Abstract
Expression of the Escherichia coli StpA protein was investigated and a functional comparison undertaken with the structurally analogous nucleoid protein H-NS. Analysis of stpA and hns expression indicated that although stpA transcript levels are much lower than those of hns, the two gene products are capable of both negative autogenous control and cross-regulation. Examination of cellular proteins in stpA, hns, or stpA-hns backgrounds revealed that StpA can repress and activate a subset of H-NS-regulated genes. Mechanistic parallels in regulation of gene expression are indicated by the ability of both proteins to inhibit transcription from promoters containing curved DNA sequences, and to form nucleoprotein structures that constrain DNA supercoils. Despite their functional similarities, each molecule is capable of independent activities. Thus, H-NS regulates a class of genes that are unaffected by StpA in vivo, whereas StpA has much stronger RNA chaperone activity in vitro. We therefore propose that in addition to its role as a molecular back-up of H-NS, StpA's superior effect on RNA may be exploited under some specific cellular conditions to promote differential gene expression.
Full text
PDF

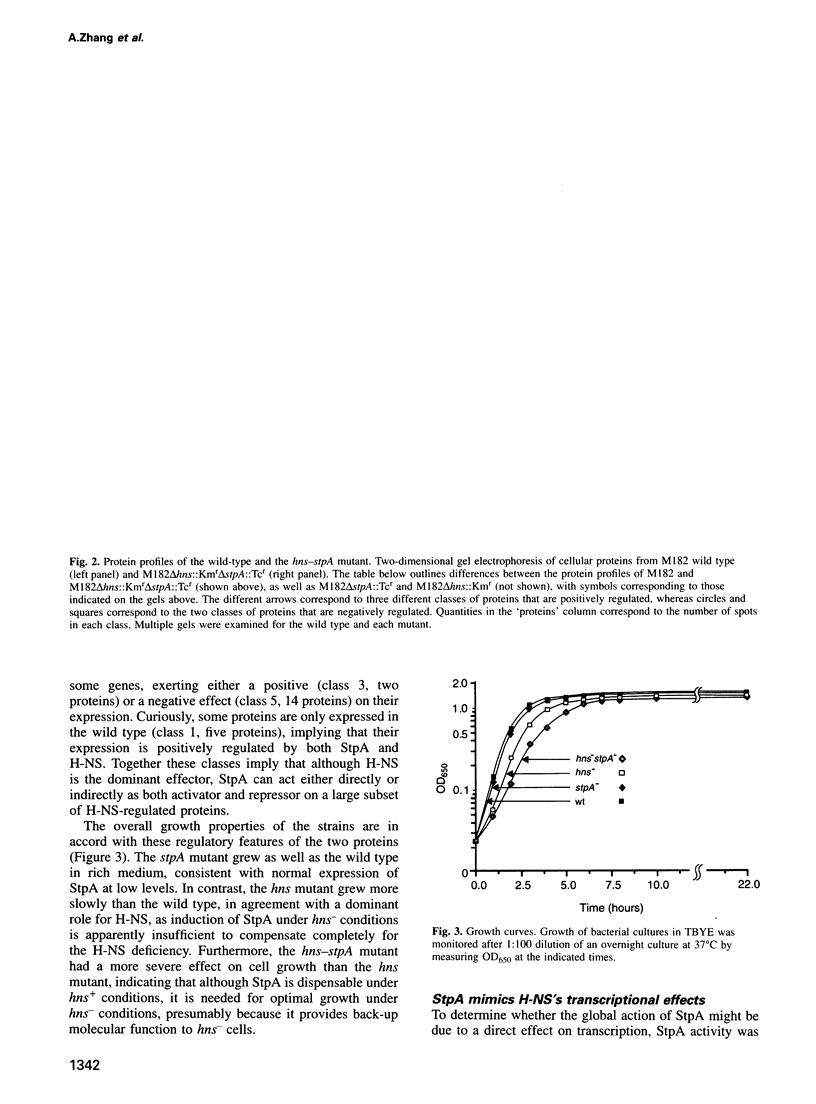
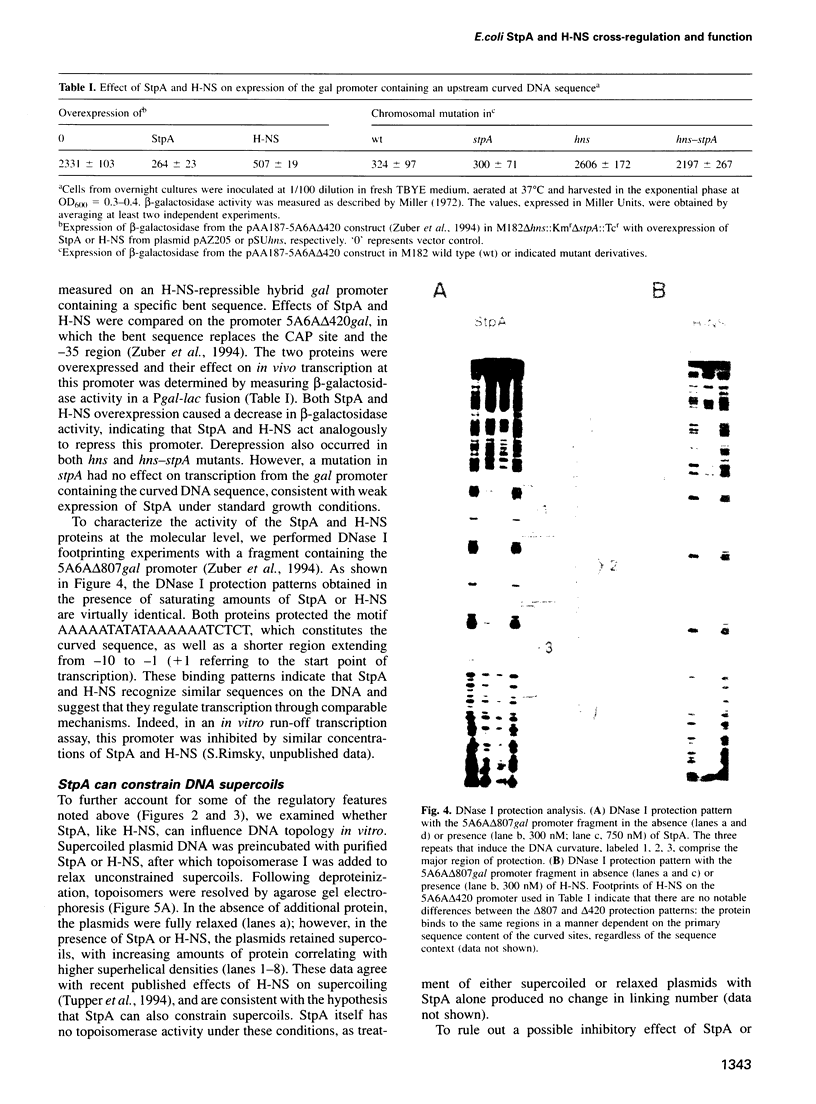






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belfort M., Chandry P. S., Pedersen-Lane J. Genetic delineation of functional components of the group I intron in the phage T4 td gene. Cold Spring Harb Symp Quant Biol. 1987;52:181–192. doi: 10.1101/sqb.1987.052.01.023. [DOI] [PubMed] [Google Scholar]
- Belfort M., Ehrenman K., Chandry P. S. Genetic and molecular analysis of RNA splicing in Escherichia coli. Methods Enzymol. 1990;181:521–539. doi: 10.1016/0076-6879(90)81149-o. [DOI] [PubMed] [Google Scholar]
- Belfort M., Moelleken A., Maley G. F., Maley F. Purification and properties of T4 phage thymidylate synthetase produced by the cloned gene in an amplification vector. J Biol Chem. 1983 Feb 10;258(3):2045–2051. [PubMed] [Google Scholar]
- Bertin P., Lejeune P., Laurent-Winter C., Danchin A. Mutations in bglY, the structural gene for the DNA-binding protein H1, affect expression of several Escherichia coli genes. Biochimie. 1990 Dec;72(12):889–891. doi: 10.1016/0300-9084(90)90008-5. [DOI] [PubMed] [Google Scholar]
- Bracco L., Kotlarz D., Kolb A., Diekmann S., Buc H. Synthetic curved DNA sequences can act as transcriptional activators in Escherichia coli. EMBO J. 1989 Dec 20;8(13):4289–4296. doi: 10.1002/j.1460-2075.1989.tb08615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee T., Herschlag D., Belfort M. Escherichia coli proteins, including ribosomal protein S12, facilitate in vitro splicing of phage T4 introns by acting as RNA chaperones. Genes Dev. 1994 Jul 1;8(13):1575–1588. doi: 10.1101/gad.8.13.1575. [DOI] [PubMed] [Google Scholar]
