Abstract
Background and Purpose
3-Iodothyronamine (3-T1AM) is an endogenous thyroid hormone derivative reported to induce strong hypothermia and bradycardia within minutes upon injection in rodents. Although 3-T1AM is rapidly converted to several other metabolites in vivo, these strong pharmacological responses were solely attributed to 3-T1AM, leaving potential contributions of downstream products untested. We therefore examined the cardiometabolic effects of 3-iodothyroacetic acid (TA1), the main degradation product of 3-T1AM.
Experimental Approach
We used a sensitive implantable radiotelemetry system in C57/Bl6J mice to study the effects of TA1 on body temperature and heart rate, as well as other metabolic parameters.
Key Results
Interestingly, despite using pharmacological TA1 doses, we observed no effects on heart rate or body temperature after a single TA1 injection (50 mg·kg−1, i.p.) compared to sham-injected controls. Repeated administration of TA1 (5 mg·kg−1, i.p. for 7 days) likewise did not alter body weight, food and water intake, heart rate, blood pressure, brown adipose tissue (BAT) thermogenesis or body temperature. Moreover, mRNA expression of tissue specific genes in heart, kidney, liver, BAT and lung was also not altered by TA1 compared to sham-injected controls.
Conclusions and Implications
Our data therefore conclusively demonstrate that TA1 does not contribute to the cardiovascular or thermoregulatory effects observed after 3-T1AM administration in mice, suggesting that the oxidative deamination constitutes an important deactivation mechanism for 3-T1AM with possible implications for cardiovascular and thermoregulatory functions.
Tables of Links
| Targets | |
|---|---|
| GPCRsa | Transportersd |
| β1-adrenoceptor | Ca2+-ATPase |
| β2-adrenoceptor | Na+/K+-ATPase |
| β3-adrenoceptor | UCP1 (uncoupling protein 1) |
| M1 receptor | Enzymese |
| Ion channelsb | AC (adenylyl cyclase) |
| HCN2 | ACC1 |
| HCN4 | ACE (angiotensin-converting enzyme) |
| Voltage-gated potassium channels | DIO1 (deiodinase, type 1) |
| Nuclear hormone receptorsc | Malonyl-CoA decarboxylase (MLYCD) |
| Thyroid hormone receptor α | Renin |
| Thyroid hormone receptor β |
| Ligands |
|---|
| 3-iodothyronamine |
| 3,5,3′-triiodothyroacetic acid |
| Angiotensin |
| Triiodothyronine |
| TSH |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Pawson et al., 2014) and are permanently archived in the Concise Guide to PHARMACOLOGY 2013/14 (a,b,c,d,eAlexander et al., 2013a,b,c,d,e).
Introduction
Thyronamines (TAMs) are decarboxylated and deiodinated metabolites of thyroid hormone (TH), and were initially discovered in the early 1950s (Hillmann et al., 1958). However, clinical and basic research has only recently shown an interest in thyronamines (Scanlan et al., 2004); several studies on rodents have reported that 3-iodothyronamine (3-T1AM) elicits rapid endocrine, metabolic and behavioural effects (Scanlan et al., 2004; Chiellini et al., 2007; Braulke et al., 2008; Musilli et al., 2014).
The most pronounced effects of 3-T1AM were observed on body temperature and cardiac parameters: a single i.p. injection of 3-T1AM at 50 mg·kg−1 led to a rapid and drastic decrease in body temperature, severe bradycardia and a reduction in cardiac output (Scanlan et al., 2004; Chiellini et al., 2007). As these properties may have beneficial effects when treating ischaemic injuries such as stroke (Doyle et al., 2007), 3-T1AM became a highly interesting molecule from a clinical perspective. However, it has been demonstrated in vitro, ex vivo and in vivo that 3-T1AM can be rapidly metabolized via oxidative deamination followed by aldehyde oxidation to the corresponding 3-iodothyroacetic acid (TA1) (Wood et al., 2009; Saba et al., 2010; Agretti et al., 2011; Hackenmueller and Scanlan, 2012). Importantly, a recent study has proposed that TA1 might elicit the same behavioural effects including amnesia, stimulation of learning and hyperalgesia as 3-T1AM, suggesting that TA1 constitutes a 3-T1AM derivative with biological activity (Musilli et al., 2014). However, it remains unknown whether TA1 also contributes to the profound thermoregulatory and cardiac effects observed after 3-T1AM administration.
