Abstract
Background
Currently, obesity has become a worldwide health problem and yet little is known about the impact of changes in obesity indicator on incident hypertension. The aim of this study was to compare the impact of changes in the WC and BMI on incident hypertension in a cohort population.
Methods
After a baseline investigation, we conducted the first and the second follow-up assessments for subjects after 2 and 5 years, respectively. The associations between the changes in the WC and BMI (measured as the D-value, i.e., the value at the first follow-up minus the value at baseline) and the hazard ratio (HR) of incident hypertension were analyzed with a multilevel Cox proportional hazards regression model.
Results
Among 2778 participants without hypertension, 660 developed hypertension between the first and the second follow-up assessments. When both the BMI and WC D-values were included in the regression model, the WC D-value was a predictor of hypertension incidence in both sexes (OR= 1.03 and P values < 0.05 for men and women), but the BMI D-value was no longer a predictor of hypertension incidence in either sex (OR=1.04 for men and 1.01 for women, both P values >0.05). In both sexes, hypertension risk was higher for subjects whose BMI was modified but WC was categorically increasing than for subjects whose WC and BMI were both modified.
Conclusions
Both WC and BMI changes were associated with hypertension, but a change in the WC was a better predictor of the hypertension.
Keywords: Body mass index, Change, Hypertension, Waist circumference
Introduction
Body fat content is a well-established risk factor for the development of hypertension (1, 2), and recent studies have suggested that abdominal visceral fat has a stronger association with hypertension incidence than does total fat mass (3–5). Although BMI is significantly related to an individual’s body fat content (6, 7), some studies have suggested that the WC is a better predictor than BMI of the amount of abdominal visceral fat determined using computed tomography; furthermore, the WC can be easily measured and interpreted (8, 9). The impact of changes in the WC or BMI on the incidence of hypertension has been observed in previous clinical studies but not in cohort studies (10–12). On the population level, the association between changes in the WC or BMI and incident hypertension is unclear, and such an association may be impacted by a change in lifestyle or a targeted intervention.
The aim of this study was to compare the impact of changes in the WC or BMI on incident hypertension utilizing data from the Prevention of Multiple Metabolic Disorders and MS in Jiangsu Province (PMMJS) project.
Methods
Study Cohort
A multi-stage sampling method was employed for the present study. In stage one; we randomly selected 3 districts from 13 urban districts and 9 counties from the 52 counties of Jiangsu province based on the economic conditions in different regions. In the second stage, one community (such as a street district or a residential community) from each city and one rural township from each county were sampled randomly. In the final stage, households were randomly chosen from the selected communities and townships; only one participant was randomly selected, without replacement, from each household. Simple random sampling methods were used at each stage. The local public health administrative institutes possess the household registrations, which include the addresses and telephone numbers for all participants, allowing the health status of each participant to be tracked easily in follow-up assessments. Individuals who suffered from cancer, severe disability, or a severe psychiatric disturbance were excluded.
Data on demographic characteristics, lifestyle risk factors, personal medical history and family history of hypertension for all participants were obtained using a standard questionnaire administered by trained staff. Three sitting blood pressure (BP) measurements were taken at 30-second intervals by trained observers using a standard mercury sphygmomanometer after the subjects had been resting for 5 min according to a standard protocol. The first and fifth Korotkoff sounds were recorded as the systolic (SBP) and diastolic (DBP) blood pressures, respectively. The mean of the three BP measurements was used in the analysis. Body weight and height were measured by using standard methods (13), and the BMI was calculated as the weight in kilograms divided by the square of the height in meters. The WC was measured two times at 1 cm above the umbilicus at minimal respiration by trained observers with the subjects standing and breathing normally during the physical examination.
Blood samples were collected in the morning after at least 8 hours of fasting. All plasma and serum samples were frozen at -80 °C until laboratory testing was performed. Plasma glucose was measured using an oxidase enzymatic method. The concentrations of HDL cholesterol and triglycerides were assessed enzymatically using an automatic biochemistry analyzer (Hitachi Inc, Tokyo, Japan) and commercial reagents. The Friedewald equation (14) was used to calculate the LDL-C from the total cholesterol, HDL cholesterol, and triglycerides. All analyses were performed by the same lab. All of the participants signed the informed consent form. The study was approved by the Soochow University ethics committee.