- Dersch P., Schmidt K., Bremer E. Synthesis of the Escherichia coli K-12 nucleoid-associated DNA-binding protein H-NS is subjected to growth-phase control and autoregulation. Mol Microbiol. 1993 May;8(5):875–889. doi: 10.1111/j.1365-2958.1993.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Ni Bhriain N., Higgins C. F. DNA supercoiling and environmental regulation of virulence gene expression in Shigella flexneri. Nature. 1990 Apr 19;344(6268):789–792. doi: 10.1038/344789a0. [DOI] [PubMed] [Google Scholar]
- Dürrenberger M., La Teana A., Citro G., Venanzi F., Gualerzi C. O., Pon C. L. Escherichia coli DNA-binding protein H-NS is localized in the nucleoid. Res Microbiol. 1991 May;142(4):373–380. doi: 10.1016/0923-2508(91)90106-k. [DOI] [PubMed] [Google Scholar]
- Falconi M., Higgins N. P., Spurio R., Pon C. L., Gualerzi C. O. Expression of the gene encoding the major bacterial nucleotide protein H-NS is subject to transcriptional auto-repression. Mol Microbiol. 1993 Oct;10(2):273–282. [PubMed] [Google Scholar]
- Falconi M., McGovern V., Gualerzi C., Hillyard D., Higgins N. P. Mutations altering chromosomal protein H-NS induce mini-Mu transposition. New Biol. 1991 Jun;3(6):615–625. [PubMed] [Google Scholar]
- Forsman K., Sondén B., Göransson M., Uhlin B. E. Antirepression function in Escherichia coli for the cAMP-cAMP receptor protein transcriptional activator. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9880–9884. doi: 10.1073/pnas.89.20.9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway Salvo J. L., Coetzee T., Belfort M. Deletion-tolerance and trans-splicing of the bacteriophage T4 td intron. Analysis of the P6-L6a region. J Mol Biol. 1990 Feb 5;211(3):537–549. doi: 10.1016/0022-2836(90)90264-m. [DOI] [PubMed] [Google Scholar]
- Göransson M., Sondén B., Nilsson P., Dagberg B., Forsman K., Emanuelsson K., Uhlin B. E. Transcriptional silencing and thermoregulation of gene expression in Escherichia coli. Nature. 1990 Apr 12;344(6267):682–685. doi: 10.1038/344682a0. [DOI] [PubMed] [Google Scholar]
- Herschlag D. RNA chaperones and the RNA folding problem. J Biol Chem. 1995 Sep 8;270(36):20871–20874. doi: 10.1074/jbc.270.36.20871. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hinton J. C., Hulton C. S., Owen-Hughes T., Pavitt G. D., Seirafi A. Protein H1: a role for chromatin structure in the regulation of bacterial gene expression and virulence? Mol Microbiol. 1990 Dec;4(12):2007–2012. doi: 10.1111/j.1365-2958.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Hinton J. C., Santos D. S., Seirafi A., Hulton C. S., Pavitt G. D., Higgins C. F. Expression and mutational analysis of the nucleoid-associated protein H-NS of Salmonella typhimurium. Mol Microbiol. 1992 Aug;6(16):2327–2337. doi: 10.1111/j.1365-2958.1992.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Hulton C. S., Seirafi A., Hinton J. C., Sidebotham J. M., Waddell L., Pavitt G. D., Owen-Hughes T., Spassky A., Buc H., Higgins C. F. Histone-like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990 Nov 2;63(3):631–642. doi: 10.1016/0092-8674(90)90458-q. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Cukier-Kahn R., Pla J., Gros F. A thermostable protein factor acting on in vitro DNA transcription. Biochem Biophys Res Commun. 1971 Dec 17;45(6):1597–1607. doi: 10.1016/0006-291x(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Jordi B. J., Dagberg B., de Haan L. A., Hamers A. M., van der Zeijst B. A., Gaastra W., Uhlin B. E. The positive regulator CfaD overcomes the repression mediated by histone-like protein H-NS (H1) in the CFA/I fimbrial operon of Escherichia coli. EMBO J. 1992 Jul;11(7):2627–2632. doi: 10.1002/j.1460-2075.1992.tb05328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y., Yasuzawa K., Tanaka H., Imamoto F. Propagation of phage Mu in IHF-deficient Escherichia coli in the absence of the H-NS histone-like protein. Gene. 1993 Apr 15;126(1):93–97. doi: 10.1016/0378-1119(93)90594-s. [DOI] [PubMed] [Google Scholar]
- Liu Q., Richardson C. C. Gene 5.5 protein of bacteriophage T7 inhibits the nucleoid protein H-NS of Escherichia coli. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1761–1765. doi: 10.1073/pnas.90.5.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht J. M., Dersch P., Kempf B., Bremer E. Interactions of the nucleoid-associated DNA-binding protein H-NS with the regulatory region of the osmotically controlled proU operon of Escherichia coli. J Biol Chem. 1994 Mar 4;269(9):6578–6578. [PubMed] [Google Scholar]
- May G., Dersch P., Haardt M., Middendorf A., Bremer E. The osmZ (bglY) gene encodes the DNA-binding protein H-NS (H1a), a component of the Escherichia coli K12 nucleoid. Mol Gen Genet. 1990 Oct;224(1):81–90. doi: 10.1007/BF00259454. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olsén A., Arnqvist A., Hammar M., Sukupolvi S., Normark S. The RpoS sigma factor relieves H-NS-mediated transcriptional repression of csgA, the subunit gene of fibronectin-binding curli in Escherichia coli. Mol Microbiol. 1993 Feb;7(4):523–536. doi: 10.1111/j.1365-2958.1993.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Owen-Hughes T. A., Pavitt G. D., Santos D. S., Sidebotham J. M., Hulton C. S., Hinton J. C., Higgins C. F. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell. 1992 Oct 16;71(2):255–265. doi: 10.1016/0092-8674(92)90354-f. [DOI] [PubMed] [Google Scholar]
- Pon C. L., Calogero R. A., Gualerzi C. O. Identification, cloning, nucleotide sequence and chromosomal map location of hns, the structural gene for Escherichia coli DNA-binding protein H-NS. Mol Gen Genet. 1988 May;212(2):199–202. doi: 10.1007/BF00334684. [DOI] [PubMed] [Google Scholar]