Here we demonstrated that pharmacological doses of TA1 do not significantly affect the cardiovascular function or temperature regulation in mice. Our findings clearly demonstrate that TA1 is not involved in mediating the effects of 3-T1AM, indicating that the amino group ethylamine side chain is essential for the rapid effects of 3-T1AM.
Methods
Animal husbandry
C57BL/6J male mice at the age of 3–4 months were housed in single cages at 21–22°C on a 12 h light/12 h dark cycle, and had ad libitum access to food and water. Animal care procedures were in accordance with the guidelines set by the European Community Council Directives (86/609/EEC) and were approved by Stockholm's Norra Djurförsöksetiska Nämnd. The total number of animals used in this study was 38. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Reagents and drugs
TA1 was synthesized as previously described (Wood et al., 2009) by Alinda Chemical Limited (Moscow, Russia) and dissolved in 60% DMSO and 40% physiological saline (pH 7.4) for in vivo studies. The purity of TA1 determined by NMR analysis was <95%. 3-T1AM was kindly provided by Thomas S. Scanlan (OHSU, Portland, OR, USA). T3 was purchased from Sigma-Aldrich (Munich, Germany).
Single-injection experiment
Implantable radio transmitters and receiver plates (Mini Mitter Respironics, Bend, OR, USA) were used to determine heart rate and body temperature of conscious and freely moving mice (Mittag et al., 2013). The mice were anaesthetized using isoflurane 4% to induce anaesthesia, then 2.5% during surgery; depth of anaesthesia was controlled using toe-pinch reflex.The transmitters were implanted into the peritoneal cavity with the electrodes sutured to the right shoulder and the lower left chest wall. Subsequently, the animals were allowed to recover for 7 days before recording started. Mice received a single i.p. injection of TA1 (50 mg·kg−1, 5 μL·g−1 body weight), 3-T1AM (50 mg·kg−1, 5 μL·g−1 body weight) or the same volume of vehicle (60% DMSO and 40% saline, pH 7.4 for TA1 or 30% ethanol for T1AM) and were returned to their home cages for recordings.
Repeated injection experiment
Body weight and food and water intake were measured daily for 7 days before and during treatment with TA1 (5 mg·kg−1 i.p. daily, 5 μL·g−1 body weight) or the same volume of vehicle. Brown fat, tail and inner ear temperature were measured non-invasively using an infrared camera (T335, FLIR Systems Termisk Systemteknik, Linköping, Sweden, ± 0.05°C sensitivity). Rectal temperature was measured using a thermometer probe. Systolic, diastolic, mean arterial pressure and pulse rate were recorded non-invasively using a tail-cuff system on a platform at 34°C (SC1000, Hatteras Instruments, Cary, NC, USA) (Warner et al., 2013). The mice were killed 24 h after the last injection, and organs were collected for subsequent analysis.
Quantitative real-time PCR (qPCR)
RNA was isolated from snap-frozen tissues using the RNeasy Mini Kit (Qiagen, Solna, Sweden). Subsequent cDNA synthesis was carried out using oligo(dT) primers and the transcriptor first-strand cDNA synthesis kit (Roche, Stockholm, Sweden). qPCR was performed with the 7300 real-time PCR system (Applied Biosystems, Stockholm, Sweden) and SYBR Green PCR master mix (Roche) using a two-step PCR protocol with 40 cycles and a temperature of 60°C for annealing and extension. Primer sequences have been published previously (Sjogren et al., 2007; Mittag et al., 2010). A standard curve was used to correct for PCR efficiency, and the results were normalized using Hprt as reference gene. T3-treated animals were used as control (Vujovic et al., 2009). A melting curve was recorded to confirm the specificity of the reaction. Nomenclature of receptors adheres to the Concise Guide to Pharmacology 2013/2014 (Alexander et al., 20132013a).
T4 and T3 elisa for mouse serum analysis
Serum total T4 (EIA 1781, DRG Instruments GmbH, Marburg, Germany) and total T3 (DNOV053, NovaTec Immundiagnostica GmbH, Dietzenbach, Germany) were determined by commercial elisa kits according to the manufacturer's instructions.
