Scintillation fluid was added, and radioactivity was counted on a β-counter. Non-specific uptake was determined for each experiment in the presence of 10 μM fluoxetine for SERT cells, 10 μM nisoxetine for NET cells and 10 μM mazindol for DAT cells and subtracted from the total counts to yield specific uptake (100%). The data were fitted by non-linear regression to variable slope sigmoidal dose–response curves, and IC50 values were calculated using Prism software (GraphPad, San Diego, CA, USA). DAT : SERT inhibition ratios were calculated as 1/DAT IC50:1/SERT IC50. Higher relative potency at the DAT indicates a higher abuse potential, whereas relatively increased activity of the 5-HT system is linked to a reduction in abuse potential and more MDMA-like psychotropic effects (Wee et al., 2005). Stimulant amphetamines, such as methamphetamine, have a DAT : SERT inhibition ratio <10, whereas MDMA and other substances with MDMA-like psychotropic effects have a DAT : SERT inhibition ratio close to 0.1 (Baumann et al., 2012; Simmler et al., 2013; 2014a,b).
Transporter-mediated monoamine release
We studied the effects of a single high dose (100 μM) of the test compounds on transporter-mediated NA, 5-HT and dopamine efflux in HEK 293 cells that overexpressed the respective human monoamine transporter, as previously reported in detail (Simmler et al., 2013). Briefly, adherent cells were incubated with the respective radiolabeled monoamine (10 nM [3H]-NA and 10 μM unlabelled NA, 10 nM [3H]-dopamine and 1 μM unlabelled dopamine, and 10 nM [3H]-5-HT) for 20 min at 37°C. We then washed the cells twice with buffer and added 1 mL of buffer that contained the test compound (100 μM final concentration). We stopped [3H]-5-HT and [3H]-dopamine release after 15 min and [3H]-NA release after 45 min by washing twice with ice-cold buffer. We quantified the radioactivity that remained in the cells. Non-specific ‘pseudo-efflux’, which arises from non-specific substrate release and subsequent reuptake inhibition (Scholze et al., 2000), was assessed for each experiment using the transporter inhibitors nisoxetine (NET cells), citalopram (SERT cells) and mazindol (DAT cells) at 10 μM as negative control conditions. anova followed by the Holm–Sidak test was used to compare compound-induced release with the negative controls. Substances that induced significantly higher monoamine efflux compared with the negative control were considered monoamine releasers.
Radioligand binding assays
The radioligand binding assays were performed as described previously (Hysek et al., 2012c; Simmler et al., 2013). Briefly, membrane preparations of HEK 293 cells (Invitrogen, Zug, Switzerland) that overexpress the respective transporters (Tatsumi et al., 1997) or receptors (human genes, except rat and mouse genes for TA1 receptor; Revel et al., 2011) were incubated with the radiolabeled selective ligands at concentrations equal to Kd, and ligand displacement by the compounds was measured. Specific binding of the radioligand to the target receptor was defined as the difference between the total binding and non-specific binding determined in the presence of selected competitors, in excess. The following radioligands and competitors, respectively, were used: N-methyl-[3H]-nisoxetine and indatraline (NET), [3H]-citalopram and indatraline (SERT), [3H]WIN35,428 and indatraline (DAT), [3H]-8-hydroxy-2-(di-n-propylamino)tetralin and indatraline (5-HT1A receptor), [3H]-ketanserin and spiperone (5-HT2A receptor), [3H]-mesulergine and mianserin (5-HT2C receptor), [3H]-prazosin and risperidone (α1 adrenoceptor), [3H]-rauwolscine and phentolamine (α2 adrenoceptor), [3H]-SCH 23390 and butaclamol (D1 receptor), [3H]-spiperone and spiperone (D2 and D3 receptors), [3H]-pyrilamine and clozapine (histamine H1 receptor), and [3H]-RO5166017 and RO5166017 (TA1 receptor). IC50 values were determined by calculating non-linear regression curves for a one-site model using three to five independent 10-point concentration–response curves for each compound. Ki (affinity) values, which correspond to the dissociation constants, were determined using the Cheng–Prusoff equation.
Functional 5-HT2A and 5-HT2B receptor activity
The 5-HT2B receptor functional assay was performed as described previously (Jensen et al., 2008). Briefly, human 5-HT2B receptor-expressing HEK 293 cells were incubated at 37°C in 96-well plates coated with poly-D-lysine. The growth medium was removed by snap inversion, and 100 μL of Fluo-4 solution (calcium indicator; Molecular Probes, Eugene, OR, USA) was added to each well. The plates were incubated for 45 min at 31°C. The Fluo-4 solution was removed by snap inversion, and 100 μL of Fluo-4 solution was added a second time. The cells were then incubated for another 45 min at 31°C. Immediately before testing, the cells were washed with HBSS (Gibco) and 20 mM HEPES (assay buffer; Gibco, Life Technologies, Zug, Switzerland) using an EMBLA cell washer, and 100 μL assay buffer was added. The plate was placed in a fluorescence imaging plate reader (FLIPR), and 25 μL of the test substances diluted in assay buffer was added online. The increase in fluorescence was then measured. EC50 values were derived from the concentration–response curves using non-linear regression. Efficacy (maximal activity) is expressed relative to the activity of 5-HT, which was used as a control set to 100%.
Cytotoxicity
To confirm cell integrity during the pharmacological assays, cytotoxicity was assessed using the ToxiLightTM bioassay (Lonza, Basel, Switzerland) according to the manufacturer's instructions. The assay quantitatively measures the release of adenylate kinase from damaged cells providing a highly sensitive method for measuring cytolysis (Crouch et al., 1993; Hysek et al., 2012c; Felser et al., 2014). Cells grown in 96-well plates were exposed to the compounds at a high concentration of 100 μM. All test conditions contained DMSO 0.1% (v:v) which is non-toxic and was also used as negative control. TritonTM X-100 (0.1%, Sigma-Aldrich, Buchs, Switzerland) lyses cells and was used as positive control. After 4 h of incubation at 37°C, 10 μL of supernatant per well was removed and combined with 50 μL of ToxiLightTM reagent and luminescence recorded using a Tecan InfiniteTM 200 Pro (Tecan, Männedorf, Switzerland) plate reader.
Statistical analyses
The uptake transporter inhibition data were fit by non-linear regression to variable-slope sigmoidal dose-response curves, and IC50 values were calculated using Prism software (GraphPad, San Diego, CA, USA). ANOVA followed by the Holm-Sidak test was used to compare compound-induced release with the negative controls. Substances that induced significantly higher monoamine efflux compared with the negative control were considered monoamine releasers. IC50 values for radioligand binding were determined by calculating nonlinear regression curves for a one-site model using three to five independent 10-point concentration-response curves for each compound. Ki (affinity) values, which correspond to the dissociation constants, were determined using the Cheng-Prusoff equation. EC50 values for 5-HT2 receptor activation were derived from the concentration-response curves using nonlinear regression.