- Shi X., Bennett G. N. Plasmids bearing hfq and the hns-like gene stpA complement hns mutants in modulating arginine decarboxylase gene expression in Escherichia coli. J Bacteriol. 1994 Nov;176(21):6769–6775. doi: 10.1128/jb.176.21.6769-6775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sledjeski D., Gottesman S. A small RNA acts as an antisilencer of the H-NS-silenced rcsA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2003–2007. doi: 10.1073/pnas.92.6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky A., Rimsky S., Garreau H., Buc H. H1a, an E. coli DNA-binding protein which accumulates in stationary phase, strongly compacts DNA in vitro. Nucleic Acids Res. 1984 Jul 11;12(13):5321–5340. doi: 10.1093/nar/12.13.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tupper A. E., Owen-Hughes T. A., Ussery D. W., Santos D. S., Ferguson D. J., Sidebotham J. M., Hinton J. C., Higgins C. F. The chromatin-associated protein H-NS alters DNA topology in vitro. EMBO J. 1994 Jan 1;13(1):258–268. doi: 10.1002/j.1460-2075.1994.tb06256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi C., Kakeda M., Mizuno T. Autoregulatory expression of the Escherichia coli hns gene encoding a nucleoid protein: H-NS functions as a repressor of its own transcription. Mol Gen Genet. 1993 Jan;236(2-3):171–178. doi: 10.1007/BF00277109. [DOI] [PubMed] [Google Scholar]
- Ueguchi C., Mizuno T. The Escherichia coli nucleoid protein H-NS functions directly as a transcriptional repressor. EMBO J. 1993 Mar;12(3):1039–1046. doi: 10.1002/j.1460-2075.1993.tb05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. J., Nedospasov S. A., Bakayev V. V., Bakayeva T. G., Georgiev G. P. Histone-like proteins in the purified Escherichia coli deoxyribonucleoprotein. Nucleic Acids Res. 1977 Aug;4(8):2725–2745. doi: 10.1093/nar/4.8.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Muramatsu S., Mizuno T. An Escherichia coli protein that preferentially binds to sharply curved DNA. J Biochem. 1990 Sep;108(3):420–425. doi: 10.1093/oxfordjournals.jbchem.a123216. [DOI] [PubMed] [Google Scholar]
- Yamada H., Yoshida T., Tanaka K., Sasakawa C., Mizuno T. Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol Gen Genet. 1991 Nov;230(1-2):332–336. doi: 10.1007/BF00290685. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Ueguchi C., Yamada H., Mizuno T. Function of the Escherichia coli nucleoid protein, H-NS: molecular analysis of a subset of proteins whose expression is enhanced in a hns deletion mutant. Mol Gen Genet. 1993 Feb;237(1-2):113–122. doi: 10.1007/BF00282791. [DOI] [PubMed] [Google Scholar]
- Zhang A., Belfort M. Nucleotide sequence of a newly-identified Escherichia coli gene, stpA, encoding an H-NS-like protein. Nucleic Acids Res. 1992 Dec 25;20(24):6735–6735. doi: 10.1093/nar/20.24.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A., Derbyshire V., Salvo J. L., Belfort M. Escherichia coli protein StpA stimulates self-splicing by promoting RNA assembly in vitro. RNA. 1995 Oct;1(8):783–793. [PMC free article] [PubMed] [Google Scholar]
- Zuber F., Kotlarz D., Rimsky S., Buc H. Modulated expression of promoters containing upstream curved DNA sequences by the Escherichia coli nucleoid protein H-NS. Mol Microbiol. 1994 Apr;12(2):231–240. doi: 10.1111/j.1365-2958.1994.tb01012.x. [DOI] [PubMed] [Google Scholar]