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA) was used to analyse the data. All data are represented as mean ± SEM. Statistical significance was defined as P < 0.05 (*), P < 0.01 (**) or P < 0.001 (***).
Results
TA1 does not alter heart rate and body temperature after a single injection
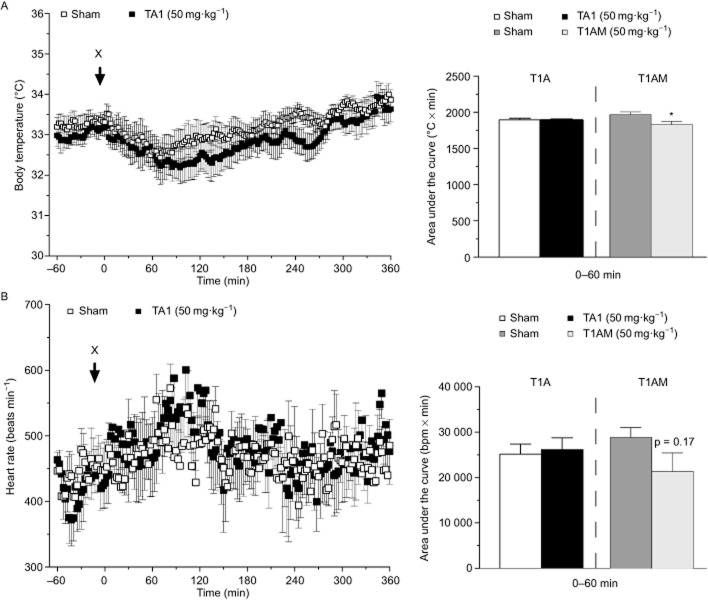
To identify any rapid TA1-mediated effects on thermoregulation or cardiac function, radio transmitters were implanted to measure heart rate and body temperature of conscious and freely moving mice before and during a 6 h post-injection period. However, the results revealed no obvious alterations in body temperature (Figure 1A) or heart rate (Figure 1B) after an i.p. TA1 (50 mg·kg−1) injection when compared with sham-injected controls. As expected from previous studies (Scanlan et al., 2004), an i.p. injection of 3-T1AM caused a significant reduction in body temperature (Figure 1A), and a minor albeit not significant bradycardia (Figure 1B).
Figure 1.
Effects of single TA1 injection on heart rate and body temperature. (A) Body temperature and (B) heart rate were measured using radiotelemetry before and during 6 h post injection (X) in TA1-injected (50 mg·kg−1) compared with sham-injected animals. Data are presented as mean ± SEM of 8–12 male mice for each group. The area under the curve was calculated for the first hour post injection, including a positive control using 3-T1AM (50 mg·kg−1, grey bars). *P < 0.05 (Student's t-test)
Repeated administration of TA1 does not affect metabolic function, heart rate or body temperature
To test if TA1 exerts effects on cardiovascular function and thermoregulation after repeated administration, we measured metabolic, cardiac and thermogenic parameters for 7 days before and during a daily treatment with 5 mg·kg−1 TA1 in a comparison with sham-injected controls. No significant effect of TA1 was observed on body weight (Figure 2A left panel) or food or water intake (Figure 2A right panel). Furthermore, heart rate and blood pressure (systolic, diastolic and mean arterial blood pressure) were not affected by TA1 injection as assessed 24 h after TA1 injection by a tail-cuff system. Infrared thermography showed no significant effect of TA1 on thermoregulation: daily inner ear temperature (Figure 2C upper panel), temperature of the skin overlaying the interscapular brown adipose tissue (BAT) (Figure 2C middle panel), tail tip temperature (Figure 2C lower panel) and rectal temperature measured on the last day (sham 32.8 ± 0.9°C; TA1 33.5 ± 1.0°C; P < 0.05, unpaired Student's t-test) were similar between the treatment groups.
Figure 2.