Drugs
MDMA, MDA, β-keto-MDA, methamphetamine and 2C-B-FLY were obtained from Lipomed (Arlesheim, Switzerland). 6-APB, 6-APDB, 5-APB, 5-APDB, 4-APB, 7-APB and 5-MAPDB were obtained from Cayman Chemicals (Ann Arbor, MI, USA). 5-EAPB was obtained from the Forensic Institute (Zurich, Switzerland). All of the drugs were used as racemic hydrochloride salts, with the exception of d-methamphetamine. Purity was at least 98% for all of the substances, with the exception of 2C-B-FLY, whose purity was approximately 95% as determined by HPLC.
Results
Monoamine uptake transporter inhibition
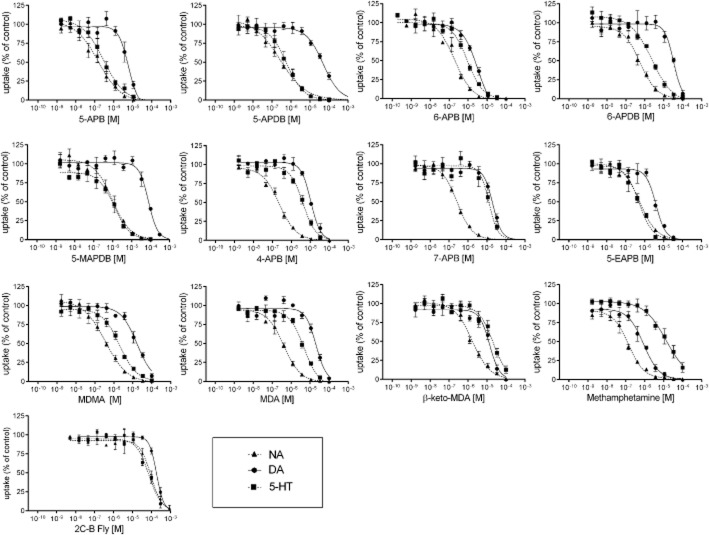
Uptake inhibition curves are depicted in Figure 2, and the corresponding IC50 values and DAT : SERT inhibition ratios are listed in Table 1. All of the benzofurans inhibited the NET at submicromolar concentrations, similar to MDMA, MDA and methamphetamine. All of the benzofurans were weak DAT inhibitors compared with methamphetamine and more similar to MDMA, which was also a weak DAT inhibitor. Only 5-APB, 6-APB and 5-EAPB were more potent at the DAT compared with MDMA and MDA. In contrast, the dihydrobenzofurans 5-APDB, 6-APDB and 5-MAPDB were inactive at the DAT (IC50 <30 μM). 5-APB, 5-APDB, 6-APB and 5-EAPB inhibited the SERT at submicromolar concentrations and more potently than MDMA. 6-APDB and 5-MAPDB inhibited the SERT in the 1–3 micromolar concentration range, similar to MDMA. 4-APB and 7-APB exhibited low potency at the SERT, more similar to methamphetamine. The DAT : SERT inhibition ratio for all of the benzofurans was low, consistent with greater 5-hydroxytryptaminergic versus dopaminergic activity that is overall similar to MDMA. The dihydrobenzofurans (5-APDB, 6-APDB and 5-MAPDB) and 5-APB exhibited the lowest DAT : SERT inhibition ratios (lower than MDMA). In contrast, 4-APB and 7-APB exhibited the highest DAT : SERT inhibition ratios, consistent with their low potency at the SERT and showing a profile that is between MDMA and methamphetamine with regard to 5-hydroxytryptaminergic versus dopaminergic activity. In terms of structure–activity relationships, the dihydro-compounds 5-APDB and 6-APDB had similar noradrenergic and 5-hydroxytryptaminergic activities compared with their analogues 5-APB and 6-APB but were markedly less potent at the DAT. The monoamine transporter inhibition potencies of the positional isomers 4-APB and 7-APB were reduced, particularly for the SERT, compared with their analogues 5-APB and 6-APB. Additionally, the oxygen in the para-position for 5-APB and 5-APDB resulted in higher absolute and relative potency at the SERT compared with 6-APB and 6-APDB respectively. β-Keto-substitution in the β-keto-MDA versus MDA structures increased dopaminergic versus 5-hydroxytryptaminergic activity. The benzodifuran 2C-B-FLY was inactive at all of the monoamine transporters (IC50 <50 μM).
Figure 2.
Monoamine uptake transporter inhibition. Concentration–response curves show the uptake inhibition of [3H]-NA, [3H]-dopamine and [3H]-5-HT in HEK 293 cells transfected with the respective monoamine transporter. The data are expressed as the mean ± SEM of three to four independents experiments. Curves were fitted to the data with non-linear regression. The corresponding IC50 values are shown in Table 1.
Table 1.
Monoamine transporter inhibition
| NET | DAT | SERT | DAT/SERT inhibition ratio | |
|---|---|---|---|---|
| IC50 (μM) (95% CI) | IC50 (μM) (95% CI) | IC50 (μM) (95% CI) | Ratio (95% CI) | |
| Benzofurans | ||||
| 5-APB | 0.16 (0.08–0.3) | 6.1 (4–9) | 0.29 (0.17–0.5) | 0.05 (0.02–1.2) |
| 5-APDB | 0.29 (0.2–0.5) | 49 (33–73) | 0.58 (0.4–0.9) | 0.01 (0.005–0.03) |
| 6-APB | 0.19 (0.1–0.3) | 3.3 (2.4–4.5) | 0.93 (0.7–1.3) | 0.29 (0.16–0.54) |
| 6-APDB | 0.56 (0.4–0.8) | 33 (25–43) | 2.3 (1.4–3.9) | 0.07 (0.03–0.16) |
| 5-MAPDB | 0.96 (0.5–1.7) | 77 (62–96) | 1.2 (0.7–2) | 0.02 (0.01–0.03) |
| 4-APB | 0.24 (0.2–0.3) | 12 (9–16) | 5.5 (3.4–8.7) | 0.46 (0.21–1.0) |
| 7-APB | 0.27 (0.2–0.3) | 20 (16–26) | 13 (9–18) | 0.65 (0.35–1.1) |
| 5-EAPB | 0.56 (0.4–0.7) | 4.9 (3–8) | 0.72 (0.5–1.1) | 0.15 (0.07–0.35) |
| Benzodifuran | ||||
| 2C-B-FLY | 94 (72–124) | 187 (161–217) | 73 (58–92) | 0.39 (0.27–0.57) |
| Related amphetamines | ||||
| MDMA | 0.36 (0.2–0.6) | 16.7 (16.3–17) | 2.4 (1.4–3.0) | 0.14 (0.08–0.18) |
| MDA | 0.42 (0.3–0.6) | 20.5 (20.3–20.6) | 4.9 (3.5–6.8) | 0.24 (0.17–0.33) |
| β-Keto-MDA | 1.6 (1.1–2.3) | 14 (10–18) | 21 (15–28) | 1.5 (0.8–2.8) |
| Methamphetamine | 0.14 (0.09–0.2) | 0.87 (0.84–0.91) | 13.6 (13.5–13.8) | 15.6 (14.8–16.4) |
Values are means of three to four independent experiments and 95% confidence intervals (CI). DAT/SERT inhibition ratio = 1/DAT IC50: 1/SERT IC50.