Follow-up Assessments
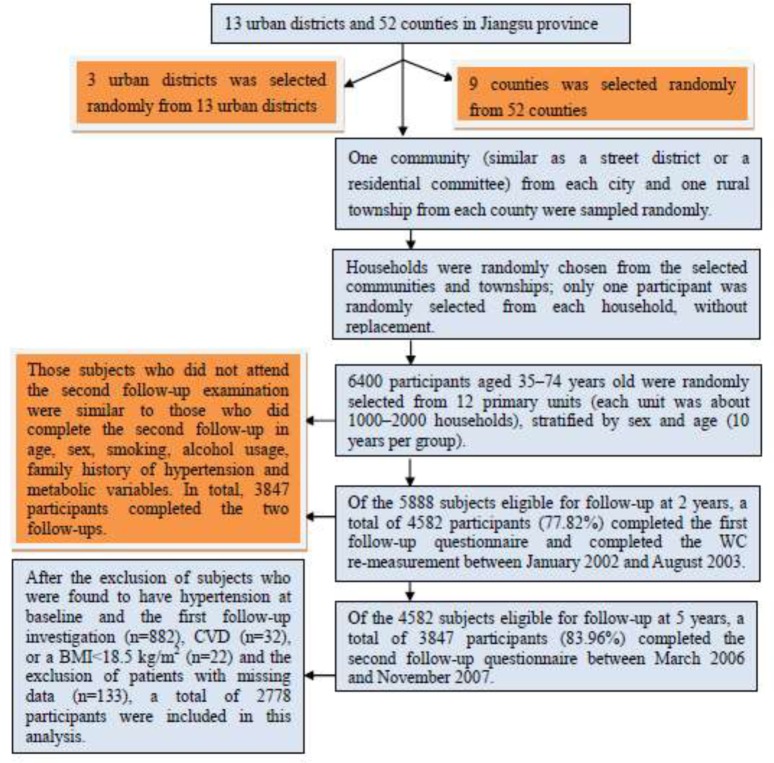
Of the 5888 subjects eligible for follow-up at 2 years, a total of 4582 participants (77.82%) completed the first follow-up questionnaire and completed the WC re-measurement between January 2002 and August 2003. The contents and methods of the questionnaire and WC measurements in the first follow-up investigation were the same as those at baseline. In addition, data on participant’s blood pressure and development of hypertension and cardiovascular diseases were collected at the first follow-up. Of the 4582 subjects eligible for follow-up at 5 years, a total of 3847 participants (83.96%) completed the second follow-up questionnaire between March 2006 and November 2007. In this survey, we mainly collected information on participant’s current blood pressure and the incidence of hypertension and cardiovascular diseases over the past five years. Those subjects who did not attend the second follow-up examination were similar to those who did complete the second follow-up in age, sex, smoking, alcohol usage, family history of hypertension and metabolic variables. In total, 3847 participants completed the two follow-ups. After the exclusion of subjects who were found to have hypertension at baseline and the first follow-up investigation (n=882), CVD (n=32), or a BMI<18.5 kg/m2 (n=22) and the exclusion of patients with missing data (n=133), a total of 2778 participants (1097 males and 1681 females) were included in this analysis, A detailed description of the multi-stage sampling method and subjects selection was shown in Fig. 1. The median duration of follow-up was 3.8 years from the first follow-up to the second follow-up. The study outcome was defined as incident hypertension during the period between the first and second follow-up assessments.
Fig. 1.
A flowchart of this multi-stage sampling method and subject selection
Definitions
Abnormal WC (abdominal obesity) was defined as a WC≥90 cm for males and ≥80 cm for females (15). Abnormal BMI (overweight or obesity) was defined as a BMI≥25 kg/m2 (16). Hypertension was defined as SBP ≥140 mmHg and/or DBP ≥90 mmHg and/or the use of antihypertensive medication, as reported in the questionnaires (17).
Statistical Analysis
The difference values (D-value) for the WC and BMI between the first follow-up and baseline were calculated to evaluate the changes in WC or BMI from the baseline to the first follow-up and to ensure that the change in WC or BMI happened before hypertension developed.
A D-value >0 signified that the WC or BMI increased between baseline and the first follow-up, and a greater D-value implied a larger WC or BMI increase. A D-value <0 signified that the WC or BMI decreased between baseline and the first follow-up, and a lower D-value implied a larger WC or BMI decrease. Subjects with abnormal WC or BMI at the first follow up no matter whether baseline WC or BMI was normal were defined as non-modificatory group. Subjects with normal WC or BMI at the first follow-up no matter whether baseline WC or BMI was normal were defined as modificatory group.
Statistical analyses were performed with the statistical program SAS for Windows V9.12. The means and standard deviations (SDs) for continuous variables were normally distributed, and the percentages for categorical variables were calculated and compared between participants with and without abdominal obesity and between participants with and without an abnormal BMI. Medians and interquartile ranges were calculated for continuous variables that were not normally distributed. Significant differences for characteristics among four groups were examined using one-way ANOVA, the rank test and the χ2 test. Logistic regression was used to examine the association between WC and BMI D-values and incident hypertension, odds ratio (OR) and 95% confidence intervals (95%CIs) were calculated. The multilevel Cox proportional hazards regression model was used to examine the association between changes in WC or BMI and incident hypertension; hazards ratios (HR) and 95% confidence intervals (95% CIs) were calculated. Potential confounding factors including age, sex and family history of hypertension were adjusted for in the analysis. All reported P-values were two-tailed, and those less than 0.05 were considered statistically significant.