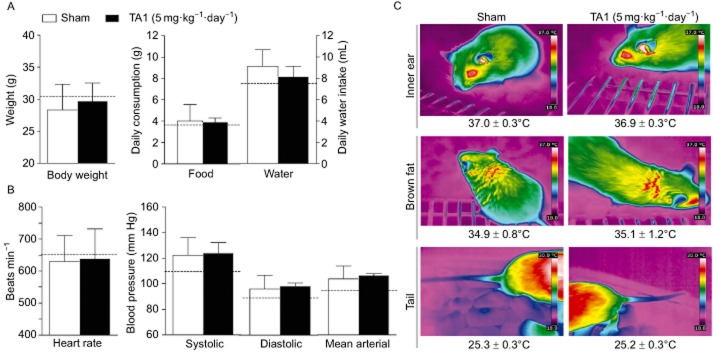
Effects of repeated TA1 treatment on metabolic effects, cardiac function and thermoregulation. (A) There was no significant difference between sham-injected and TA1-injected animals (5 mg·kg−1) in body weight, food or water intake. (B) No significant effect was observed on heart rate and blood pressure (systolic, diastolic and mean arterial blood pressure). (C) Infrared thermography was used to quantify surface heat over the interscapular brown fat depot, inner ear and tail tip. Data are presented as the average of the last three injection days of five animals for each group, dashed line indicates the mean value of all animals before treatment (n.s.: P < 0.05).
Analysis of TH-regulated target genes and total T4 and T3 serum concentrations
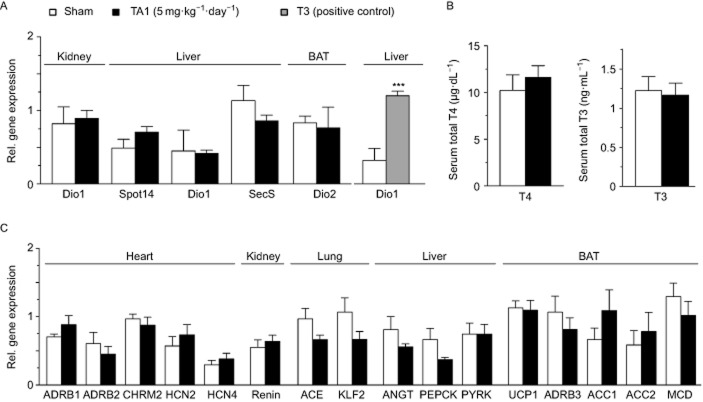
To determine whether repeated administration of TA1 could interfere with peripheral TH metabolism, we analysed the mRNA expression levels of TH-responsive genes SPOT14, deiodinase type I (DIO1) and selenoprotein S in liver, as well as renal DIO1 and brown fat deiodinase type II. qPCR revealed no change upon TA1 treatment, indicating unaffected TH signalling, whereas as expected hepatic DIO1 was elevated by T3 treatment used as positive control (Figure 3A). The lack of effect of TA1 on TH signalling was further supported by comparable levels of serum total T4 and total T3 concentrations in sham and TA1-injected animals (Figure 3B).
Figure 3.
Effect of repeated TA1 treatment on gene expression and thyroid hormone levels. (A) mRNA expression of TH-regulated genes assessed by real-time PCR, (B) total T4 and T3 serum levels, and (C) mRNA expression of cardiac, blood pressure and metabolic genes in sham-injected and TA1-injected (5 mg·kg−1) animals. Data are represented as mean ± SEM of five animals for each group. ACC1/2, acetyl-CoA carboxylase; ADRB1/2/3, β1/2/3-adrenoceptors; ANGT, angiotensinogen; CHRM2, muscarinic M2 receptor; DIO 1/2, deiodinase type 1/2; HCN2/4; potassium/sodium hyperpolarization-activated cyclic nucleotide-gated ion channel 2/4; Klf2, krüppel-like factor 2; MCD, malonyl-CoA decarboxylase; PEPCK, phosphoenolpyruvate carboxykinase; PYRK, pyruvate kinase; SecS, selenoprotein S; UCP1, uncoupling protein 1. ***P < 0.001 (Student's t-test).
Expression profiling in tissues after repeated TA1 administration
To identify tissues potentially affected by the repeated TA1 administration, gene expression analysis was performed on the heart, kidney, lung, liver and BAT (Figure 3C). The results showed no effect of TA1 on the expression of cardiac genes involved in heart rate regulation: the expression levels of β1-adrenoceptors, β2-adrenoceptors, muscarinic M2 receptors, the potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 2 (HCN2) or HCN4 mRNA were unaltered by TA1.
