Abstract
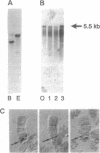
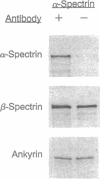
We report the identification and initial characterization of Drosophila melanogaster ankyrin. Oligonucleotide primers based on the spectrin-binding domain of human brain ankyrin were used to amplify Drosophila genomic DNA. A cloned 184-bp PCR product was used to isolate Drosophila ankyrin cDNAs. Ankyrin cDNA probes detected a 5.5-kb transcript from embryonic poly(A)+ RNA and a single polytene chromosome locus (101F-102A). The cDNA sequence encodes a 170-kDa protein that is 53% identical to human brain ankyrin (Ank2). Antibodies directed against a recombinant fragment of Drosophila ankyrin reacted with a 170-kDa polypeptide from Drosophila embryos, larvae, S2 cells, and adult flies. The ankyrin antibody coimmunoprecipitated alpha- and beta-spectrin with ankyrin in detergent extracts of Drosophila embryo membranes. Antibodies against Drosophila ankyrin, alpha-spectrin and beta-spectrin were used to detect these proteins in wild-type and alpha-spectrin-mutant larvae. alpha-Spectrin levels were greatly diminished in mutant larvae, but levels of ankyrin and beta-spectrin were indistinguishable from wild type. The persistence of ankyrin and beta-spectrin may explain the relatively mild phenotype of alpha-spectrin mutants during early Drosophila development.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V. Ankyrins. Adaptors between diverse plasma membrane proteins and the cytoplasm. J Biol Chem. 1992 May 5;267(13):8703–8706. [PubMed] [Google Scholar]
- Bennett V., Gilligan D. M. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. Physiol Rev. 1990 Oct;70(4):1029–1065. doi: 10.1152/physrev.1990.70.4.1029. [DOI] [PubMed] [Google Scholar]
- Bieber A. J., Snow P. M., Hortsch M., Patel N. H., Jacobs J. R., Traquina Z. R., Schilling J., Goodman C. S. Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989 Nov 3;59(3):447–460. doi: 10.1016/0092-8674(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., 4th, Birkenmeier C. S., Barker J. E. Spectrin deficient inherited hemolytic anemias in the mouse: characterization by spectrin synthesis and mRNA activity in reticulocytes. Cell. 1984 Jul;37(3):721–729. doi: 10.1016/0092-8674(84)90408-2. [DOI] [PubMed] [Google Scholar]
- Brown N. H., Kafatos F. C. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988 Sep 20;203(2):425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Byers T. J., Brandin E., Lue R. A., Winograd E., Branton D. The complete sequence of Drosophila beta-spectrin reveals supra-motifs comprising eight 106-residue segments. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6187–6191. doi: 10.1073/pnas.89.13.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers T. J., Dubreuil R., Branton D., Kiehart D. P., Goldstein L. S. Drosophila spectrin. II. Conserved features of the alpha-subunit are revealed by analysis of cDNA clones and fusion proteins. J Cell Biol. 1987 Nov;105(5):2103–2110. doi: 10.1083/jcb.105.5.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K. A., Lux S. E. Hereditary disorders of the red cell membrane skeleton. Trends Genet. 1989 Jul;5(7):222–227. doi: 10.1016/0168-9525(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Davis J. Q., McLaughlin T., Bennett V. Ankyrin-binding proteins related to nervous system cell adhesion molecules: candidates to provide transmembrane and intercellular connections in adult brain. J Cell Biol. 1993 Apr;121(1):121–133. doi: 10.1083/jcb.121.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R. R., Byers T. J., Sillman A. L., Bar-Zvi D., Goldstein L. S., Branton D. The complete sequence of Drosophila alpha-spectrin: conservation of structural domains between alpha-spectrins and alpha-actinin. J Cell Biol. 1989 Nov;109(5):2197–2205. doi: 10.1083/jcb.109.5.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R., Byers T. J., Branton D., Goldstein L. S., Kiehart D. P. Drosophilia spectrin. I. Characterization of the purified protein. J Cell Biol. 1987 Nov;105(5):2095–2102. doi: 10.1083/jcb.105.5.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon R. G., Dawson I. A., Artavanis-Tsakonas S. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development. 1994 Mar;120(3):545–557. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- Kidd S. Characterization of the Drosophila cactus locus and analysis of interactions between cactus and dorsal proteins. Cell. 1992 Nov 13;71(4):623–635. doi: 10.1016/0092-8674(92)90596-5. [DOI] [PubMed] [Google Scholar]
- Lee J. K., Coyne R. S., Dubreuil R. R., Goldstein L. S., Branton D. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J Cell Biol. 1993 Dec;123(6 Pt 2):1797–1809. doi: 10.1083/jcb.123.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990 Mar 1;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- McNeill H., Ozawa M., Kemler R., Nelson W. J. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990 Jul 27;62(2):309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Morgans C. W., Kopito R. R. Association of the brain anion exchanger, AE3, with the repeat domain of ankyrin. J Cell Sci. 1993 Aug;105(Pt 4):1137–1142. doi: 10.1242/jcs.105.4.1137. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987 Aug 6;328(6130):533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Veshnock P. J. Dynamics of membrane-skeleton (fodrin) organization during development of polarity in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1986 Nov;103(5):1751–1765. doi: 10.1083/jcb.103.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neil M. T., Belote J. M. Interspecific comparison of the transformer gene of Drosophila reveals an unusually high degree of evolutionary divergence. Genetics. 1992 May;131(1):113–128. doi: 10.1093/genetics/131.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto E., Kunimoto M., McLaughlin T., Bennett V. Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J Cell Biol. 1991 Jul;114(2):241–253. doi: 10.1083/jcb.114.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palek J., Lambert S. Genetics of the red cell membrane skeleton. Semin Hematol. 1990 Oct;27(4):290–332. [PubMed] [Google Scholar]
- Peters L. L., Turtzo L. C., Birkenmeier C. S., Barker J. E. Distinct fetal Ank-1 and Ank-2 related proteins and mRNAs in normal and nb/nb mice. Blood. 1993 Apr 15;81(8):2144–2149. [PubMed] [Google Scholar]
- Ron D., Dressler H. pGSTag--a versatile bacterial expression plasmid for enzymatic labeling of recombinant proteins. Biotechniques. 1992 Dec;13(6):866–869. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Smith P. R., Saccomani G., Joe E. H., Angelides K. J., Benos D. J. Amiloride-sensitive sodium channel is linked to the cytoskeleton in renal epithelial cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6971–6975. doi: 10.1073/pnas.88.16.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan Y., Elmer L., Davis J., Bennett V., Angelides K. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988 May 12;333(6169):177–180. doi: 10.1038/333177a0. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Yue L., Spradling A. C. hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 1992 Dec;6(12B):2443–2454. doi: 10.1101/gad.6.12b.2443. [DOI] [PubMed] [Google Scholar]