Monoamine release
At high concentrations, all of the benzofurans released at least one of the monoamines through the respective monoamine transporter, similar to the amphetamines (Figure 3). In contrast, 2C-B-FLY was not a monoamine releaser.
Figure 3.
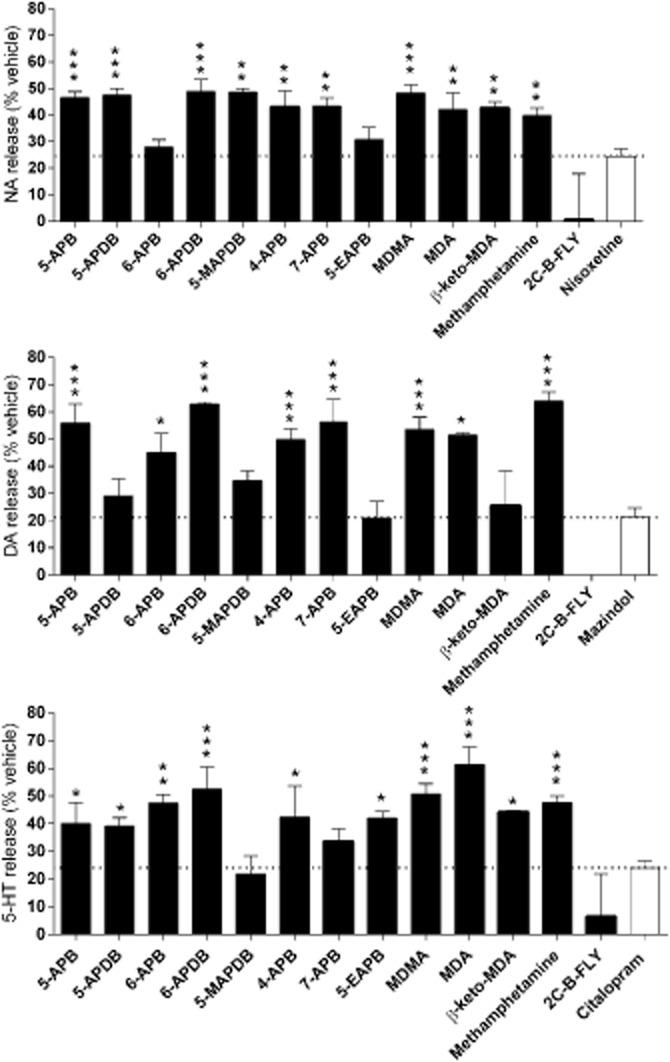
Monoamine release. Monoamine release was induced by a high concentration of the compound (100 μM) after preloading the transporter-transfected cells with the respective radiolabelled monoamine. All of the benzofurans released NA, dopamine and 5-HT similarly to methamphetamine and MDMA. In contrast, the benzodifuran 2CB-FLY was not a monoamine releaser. Transporter blockers induced non-specific ‘pseudo-efflux’ (horizontal dashed line, open bars), which arises from substrate that diffuses out of the cells and from subsequent reuptake inhibition. Compounds that produced significantly more monoamine efflux (*P < 0.05, **P < 0.01, ***P < 0.001) compared with the respective non-releasing uptake inhibitors (negative controls, open bars) were considered monoamine releasers. The data are expressed as the mean ± SEM of three to four independent experiments.
Binding affinities
The benzofurans interacted with the monoamine transporters but also with several monoamine receptors (Tables 2013a and 2013b). All of the benzofurans exhibited submicromolar affinity for the TA1 receptor, except for 5-EAPB, which was inactive at mouse TA1 receptos. Benzofurans showed mostly higher potency at TA1 receptors than the classic amphetamines. All of the benzomonofurans exhibited binding affinities for the 5-HT2A receptor in the micromolar range (0.8–3.4 μM). Functionally, most of them acted as low-potency partial agonists similar to MDMA and MDA but unlike methamphetamine. Most of the benzofurans were also partial agonists at the 5-HT2B receptor. In contrast MDMA and methamphetamine did not stimulate 5-HT2B receptors. With the exception of 7-APB and 5-EAPB, the benzofurans exhibited submicromolar binding affinities at the 5-HT2C receptor. Binding potencies at the 5-HT1 receptor varied among different benzofurans. Only 7-APB showed submicromolar binding affinity. Potent binding to most of the assessed 5-HT receptor subtypes distinctly discriminated the benzofurans from the pharmacological profiles of their related amphetamines, which exhibited no or low 5-HT1A affinity and did not bind to 5-HT2B or 5-HT2C receptors except for MDA with a Ki value of 3 μM at 5-HT2C. Most of the benzofurans bound to α1A- and α2A-adrenoceptors in the 3–12 and 0.1–6 μM ranges respectively. There was no binding to dopamine receptors and only low-affinity binding to histamine H1 receptors (<10 μM for most of the drugs). The benzodifuran 2C-B-FLY did not bind to the monoamine transporters but interacted with all of the receptors tested in the present study and particularly exhibited high affinity for TA1 receptors and all of the 5-HT2 receptors. Importantly, 2C-B-FLY was a very potent agonist at the 5-HT2A receptor. 2C-B-FLY thus exhibited a pharmacological profile that was distinct from the mono-benzofurans and related amphetamines.
Table 2.