Expression of pulmonary ACE, renal renin, renal krüppel-like factor 2 and liver angiotensin remained likewise unaffected, concurring with the lack of TA1 effect on blood pressure. Similarly, as expected from the infrared thermography, TA1 did not alter uncoupling protein 1 (UCP1) and β3-adrenoceptor mRNA levels in BAT. Liver and BAT genes involved in energy metabolism, such as phosphoenolpyruvate carboxykinase, pyruvate kinase, acetyl-CoA carboxylase 1 and 2 (ACC1, ACC2), and malonyl-CoA decarboxylase, were also similarly expressed in both groups.
Discussion and conclusion
Our data show that TA1 lacks the cardiovascular and thermoregulatory properties of 3-T1AM as it did not induce bradycardia and hypothermia in mice upon single (50 mg·kg−1, i.p.) and repeated (5 mg·kg−1, i.p. 7 days) administration. The dose was chosen based on the published cardiac and thermoregulatory effects of 50 mg·kg−1 3-T1AM (Scanlan et al., 2004), as we hypothesized that the conversion to TA1 could play an important role in these processes. For the long-term application, a 10-fold lower dose was used for 7 days, which is still in the pharmacological range and several fold higher than what would be used for the experimental induction of thyrotoxicosis by TH.
However, despite the relatively high doses, neither the highly sensitive radiotelemetry nor the non-invasive infrared camera were able to detect any difference in cardiovascular or thermoregulatory systems between the treatment groups. This concurs with previous findings showing that TA1 also has no direct effect on oxygen uptake of cardiac muscle in chicks (Newcomer and Barrett, 1960). Furthermore, in contrast to other TH metabolites (Mendoza et al., 2013), TA1 (5 mg·kg−1, i.p. 7 days) does not interfere with TH metabolism, as TH-regulated genes and T4 as well as T3 serum levels were unaltered in our experiments.
THs are well known for their pronounced cardiovascular and thermoregulatory effects (Kahaly and Dillmann, 2005; Mullur et al., 2014). The biological active triiodothyronine (T3) increases facultative and obligatory thermogenesis as well as cardiac output by affecting vascular resistance, blood volume, cardiac contractility and heart rate (Kahaly and Dillmann, 2005). At the molecular level, both non-genomic and genomic cardiovascular effects have been observed to play a role in T3 action in the heart (Klein and Ojamaa, 2001a; Dillmann, 2010). Non-genomically, T3 acts at the cardiomyocyte cell membrane on ion channels for sodium, potassium and calcium ions in the heart, which can increase inotropy and chronotropy (Klein and Ojamaa, 2001b). Genomically, T3 effects are largely mediated by thyroid hormone receptor α1, which is the main isoform in heart (Makino et al., 2012). Several genes encoding ion channels involved in cardiac contractile activity have been shown to be positively or negatively regulated by T3 in the adult mouse heart including those for Ca2+-ATPase and phospholamban, myosin, β-adrenoceptor, AC, guanine nucleotide-binding proteins, Na+/Ca2+ exchanger, Na+/K+-ATPase and voltage-gated potassium channels (Klein and Ojamaa, 2001b). Of particular interest is the potassium/sodium HCN2, which constitutes a component of the pacemaker in the sinoatrial node of the heart and is positively regulated by T3, thus mediating the positive chronotropic effect of the hormone. However, we did not observe any change in the expression of HCN2 or other cardiac TH target genes upon TA1 injection, demonstrating that the compound does not interfere with cardiac TH signalling.
In contrast to TA1, the three times iodinated 3,5,3′-triiodothyroacetic acid (TRIAC) exerts cardiac and thermogenic effects (Symons et al., 1975; Liang et al., 1997; Medina-Gomez et al., 2008). Interestingly, TRIAC has about a 1.5-fold higher affinity for the thyroid hormone receptor α and about a 3.5-fold higher affinity for the thyroid hormone receptor β than T3 (Schueler et al., 1990). In rats, TRIAC has different effects on cardiac DIO1 activity and on cardiac function, resulting in significantly less increase of heart weight with TRIAC than with T3 or T4. This discrepancy might be due to the rapid metabolic clearance rate and short half-life of TRIAC, resulting in only a transient activation of the nuclear receptors (Liang et al., 1997). Our findings, however, suggest that TA1 does not bind to nuclear thyroid receptors, as none of the target genes were altered.
Using brown adipocytes, it was also shown that TRIAC has higher thermogenic potency than T3 towards the adrenergic stimulation of UCP1 mRNA without concomitant inhibition of TSH or hypothyroxinemia (Medina-Gomez et al., 2008). Again, we did not observed any effect of TA1 on body temperature or UCP1 mRNA expression, demonstrating that TA1 displays no thermoregulatory action upon repeated administration.
Like TRIAC, 3-T1AM is a potent modulator of cardiovascular and thermoregulatory action. It has been reported that 3-T1AM induces hypothermia and reduces cardiac output, heart rate, systolic pressure and coronary flow in isolated heart preparation within minutes as a result of the reduced amplitude and duration of the calcium transients (Scanlan et al., 2004; Chiellini et al., 2007; Ghelardoni et al., 2009; Zucchi et al., 2010). Hence, the inactivation of 3-T1AM is physiologically of great relevance to terminate the rapid effects of this potent compound. Detailed ex vivo studies have demonstrated that TA1 is indeed produced from 3-T1AM in rat cardiac tissue (Saba et al., 2010), suggesting that oxidative deamination followed by aldehyde oxidation constitutes an important inactivation mechanism for 3-T1AM directly in the heart (Scanlan et al., 2004; Chiellini et al., 2007; Frascarelli et al., 2011).
At the molecular crossroad downstream of 3-T1AM and TRIAC metabolism, TA1 constitutes a major metabolite (Wood et al., 2009; Saba et al., 2010; Hackenmueller and Scanlan, 2012), which has recently been identified endogenously in human serum and mouse brain (Wood et al., 2009; Musilli et al., 2014). However, the exact biosynthesis of TA1 as well as its export and uptake in different tissues is still enigmatic, and may differ substantially from 3-T1AM, which is taken up by several organs such as gallbladder, stomach, liver, kidney, muscle and adipose tissue (Chiellini et al., 2012). Therefore, at this point, we cannot fully exclude that the levels of TA1 after i.p. injection might differ from the ones obtained after i.p. injection of the precursor 3-T1AM.
In vivo, TA1 is further metabolized by deiodinases to the iodine-free thyroacetic acid TA0, the likely end product of TH metabolism (Pittman et al., 1972). Although TA0 is excreted via the urine, the released iodine can be recycled for TH synthesis within the thyroid gland (Pittman et al., 1972). Our results that TA1 lacks any significant cardiovascular or thermoregulatory activity are thus quite significant: they demonstrate that TA1 can in fact constitute an inactivation product, which terminates the powerful cardiovascular and thermoregulatory effects of other TH derivates such as 3-T1AM or TRIAC. Moreover, our results provide molecular evidence that the ethylamine side chain is essential for the rapid cardiac and thermogenic effects of 3-T1AM.
Acknowledgments
We thank the staff of the CMB animal facility for technical assistance. This work is supported by grants from the Deutsche Forschungsgemeinschaft (C. S. H.: HO 5096/1-1, J. M.: Mi1242/2-1 and Mi1242/3-1), Karolinska Institutets Foundation (C. S. H., A. W. and J. M.), the Swedish Research Council (A. W., B. V. and J. M.) and Hjärt-och Lungfonden (B. V., J. M.).
Glossary
- 3-T1AM
3-iodothyronamine
- ACC1
acetyl-CoA carboxylase 1
- BAT
brown adipose tissue
- DIO1
deiodinase type I
- TA1
3-iodothyroacetic acid
- TH
thyroid hormone
- TRIAC
3,5,3′-triiodothyroacetic acid
Author contributions
C. S. H. and J. M. designed research. C. S. H., S. F. J., A. W., L. H. and N. S. performed the research. B. V. and J. M. contributed new reagents/analytic tools. C. S. H., S. F. J., A. W. and J. M. analysed the data. C. S. H., B. V. and J. M. wrote the paper.
Conflict of interest
None.