Monoamine transporter and receptor-binding affinities
| NET | DAT | SERT | α1A | α2A | D1 | D2 | D3 | H1 | TA1rat | TA1mouse | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Benzofurans | |||||||||||
| 5-APB | 3.1 ± 0.2 | 2.6 ± 0.3 | 3.2 ± 0.4 | 3.5 ± 0.5 | 2.9 ± 0.1 | <14 | <10 | <17 | 8.4 ± 0.8 | 0.04 ± 0.01 | 0.11 ± 0.01 |
| 5-APDB | 28 ± 5 | <30 | 4.0 ± 0.3 | 11 ± 3 | 4.2 ± 0.5 | <14 | <10 | <17 | 21 ± 2 | 0.49 ± 0.05 | 0.77 ± 0.06 |
| 6-APB | 1.8 ± 0.4 | 0.60 ± 0.05 | 12 ± 1 | 7.3 ± 3.4 | 0.38 ± 0.02 | <14 | <10 | <17 | 15 ± 2 | 0.05 ± 0.02 | 0.06 ± 0.02 |
| 6-APDB | 18 ± 1 | <30 | 23 ± 1 | <15 | 0.65 ± 0.07 | <14 | <10 | <17 | <25 | 1.0 ± 0.04 | 0.21 ± 0.04 |
| 5-MAPDB | 26 ± 5 | <30 | 6.3 ± 0.6 | 4.9 ± 1.5 | 6.4 ± 1.8 | 23 ± 3 | <10 | <17 | 4.9 ± 0.1 | 0.67 ± 0.09 | 3.5 ± 0.1 |
| 4-APB | 3.9 ± 0.5 | 7.4 ± 0.6 | 7.7 ± 0.5 | 12 ± 3 | 0.87 ± 0.22 | <14 | <10 | <17 | 16 ± 1 | 0.11 ± 0.02 | 2.08 ± 0.14 |
| 7-APB | 5.3 ± 0.1 | 14 ± 2 | 14 ± 1 | 9.6 ± 2.4 | 0.14 ± 0.02 | <14 | 8.2 ± 3.2 | <17 | 25 ± 5 | 0.07 ± 0.01 | 0.13 ± 0.02 |
| 5-EAPB | 1.0 ± 0.3 | 0.34 ± 0.02 | 0.52 ± 0.03 | 3.3 ± 0.5 | 2.7 ± 0.7 | 16 ± 3 | <10 | <17 | 2.4 ± 0.4 | 0.81 ± 0.08 | <15 |
| Benzodifuran | |||||||||||
| 2C-B-FLY | 17 ± 4 | <26 | 10 ± 3 | 11 ± 1 | 0.78 ± 0.3 | 1.4 ± 0.2 | 1.9 ± 0.3 | 6.8 ± 1.2 | 3.4 ± 0.5 | 0.03 ± 0.01 | 0.71 ± 0.23 |
| Related amphetamines | |||||||||||
| MDMAa | 27 ± 9 | 8.4 ± 3.3 | 13 ± 2 | <5 | 15 ± 10 | <12 | <20 | <17 | <13 | 0.37 ± 0.12 | 2.4 ± 1.1 |
| MDA | 13 ± 3.7 | <26 | 5.6 ± 1.5 | <5 | 1.1 ± 0.1 | <12 | <20 | <17 | <13 | 0.25 ± 0.04 | 0.16 ± 0.01 |
| β-Keto-MDA | <30 | 11 ± 2 | <30 | <5 | 15 ± 2 | <12 | <20 | <17 | <13 | 4.8 ± 0.9 | 6.5 ± 2.8 |
| Methamphetaminea | 3.0 ± 2.2 | 1.8 ± 0.7 | 25 ± 10 | <5 | 6.1 ± 1.6 | <12 | <20 | <17 | <13 | 0.35 ± 0.12 | 0.55 ± 0.24 |
Values are Ki given as μM (mean ± SD).
Values are from Simmler et al., 2014a.
Table 3.
5-HT receptor interactions
| 5-HT1A | 5-HT2A | 5-HT2B | 5-HT2C | ||||
|---|---|---|---|---|---|---|---|
| Receptor-binding Ki (μM) | Receptor-binding Ki (μM) | Activation potency EC50 (μM) | Activation efficacy % maximum | Activation potency EC50 (μM) | Activation efficacy % maximum | Receptor-binding Ki (μM) | |
| Benzofurans | |||||||
| 5-APB | 3.3 ± 0.2 | 0.84 ± 0.27 | 6.3 ± 2.1 | 54 ± 35 | 0.28 ± 0.12 | 61 ± 17 | 0.88 ± 0.33 |
| 5-APDB | 20 ± 4 | 3.4 ± 1.0 | 11 ± 2 | 24 ± 17 | 1.2 ± 0.6 | 50 ± 21 | 0.06 ± 0.02 |
| 6-APB | 1.5 ± 0.2 | 0.97 ± 0.23 | 5.9 ± 1.8 | 43 ± 23 | 0.14 ± 0.06 | 70 ± 9 | 0.27 ± 0.05 |
| 6-APDB | 9.2 ± 1.5 | 2.0 ± 1.0 | 5.9 ± 1.1 | 62 ± 36 | 0.12 ± 0.03 | 66 ± 17 | 0.06 ± 0.02 |
| 5-MAPDB | 26 ± 6 | 4.8 ± 2.1 | <20 | 0 | <20 | 0 | 0.10 ± 0.02 |
| 4-APB | 1.2 ± 0.1 | 0.96 ± 0.17 | 13 ± 2 | 30 ± 9 | 1.0 ± 0.5 | 38 ± 16 | 0.06 ± 0.02 |
| 7-APB | 0.28 ± 0.05 | 0.91 ± 0.20 | 5.7 ± 2.0 | 43 ± 21 | 0.28 ± 0.52 | 52 ± 17 | 3.3 ± 0.3 |
| 5-EAPB | 3.1 ± 0.6 | 2.7 ± 1.5 | 7.6 ± 3.2 | 29 ± 7 | <20 | 0 | 4.6 ± 1.3 |
| Benzodifuran | |||||||
| 2C-B-FLY | 0.35 ± 0.04 | 0.011 ± 0.002 | 0.0015 ± 0.0002 | 82 ± 12 | 0.040 ± 0.036 | 56 ± 3 | 0.012 ± 0.004 |
| Related amphetamines | |||||||
| MDMA | 12 ± 0.8a | 6.3 ± 2.4 | 6.1 ± 0.3 | 55 ± 9 | <20 | 0 | <13a |
| MDA | 4.9 ± 0.9 | 3.3 ± 0.8 | 0.63 ± 0.24 | 77 ± 16 | 0.85 ± 0.11 | 52 ± 12 | 3.0 ± 0.9 |
| β-Keto-MDA | <17 | <13 | <20 | 0 | <20 | 0 | <13 |
| Methamphetamine | 8.1 ± 0.7a | <13 | <20 | 0 | <20 | 0 | <13a |
Values are Ki given as μM (mean ± SD).
Values are from Simmler et al., 2014a and were included for comparison.
Cytotoxicity
None of the compounds investigated produced cytotoxicity, thus confirming cell integrity during the functional assays in this study.
Discussion
We determined the in vitro pharmacological profiles of new benzofurans that are recreationally abused compared with their well-known amphetamine analogues. The benzofurans blocked monoamine transporters and induced transporter-mediated monoamine release similarly to MDMA. More than MDMA and methamphetamine, the benzofurans also directly stimulated adrenoceptors and 5-HT receptors. The benzodifuran 2C-B-FLY was a potent agonist at 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors, consistent with the reported hallucinogenic properties of 2C-B-FLY.
Monoamine uptake transporter inhibition and monoamine release
All of the benzofurans inhibited the NET at submicromolar concentrations, similar to MDMA, MDA and methamphetamine. NA mediates sympathomimetic stimulation (Hysek et al., 2011), and this finding predicts the cardiostimulant and psychostimulant properties of these benzofurans, similar to MDMA and methamphetamine. Unlike the relatively constant NET inhibition, the potencies of the benzofurans to inhibit the DAT and SERT notably varied, resulting in DAT : SERT inhibition ratios that ranged from 0.01 to 0.65. Specifically, the dihydrobenzofurans 5-APDB and 5-MAPDB exhibited the highest preference for the SERT versus DAT (more selective than MDMA), followed by 5-APB, 6-APDB and 5-EAPB, which exhibited a DAT : SERT inhibition ratio similar to MDMA. With DAT : SERT ratios of 0.46 and 0.65, 4-APB and 7-APB were the benzofurans with the most dopaminergic profiles and were relatively more dopaminergic than MDMA. Stimulants like methamphetamine exhibit a DAT : SERT ratio <10, whereas MDMA and other entactogens exhibit a DAT : SERT ratio of 0.01–1 (Simmler et al., 2013; 2014a,b; Liechti, 2014b). Accordingly, based on their DAT : SERT inhibition ratios, all of the benzofurans can be expected to produce MDMA-like entactogenic subjective effects in humans. In contrast to the benzofurans, the benzodifuran 2C-B-FLY blocked monoamine transporters only at very high concentrations but had high affinity for 5-HT receptors. Thus, monoamine transporter inhibition is unlikely to contribute to the mechanism of action of 2C-B-FLY as is also the case for the structurally similar substance 2C-B and related compounds of the 2C phenethylamine series containing methoxy groups at positions 2 and 5 of the benzene ring (Acuna-Castillo et al., 2002; Hill and Thomas, 2011; Eshleman et al., 2014).
Only a few other studies have determined the monoamine transporter inhibition profiles of some of the benzofurans. Consistent with our findings, 5-APDB and 6-APDB inhibited the SERT more potently than the DAT in rat synaptosomes (Monte et al., 1993). The oxygen in the para-position in the 5-APDB and 5-APB structures enhanced the 5-hydroxytryptaminergic versus dopaminergic properties compared with 6-APDB and 6-APB, respectively, as shown in the present study and previously for 5-APDB versus 6-APDB in rat synaptosomes (Monte et al., 1993). Drug discrimination studies in rats showed that 5-APDB and 6-APDB substituted for MDMA-like 5-hydroxytryptaminergic drugs but not the more dopaminergic stimulant amphetamine (Monte et al., 1993). These behavioural findings support our hypothesis that 5-APDB and 6-APDB produce subjective effects that are similar to MDMA, and entactogenic effects have been reported by users (Bluelight, 2013a,b; Drugs-Forum, 2013). The monoamine transporter inhibition profiles for 5-APB and 6-APB were determined in one previous study (Iversen et al., 2013). In contrast to our results, this study showed that 5-APB and 6-APB inhibited the DAT more potently than the SERT (Iversen et al., 2013). However, MDMA did not show the 5-hydroxytryptaminergic preference that is typically reported by others (Rothman et al., 2001; Han and Gu, 2006; Hysek et al., 2012c; Simmler et al., 2013). Consistent with the present results, the inhibition profiles for 5-APB and 6-APB were similar to MDMA and unlike methamphetamine (Iversen et al., 2013). The reinforcing effects of benzofurans have not yet been studied in drug self-administration studies. There is a decrease in reinforcing potency and efficacy among monoamine-releasing agents when 5-HT releasing potency is increased relative to dopamine (Wee et al., 2005). The relatively high 5-hydroxytryptaminergic properties of the benzofurans in vitro would indicate lower addictive properties (Wee et al., 2005; Liechti, 2014b), more similar to MDMA, which is not a strong reinforcer in self-administration studies (Lamb and Griffiths, 1987; Cole and Sumnall, 2003) than to methamphetamine.
All of the benzofurans also released 5-HT, NA and/or dopamine through their respective transporters, similar to their amphetamine analogues and other amphetamine derivatives (Simmler et al., 2013; 2014a,b). Dopamine release has also been previously documented for 5-APB in voltammetric studies of rat brain slices (Dawson et al., 2014). In contrast to the benzofurans, 2C-B-FLY did not release monoamines. Our release assay was designed to qualitatively assess monoamine release because we used only one high concentration of the substances to induce transporter-mediated monoamine efflux. Additional studies that include the assessment of transporter-mediated ionic currents and in vivo microdialysis could be useful to further characterize and quantify monoamine release and its contribution to the mechanism of action of the benzofurans.
Receptor-binding profiles
The present study found several important high-potency interactions between the benzofurans and various monoamine receptors. 6-APB, 6-APDB, 4-APB, 7-APB and 2C-B-FLY all bound to α2A-adrenoceptors, which are known to modulate NA release and sympathomimetic activity (Hysek et al., 2012a). As expected (Monte et al., 1996), 2C-B-FLY potently interacted with 5-HT2 receptors. Specifically, 2C-B-FLY potently bound to the human 5-HT2A receptor (Ki = 0.01 μM), consistent with the previously documented nanomolar affinity for rat cortical 5-HT2A receptors (Monte et al., 1996). Even higher potency binding to 5-HT2A receptors has been shown for the benzodifuran bromo-dragonFLY in rat (Monte et al., 1996; Chambers et al., 2001) and human (Monte et al., 1996) 5-HT2A receptors. In the present study, 2C-B-FLY was also a very potent functional 5-HT2A receptor agonist. 2C-B-FLY resembles the structures of the 2C series phenethylamines, which are also potent 5-HT2A receptor agonists (Nelson et al., 1999; Acuna-Castillo et al., 2002; Hansen et al., 2014).
Consistent with the predicted lysergic acid diethylamide (LSD)-like properties of substances with high 5-HT2A receptor affinity, both 2C-B-FLY and bromo-dragonFLY completely substituted for LSD in drug discrimination studies (Monte et al., 1996). The affinity of 2C-B-FLY for the 5-HT1A receptor was relatively low, which has also been shown for rat 5-HT1A receptors (Monte et al., 1996). The 5-HT2A receptor is thought to mediate the alterations in perception induced by hallucinogens (Vollenweider et al., 1998; Nelson et al., 1999; Nichols, 2004) and therefore is likely to be the key target in the mechanism of action of benzodifuran hallucinogens. Interestingly, some of the benzofurans also exhibited micromolar affinity for the 5-HT2A receptor and were low-potency 5-HT2A receptor partial agonists similar to MDMA and MDA, but in contrast to methamphetamine. Binding to 5-HT2A receptors at micromolar concentrations has also been previously shown for 5-APB and 6-APB (Iversen et al., 2013). 5-APB also constricts the rat aorta via an agonist action on 5-HT2A receptors (Dawson et al., 2014). Thus, some benzofurans could have hallucinogenic properties because of 5-HT2A receptor stimulation, in addition to their MDMA-like entactogenic subjective effects. Psychosis and hallucinations have been reported after the use of 6-APB (Chan et al., 2013; Greene, 2013). However, in drug discrimination studies, 5-APDB and 6-APDB did not substitute for LSD in rats (Monte et al., 1993), consistent with their lower binding affinity compared with 5-APB and 6-APB. In terms of clinical toxicity, the 5-HT2A receptor agonist and possible α1-adrenoceptor agonist action could enhance the risk for vasoconstriction, hyperthermia and hypertension. Both α1 and 5-HT2A receptors are implicated in substance-induced vasoconstriction (Blessing et al., 2003; Docherty and Green, 2010; Dawson et al., 2014) and associated hypertension (Hysek et al., 2013) and hyperthermia (Liechti et al., 2000; Hysek et al., 2012b; Liechti, 2014a) in humans. In fact, hypertension, hyperpyrexia and cases of severe limb ischaemia have been reported after the use of bromo-dragonFLY (Thorlacius et al., 2008; Wood et al., 2009; Nielsen et al., 2010), a benzodifuran structurally similar to 2C-B-FLY. Direct agonist actions at the 5-HT2A receptor compared with an indirect action via 5-HT release can also be expected to result in longer lasting effects as described for 5-hydroxytryptaminergic hallucinogens (Schmid et al., 2014) and compared with MDMA (Hysek et al., 2012c).