References
- Agretti P, De Marco G, Russo L, Saba A, Raffaelli A, Marchini M, et al. 3-Iodothyronamine metabolism and functional effects in FRTL5 thyroid cells. J Mol Endocrinol. 2011;47:23–32. doi: 10.1530/JME-10-0168. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: ion channels. Br J Pharmacol. 2013b;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: nuclear hormone receptors. Br J Pharmacol. 2013c;170:1652–1675. doi: 10.1111/bph.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: transporters. Br J Pharmacol. 2013d;170:1706–1796. doi: 10.1111/bph.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The concise guide to pharmacology 2013/14: enzymes. Br J Pharmacol. 2013e;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke LJ, Klingenspor M, DeBarber A, Tobias SC, Grandy DK, Scanlan TS, et al. 3-Iodothyronamine: a novel hormone controlling the balance between glucose and lipid utilisation. J Comp Physiol [B] 2008;178:167–177. doi: 10.1007/s00360-007-0208-x. [DOI] [PubMed] [Google Scholar]
- Chiellini G, Frascarelli S, Ghelardoni S, Carnicelli V, Tobias SC, DeBarber A, et al. Cardiac effects of 3-iodothyronamine: a new aminergic system modulating cardiac function. FASEB J. 2007;21:1597–1608. doi: 10.1096/fj.06-7474com. [DOI] [PubMed] [Google Scholar]
- Chiellini G, Erba P, Carnicelli V, Manfredi C, Frascarelli S, Ghelardoni S, et al. Distribution of exogenous [125I]-3-iodothyronamine in mouse in vivo: relationship with trace amine-associated receptors. J Endocrinol. 2012;213:223–230. doi: 10.1530/JOE-12-0055. [DOI] [PubMed] [Google Scholar]
- Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev. 2010;15:125–132. doi: 10.1007/s10741-008-9125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle KP, Suchland KL, Ciesielski TM, Lessov NS, Grandy DK, Scanlan TS, et al. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke. 2007;38:2569–2576. doi: 10.1161/STROKEAHA.106.480277. [DOI] [PubMed] [Google Scholar]
- Frascarelli S, Ghelardoni S, Chiellini G, Galli E, Ronca F, Scanlan TS, et al. Cardioprotective effect of 3-iodothyronamine in perfused rat heart subjected to ischemia and reperfusion. Cardiovasc Drugs Ther. 2011;25:307–313. doi: 10.1007/s10557-011-6320-x. [DOI] [PubMed] [Google Scholar]
- Ghelardoni S, Suffredini S, Frascarelli S, Brogioni S, Chiellini G, Ronca-Testoni S, et al. Modulation of cardiac ionic homeostasis by 3-iodothyronamine. J Cell Mol Med. 2009;13:3082–3090. doi: 10.1111/j.1582-4934.2009.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackenmueller SA, Scanlan TS. Identification and quantification of 3-iodothyronamine metabolites in mouse serum using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2012;1256:89–97. doi: 10.1016/j.chroma.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmann G, Keil B, Taslimi P. [Determination of thyroxamine in the thyroid gland and plasma.] Z Naturforsch B. 1958;13B:820–821. [PubMed] [Google Scholar]
- Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein I, Ojamaa K. Thyroid hormone-targeting the heart. Endocrinology. 2001a;142:11–12. doi: 10.1210/endo.142.1.7986. [DOI] [PubMed] [Google Scholar]
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001b;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- Liang H, Juge-Aubry CE, O'Connell M, Burger AG. Organ-specific effects of 3,5,3′-triiodothyroacetic acid in rats. Eur J Endocrinol. 1997;137:537–544. doi: 10.1530/eje.0.1370537. [DOI] [PubMed] [Google Scholar]
- Makino A, Wang H, Scott BT, Yuan JX, Dillmann WH. Thyroid hormone receptor-alpha and vascular function. Am J Physiol Cell Physiol. 2012;302:C1346–C1352. doi: 10.1152/ajpcell.00292.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez G, Calvo RM, Obregon MJ. Thermogenic effect of triiodothyroacetic acid at low doses in rat adipose tissue without adverse side effects in the thyroid axis. Am J Physiol Endocrinol Metab. 2008;294:E688–E697. doi: 10.1152/ajpendo.00417.2007. [DOI] [PubMed] [Google Scholar]
- Mendoza A, Navarrete-Ramirez P, Hernandez-Puga G, Villalobos P, Holzer G, Renaud JP, et al. 3,5-T2 is an alternative ligand for the thyroid hormone receptor beta1. Endocrinology. 2013;154:2948–2958. doi: 10.1210/en.2013-1030. [DOI] [PubMed] [Google Scholar]
- Mittag J, Davis B, Vujovic M, Arner A, Vennstrom B. Adaptations of the autonomous nervous system controlling heart rate are impaired by a mutant thyroid hormone receptor-alpha1. Endocrinology. 2010;151:2388–2395. doi: 10.1210/en.2009-1201. [DOI] [PubMed] [Google Scholar]
- Mittag J, Lyons DJ, Sallstrom J, Vujovic M, Dudazy-Gralla S, Warner A, et al. Thyroid hormone is required for hypothalamic neurons regulating cardiovascular functions. J Clin Invest. 2013;123:509–516. doi: 10.1172/JCI65252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94:355–382. doi: 10.1152/physrev.00030.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musilli C, De Siena G, Manni ME, Logli A, Landucci E, Zucchi R, et al. Histamine mediates behavioral and metabolic effects of 3-iodothyroacetic acid (TA1), an endogenous end product of thyroid hormone metabolism. Br J Pharmacol. 2014;171:3476–3484. doi: 10.1111/bph.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer WS, Barrett PA. Effects of various analogues of thyroxine on oxygen uptake of cardiac muscle from chicks. Endocrinology. 1960;66:409–415. doi: 10.1210/endo-66-3-409. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, et al. NC-IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucl Acids Res. 2014;42:D1098–D1106. doi: 10.1093/nar/gkt1143. (Database Issue): [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman CS, Buck MW, Chambers JB., Jr Urinary metabolites of 14 C-labeled thyroxine in man. J Clin Invest. 1972;51:1759–1766. doi: 10.1172/JCI106977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba A, Chiellini G, Frascarelli S, Marchini M, Ghelardoni S, Raffaelli A, et al. Tissue distribution and cardiac metabolism of 3-iodothyronamine. Endocrinology. 2010;151:5063–5073. doi: 10.1210/en.2010-0491. [DOI] [PubMed] [Google Scholar]
- Scanlan TS, Suchland KL, Hart ME, Chiellini G, Huang Y, Kruzich PJ, et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat Med. 2004;10:638–642. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- Schueler PA, Schwartz HL, Strait KA, Mariash CN, Oppenheimer JH. Binding of 3,5,3′-triiodothyronine (T3) and its analogs to the in vitro translational products of c-erbA protooncogenes: differences in the affinity of the alpha- and beta-forms for the acetic acid analog and failure of the human testis and kidney alpha-2 products to bind T3. Mol Endocrinol. 1990;4:227–234. doi: 10.1210/mend-4-2-227. [DOI] [PubMed] [Google Scholar]
- Sjogren M, Alkemade A, Mittag J, Nordstrom K, Katz A, Rozell B, et al. Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor alpha1. EMBO J. 2007;26:4535–4545. doi: 10.1038/sj.emboj.7601882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons C, Olsen EG, Hawkey CM. The production of cardiac hypertrophy by tri-iodothyroacetic acid. J Endocrinol. 1975;65:341–346. doi: 10.1677/joe.0.0650341. [DOI] [PubMed] [Google Scholar]
- Vujovic M, Nordstrom K, Gauthier K, Flamant F, Visser TJ, Vennstrom B, et al. Interference of a mutant thyroid hormone receptor alpha1 with hepatic glucose metabolism. Endocrinology. 2009;150:2940–2947. doi: 10.1210/en.2008-1085. [DOI] [PubMed] [Google Scholar]
- Warner A, Rahman A, Solsjo P, Gottschling K, Davis B, Vennstrom B, et al. Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor alpha1. Proc Natl Acad Sci U S A. 2013;110:16241–16246. doi: 10.1073/pnas.1310300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood WJ, Geraci T, Nilsen A, DeBarber AE, Scanlan TS. Iodothyronamines are oxidatively deaminated to iodothyroacetic acids in vivo. Chembiochem. 2009;10:361–365. doi: 10.1002/cbic.200800607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Ghelardoni S, Chiellini G. Cardiac effects of thyronamines. Heart Fail Rev. 2010;15:171–176. doi: 10.1007/s10741-008-9120-z. [DOI] [PubMed] [Google Scholar]