In humans, MDMA is mainly inactivated by O-demethylation but also N-demethylated to the minor but active metabolite MDA (Hysek et al., 2013). Similarly, 5-MAPDB and 5-EAPB are N-dealkylated (Welter et al., 2015) to 5-APDB and 5-APB respectively. As shown in the present study, the N-dealkylated substances MDA, 5-APDB and 5-APB activate 5-HT2A and 5-HT2B receptors more potently and also more potently bind to 5-HT2C receptors than their parent compounds MDMA, 5-MAPDB and 5-EAPB respectively. Thus, the formation of active metabolites probably adds enhanced 5-HT2A and 5-HT2B receptor-associated toxicity in these cases.
2C-B-FLY and several of the benzofurans acted as partial agonists at the 5-HT2B receptor as previously shown for 5-APB (Iversen et al., 2013; Dawson et al., 2014) and 6-APB (Iversen et al., 2013). In contrast, no such 5-HT2B receptor agonist properties were observed for the classic amphetamines MDMA and methamphetamine in the present study. 5-HT2B receptors have been implicated in substance-induced heart valve fibrosis (Setola et al., 2003; Bhattacharyya et al., 2009). 5-HT2B receptor activation by 2C-B-FLY, 5-APB, 6-APB, 6-APDB and 7-APB occurred at submicromolar concentrations that are likely to be present when these drugs are used by drug users to induce subjective effects.
All of the benzofurans bound to TA1 receptors, many at even higher potency than MDMA or methamphetamine. MDMA and methamphetamine inhibit their own neurochemical and locomotor stimulant effects via TA1 receptor activation (Di Cara et al., 2011). Similar TA1 receptor-mediated ‘auto-inhibition’ may, therefore, modulate the effects of benzofurans. In contrast, for cathinones (e.g. β-keto-amphetamines), more stimulant-like and addictive properties would be expected based on their lower affinity for TA1 receptors compared with their amphetamine analogues (Simmler et al., 2013; 2014a).
In terms of structure–activity relationships ( Table 4), 3,4-substitution on the benzene ring (methylenedioxy group in MDMA and MDA, furans or dihydrofurans) strongly reduced the DAT/SERT inhibition ratio confirming previous studies (Nichols, 1994; Han and Gu, 2006; Iversen et al., 2013; Simmler et al., 2013). Additionally, the DAT/SERT inhibition ratio depended on the position of the oxygen on the benzene ring and was lowest for compounds with the oxygen in the para-(4)-position and highest for those with the oxygen in the ortho-(2)-position. This finding was consistent with the high 5-hydroxytryptaminergic activity of other para-substituted amphetamines (Nichols, 1994; Rickli et al., 2015). The dihydrobenzofurans (5-APDB, 6-APDB and 5-MAPDB) exhibited reduced monoamine transporter inhibition potency in particular at the DAT resulting in relatively more 5-hydroxytryptaminergic properties compared with their furan analogues. N-alkylation (MDMA, 5-MAPDB, 5-EAPB, methamphetamine) moderately reduced activity at 5-HT2A/B receptors and binding at 5-HT2C receptors. This has previously been shown for other phenethylamines for simple N-alkylation (e.g. methyl, ethyl) (Nelson et al., 1999). N-alkylation had no relevant effect on the interactions with the monoamine transporter as previously noted for related amphetamines (Nichols, 1994). 2,5-Substitution on the benzene ring strongly increased activity at the 5-HT2 receptors and reduced interactions with the monoamine transporters as seen in 2C-B-FLY in the present study and many other 2,5-substituted phenethylamines (2C series) (Hill and Thomas, 2011; Eshleman et al., 2014). Transporter inhibition potency was also moderately reduced when the oxygen was in the ortho-(2)-position (similar to the 2 series) at the benzene ring as in 7-APB compared with 5-APB, 6-APB or 4-APB. β-Keto-substitution increased dopaminergic versus 5-hydroxytryptaminergic activity extending previous similar findings (Simmler et al., 2013; 2014a).
Table 4.
Structure–activity relationships
| Structure | Present in | Not present in | Pharmacolocial (clinical) activity |
|---|---|---|---|
| 3,4-Substitution on benzene ring (methylenedioxy or furan) | MDMA, 5-MAPDB, 5-EAPB | Methamphetamine | Reduced DAT/SERT inhibition ratio, aincreased potency to release 5-HT, areduced potency to release dopamine (more entactogenic, less stimulant) |
| MDA, 5-APB, 6-APB, 5-APDB, 6-APDB, 7-APB, β-keto-MDA | aAmphetamine | ||
| Oxygen in para-(4)-position | 5-APB, 5-APDB, 5-MAPDB | 7-APB, 4-APB, 6-APB, 6-APDB | Reduced DAT/SERT inhibition ratio (more serotonergic) |
| Dihydrobenzofuran | 5-APDB, 6-APDB, 5-MAPDB | 5-APB, 6-APB, 4-APB, 7-APB | Reduced DAT/SERT inhibition ratio (more 5-hydroxytryptaminergic) |
| N-Alkyl group | MDMA, 5-EAPB, 5-MAPDB | MDA, 5-APB, 5-APDB | Reduced 5-HT2A/B receptor activation and 5-HT2C receptor-binding potency (less hallucinogenic) |
| 2,5-Oxy-substitution on benzene ring | 2C-B-FLY | All other compounds | Strongly increased 5-HT2A/B receptor activation, strongly increased 5-HT2C receptor-binding potency (more hallucinogenic) |
| β-Keto group | β-Keto MDA | MDA | a,bIncreased DAT/SERT ratio (more dopaminergic) |
Conclusions
Benzofurans are monoamine transporter blockers and monoamine releasers, similar to MDMA, but they also interact with 5-HT receptors. This mechanism of action predicts psychotropic and clinical toxicological effects that are similar to the entactogen MDMA but with additional hallucinogenic properties. The benzodifuran 2C-B-FLY is a potent hallucinogen, probably also associated with a risk for clinical complications related to vasoconstriction (e.g. ischaemia and hypertension). Although structure–activity relationships exist, the present study showed that structurally very similar compounds may exhibit distinct pharmacological profiles, illustrating the need for pharmacotoxicological profiling of each novel psychoactive substance.
Acknowledgments
This work was supported by the Federal Office of Public Health (no. 13.006497) and the Translational Medicine Hub Innovation Fund of F. Hoffmann-La Roche and the University of Basel. The authors thank Daniele Buchy and Sylvie Chaboz for technical assistance, Linda Simmler for critical comments on the manuscript, Lipomed (Arlesheim, Switzerland) for providing 2C-B-FLY at no cost and Michael Arends for text editing.
Glossary
- 2C-B-FLY
8-bromo-2,3,6,7-benzo-dihydro-difuran-ethylamine
- 4-APB
4-(2-aminopropyl)benzofuran
- 5-APB
5-(2-aminopropyl)benzofuran
- 5-APDB
5-(2-aminopropyl)-2,3-dihydrobenzofuran
- 5-EAPB
5-(2-ethylaminopropyl)benzofuran
- 5-MAPDB
1-(2,3-dihydrobenzofuran-5-yl)-N-methylpropan-2-amine
- 6-APB
6-(2-aminopropyl)benzofuran
- 6-APDB
6-(2-aminopropyl)-2,3-dihydrobenzofuran
- 7-APB
7-(2-aminopropyl)benzofuran
- β-keto-MDA
β-keto-3,4-methylenedioxyamphetamine
- bromo-dragonFLY
1-(8-bromobenzo[1,2-b;4,5-b′]difuran-4-yl)-2-aminopropane
- DAT
dopamine transporter
- MDMA
3,4-methylenedioxymethamphetamine
- MDA
3,4-methylenedioxyamphetamine
- NET
noradrenaline transporter
- SERT
5-HT transporter
- TA receptor
trace amine-associated receptor
Author contributions
A. R. and M. E. L. designed the research study. A. R., S. K. and M. C. H. performed the research. A. R., M. C. H. and M. E. L. analysed the data. A. R. and M. E. L. wrote the paper.
Conflict of interest
None.
References
- Acuna-Castillo C, Villalobos C, Moya PR, Saez P, Cassels BK, Huidobro-Toro JP. Differences in potency and efficacy of a series of phenylisopropylamine/phenylethylamine pairs at 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 2002;136:510–519. doi: 10.1038/sj.bjp.0704747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: transporters. Br J Pharmacol. 2013b;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen MF, Telving R, Birkler RI, Schumacher B, Johannsen M. A fatal poisoning involving Bromo-Dragonfly. Forensic Sci Int. 2009;183:91–96. doi: 10.1016/j.forsciint.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Archer JR, Dargan PI, Lee HM, Hudson S, Wood DM. Trend analysis of anonymised pooled urine from portable street urinals in central London identifies variation in the use of novel psychoactive substances. Clin Toxicol (Phila) 2014;52:160–165. doi: 10.3109/15563650.2014.885982. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Schapira AH, Mikhailidis DP, Davar J. Drug-induced fibrotic valvular heart disease. Lancet. 2009;374:577–585. doi: 10.1016/S0140-6736(09)60252-X. [DOI] [PubMed] [Google Scholar]
- Blessing WW, Seaman B, Pedersen NP, Ootsuka Y. Clozapine reverses hyperthermia and sympathetically mediated cutaneous vasoconstriction induced by 3,4-methylenedioxymethamphetamine (ecstasy) in rabbits and rats. J Neurosci. 2003;23:6385–6391. doi: 10.1523/JNEUROSCI.23-15-06385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluelight. 2013a. The Main 5-APDB Thread. Available at: http://www.bluelight.org/vb/threads/672585-The-Main-5-APDB-Thread (accessed 20/12/2014)
- Bluelight. 2013b. The Main 6-APDB Thread. Available at: http://www.bluelight.org/vb/threads/661435-The-Main-6-APDB-Thread (accessed 20/12/2014)
- Chambers JJ, Kurrasch-Orbaugh DM, Parker MA, Nichols DE. Enantiospecific synthesis and pharmacological evaluation of a series of super-potent, conformationally restricted 5-HT2A/2C receptor agonists. J Med Chem. 2001;44:1003–1010. doi: 10.1021/jm000491y. [DOI] [PubMed] [Google Scholar]
- Chan WL, Wood DM, Hudson S, Dargan PI. Acute psychosis associated with recreational use of benzofuran 6-(2-aminopropyl)benzofuran (6-APB) and cannabis. J Med Toxicol. 2013;9:278–281. doi: 10.1007/s13181-013-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. The pre-clinical behavioural pharmacology of 3,4-methylenedioxymethamphetamine (MDMA) Neurosci Biobehav Rev. 2003;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Methods. 1993;160:81–88. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- Dawson P, Opacka-Juffry J, Moffatt JD, Daniju Y, Dutta N, Ramsey J, et al. The effects of benzofury (5-APB) on the dopamine transporter and 5-HT2-dependent vasoconstriction in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:57–63. doi: 10.1016/j.pnpbp.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Di Cara B, Maggio R, Aloisi G, Rivet JM, Lundius EG, Yoshitake T, et al. Genetic deletion of trace amine 1 receptors reveals their role in auto-inhibiting the actions of ecstasy (MDMA) J Neurosci. 2011;31:16928–16940. doi: 10.1523/JNEUROSCI.2502-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty JR, Green AR. The role of monoamines in the changes in body temperature induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and its derivatives. Br J Pharmacol. 2010;160:1029–1044. doi: 10.1111/j.1476-5381.2010.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drugs-Forum. 2013. 5-EAPB (1-(benzofuran-5-yl)-N-ethylpropan-2-amine). Available at: https://www.drugs-forum.com/forum/showthread.php?t=217991 (accessed 20/12/2014)
- Elliott S, Evans J. A 3-year review of new psychoactive substances in casework. Forensic Sci Int. 2014;243C:55–60. doi: 10.1016/j.forsciint.2014.04.017. [DOI] [PubMed] [Google Scholar]
- EMCDDA. 2014. European Drug Report 2014. European Monitoring Center for Drugs and Drug Addiction (EMCDDA). Available at: www.emcdda.europa.eu (accessed 20/12/2014)
- Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB. Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function. Psychopharmacology (Berl) 2014;231:875–888. doi: 10.1007/s00213-013-3303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felser A, Lindinger PW, Schnell D, Kratschmar DV, Odermatt A, Mies S, et al. Hepatocellular toxicity of benzbromarone: effects on mitochondrial function and structure. Toxicology. 2014;324:136–146. doi: 10.1016/j.tox.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Greene SL. Benzofurans and benzodifurans. In: Dargan PI, Wood DM, editors. Novel Psychoactive Substances. Amsterdam: Elsevier; 2013. pp. 283–392. In: (eds) [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Brauner-Osborne H, et al. Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci. 2014;5:243–249. doi: 10.1021/cn400216u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila) 2011;49:705–719. doi: 10.3109/15563650.2011.615318. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, Grouzmann E, Hoener MC, Brenneisen R, et al. The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (‘ecstasy’) in humans. Clin Pharmacol Ther. 2011;90:246–255. doi: 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Brugger R, Simmler LD, Bruggisser M, Donzelli M, Grouzmann E, et al. Effects of the alpha2-adrenergic agonist clonidine on the pharmacodynamics and pharmacokinetics of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Pharmacol Exp Ther. 2012a;340:286–294. doi: 10.1124/jpet.111.188425. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Schmid Y, Rickli A, Simmler LD, Grouzmann E, Liechti ME. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. Swiss Med Forum. 2012b;12(S58):110S. doi: 10.1111/j.1476-5381.2012.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola V, Vischer N, Donzelli M, Krähenbühl S, et al. Duloxetine inhibits effects of MDMA (‘ecstasy’) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS ONE. 2012c;7:e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Fink AE, Simmler LD, Donzelli M, Grouzmann E, Liechti ME. α-Adrenergic receptors contribute to the acute effects of MDMA in humans. J Clin Psychopharmacol. 2013;33:658–666. doi: 10.1097/JCP.0b013e3182979d32. [DOI] [PubMed] [Google Scholar]
- Iversen L, Gibbons S, Treble R, Setola V, Huang XP, Roth BL. Neurochemical profiles of some novel psychoactive substances. Eur J Pharmacol. 2013;700:147–151. doi: 10.1016/j.ejphar.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jebadurai J, Schifano F, Deluca P. Recreational use of 1-(2-naphthyl)-2-(1-pyrrolidinyl)-1-pentanone hydrochloride (NRG-1), 6-(2-aminopropyl) benzofuran (benzofury/ 6-APB) and NRG-2 with review of available evidence-based literature. Hum Psychopharmacol. 2013;28:356–364. doi: 10.1002/hup.2302. [DOI] [PubMed] [Google Scholar]
- Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB, Roth BL. N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine's antidepressant activity. Neuropsychopharmacology. 2008;33:2303–2312. doi: 10.1038/sj.npp.1301646. [DOI] [PubMed] [Google Scholar]
- King LA. New phenethylamines in Europe. Drug Test Anal. 2014;6:808–818. doi: 10.1002/dta.1570. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Griffiths RR. Self-injection of d,1–3,4-methylenedioxymethamphetamine (MDMA) in the baboon. Psychopharmacology (Berl) 1987;91:268–272. doi: 10.1007/BF00518175. [DOI] [PubMed] [Google Scholar]
- Liechti ME. Effects of MDMA on body temperature in humans. Temperature. 2014a;1:179–187. doi: 10.4161/23328940.2014.955433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME. Novel psychoactive substances (designer drugs): overview and pharmacology of modulators of monoamine signalling. Swiss Med Wkly. 2014b;144:w14043. doi: 10.4414/smw.2015.14043. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA (‘Ecstasy’) after pretreatment with the 5-HT2 antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Monte AP, Marona-Lewicka D, Cozzi NV, Nichols DE. Synthesis and pharmacological examination of benzofuran, indan, and tetralin analogues of 3,4-(methylenedioxy)amphetamine. J Med Chem. 1993;36:3700–3706. doi: 10.1021/jm00075a027. [DOI] [PubMed] [Google Scholar]
- Monte AP, Marona-Lewicka D, Parker MA, Wainscott DB, Nelson DL, Nichols DE. Dihydrobenzofuran analogues of hallucinogens. 3. Models of 4-substituted (2,5-dimethoxyphenyl)alkylamine derivatives with rigidified methoxy groups. J Med Chem. 1996;39:2953–2961. doi: 10.1021/jm960199j. [DOI] [PubMed] [Google Scholar]
- Monte AP, Waldman SR, Marona-Lewicka D, Wainscott DB, Nelson DL, Sanders-Bush E, et al. Dihydrobenzofuran analogues of hallucinogens. 4. Mescaline derivatives. J Med Chem. 1997;40:2997–3008. doi: 10.1021/jm970219x. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Lucaites VL, Wainscott DB, Glennon RA. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT2B and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:1–6. doi: 10.1007/pl00005315. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Medicinal chemistry and structure-activity relationships. In: Cho AK, Segal DS, editors. Amphetamine and Its Analogs: Psychopharmacology, Toxicology and Abuse. London: Academic Press; 1994. pp. 3–41. In: (eds) [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nielsen VT, Hogberg LC, Behrens JK. Bromo-Dragonfly poisoning of 18-year-old male. Ugeskr Laeger. 2010;172:1461–1462. [PubMed] [Google Scholar]
- Parker MA, Marona-Lewicka D, Lucaites VL, Nelson DL, Nichols DE. A novel (benzodifuranyl)aminoalkane with extremely potent activity at the 5-HT2A receptor. J Med Chem. 1998;41:5148–5149. doi: 10.1021/jm9803525. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–D1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel FG, Moreau JL, Gainetdinov RR, Bradaia A, Sotnikova TD, Mory R, et al. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc Natl Acad Sci U S A. 2011;108:8485–8490. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol. 2015;25:365–376. doi: 10.1016/j.euroneuro.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Roth BL. Drugs and valvular heart disease. N Engl J Med. 2007;356:6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schmid Y, Enzler F, Gasser P, Grouzmann E, Preller KH, Vollenweider FX, et al. Acute effects of LSD in healthy subjects. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.11.015. doi: 10.1016/j.biopsych.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Scholze P, Zwach J, Kattinger A, Pifl C, Singer EA, Sitte HH. Transporter-mediated release: a superfusion study on human embryonic kidney cells stably expressing the human serotonin transporter. J Pharmacol Exp Ther. 2000;293:870–878. [PubMed] [Google Scholar]
- Seetohul LN, Pounder DJ. Four fatalities involving 5-IT. J Anal Toxicol. 2013;37:447–451. doi: 10.1093/jat/bkt053. [DOI] [PubMed] [Google Scholar]
- Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, et al. 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol Pharmacol. 2003;63:1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- Simmler L, Buser T, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology. 2014a;79:152–160. doi: 10.1016/j.neuropharm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Rickli A, Schramm Y, Hoener MC, Liechti ME. Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem Pharmacol. 2014b;88:237–244. doi: 10.1016/j.bcp.2014.01.024. [DOI] [PubMed] [Google Scholar]
- Stanczuk A, Morris N, Gardner EA, Kavanagh P. Identification of (2-aminopropyl)benzofuran (APB) phenyl ring positional isomers in internet purchased products. Drug Test Anal. 2013;5:270–276. doi: 10.1002/dta.1451. [DOI] [PubMed] [Google Scholar]
- Strano Rossi S, Odoardi S, Gregori A, Peluso G, Ripani L, Ortar G, et al. An analytical approach to the forensic identification of different classes of new psychoactive substances (NPSs) in seized materials. Rapid Commun Mass Spectrom. 2014;28:1904–1916. doi: 10.1002/rcm.6969. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Thorlacius K, Borna C, Personne M. Bromo-dragon fly – life-threatening drug. Can cause tissue necrosis as demonstrated by the first described case. Lakartidningen. 2008;105:1199–1200. [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Welter J, Kavanagh P, Meyer MR, Maurer HH. Benzofuran analogues of amphetamine and methamphetamine: studies on the metabolism and toxicological analysis of 5-APB and 5-MAPB in urine and plasma using GC-MS and LC-(HR)-MS techniques. Anal Bioanal Chem. 2015;407:1371–1388. doi: 10.1007/s00216-014-8360-0. [DOI] [PubMed] [Google Scholar]
- Wood DM, Looker JJ, Shaikh L, Button J, Puchnarewicz M, Davies S, et al. Delayed onset of seizures and toxicity associated with recreational use of Bromo-dragonFLY. J Med Toxicol. 2009;5:226–229. doi: 10.1007/BF03178273. [DOI] [PMC free article] [PubMed] [Google Scholar]