Results
A total of 2778 participants (1097 males and 1681 females) were studied; this number included 660 subjects with incident hypertension (254 males and 406 females) who developed hypertension by the second follow-up. A total of 440 subjects with a normal WC at baseline developed abdominal obesity by the first follow-up, and 112 subjects with abdominal obesity at baseline returned to a normal WC at the first follow-up. A total of 280 subjects with a normal BMI at baseline developed obesity at the first follow-up, and 164 subjects with obesity at baseline returned to a normal BMI at the first follow-up. The baseline characteristics of the 2778 study participants separated by sex are shown in Table 1.
Table 1.
Baseline characteristics of 2778 study participants grouped by sex
| Variables | Males (n=1097) | Females (n=1681) | P values |
|---|---|---|---|
| Age (yr) | 49.4±9.4 | 48.6±9.7 | 0.029 |
| TC (mmol/l) | 4.5±0.9 | 4.5±1.0 | 0.226 |
| HDL-C (mmol/l) | 1.2±0.4 | 1.3±0.3 | 0.037 |
| LDL-C (mmol/l) | 2.5±0.7 | 2.6±0.8 | 0.103 |
| FPG (mmol/l) | 5.2±1.2 | 0.351 | |
| TG (mmol/l) | 1.2 (0.9-1.8) | 1.3 (1.0-1.8) | 0.07 |
| SBP (mmHg) | 118.3±10.8 | 0.001 | |
| DBP (mmHg) | 77.1±7.8 | 75.2±7.6 | <0.001 |
| BMI (Kg/m2) | 22.2±2.9 | 22.9±3.2 | <0.001 |
| BMI D-value | 0.4±2.7 | 0.4±2.9 | 0.713 |
| WC (cm) | 76.4±8.6 | 74.9±8.7 | <0.001 |
| WC D-value | 3.2±7.3 | 3.5±7.5 | 0.323 |
| Smoking (%) | 46.9 | 5.9 | <0.001 |
| Alcohol use (%) | 44.4 | 5.8 | <0.001 |
| Family history of hypertension (%) | 26.0 | 24.7 | 0.465 |
BMI, body mass index; WC, waist circumference; TC, total cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high density lipoprotein; LDL, low density lipoprotein; FPG, fast plasma glucose; TG, triglyceride; D-value, difference value between the first follow-up and baseline./Note: median and inter quartile for TG; means± standard deviation for age, TC, HDL-C, LDL-C, FPG, SBP, DBP, WC, BMI, WC D-value and BMI D-value
The results of the multilevel proportional hazards regression model in which the WC D-value alone, the BMI D-value alone, or both the WC and BMI D-values were used as continuous variables to predict hypertension are shown in Table 2. Because the units for BMI and WC are different, the magnitude of the ORs for BMI and WC presented in Table 2 are not directly comparable. The estimated tolerance between the WC D-value and BMI D-value was high (0.63), and the VIF was low (1.58), suggesting a low level of multicollinearity. When the BMI D-value alone or the WC D-value alone was included in the multivariate regression model, the WC D-value was significantly related to hypertension, SBP and DBP in both sexes. The BMI D-value was related to hypertension, SBP and DBP in males and to SBP in females. When both the BMI and WC D-values were included in the multivariate regression model, the WC D-value remained a predictor of hypertension, SBP and DBP in both sexes. However, the BMI D-value was no longer a predictor of hypertension, SBP and DBP in either sex.
Table 2.
Four Logistic regression models predicting hypertension, SBP and DBP using WC D-value, BMI D-value or combined D-values a
| Variables | WC D-value | BMI D-value | WC D-value and BMI D-value | |
|---|---|---|---|---|
| alone | alone | WC D-value | BMI D-value | |
| Males (n=1097) | ||||
| Hypertension | 1.04 (1.02-1.07)b | 1.09 (1.04-1.18)b | 1.03 (1.01-1.06)b | 1.04 (0.97-1.12) |
| SBP | 1.03 (1.01-1.06)b | 1.08 (1.02-1.15)b | 1.02 (1.01-1.05)b | 1.04 (0.96-1.12) |
| DBP | 1.09 (1.06-1.12)b | 1.20 (1.11-1.30)b | 1.06 (1.02-1.09)b | 1.06 (0.98-1.14) |
| Females (n=1681) | ||||
| Hypertension | 1.03 (1.02-1.05)b | 1.03 (0.98-1.09) | 1.03 (1.01-1.05)b | 1.01 (0.97-1.06) |
| SBP | 1.04 (1.02-1.05)b | 1.08 (1.03-1.13)b | 1.03 (1.01-1.05)b | 1.03 (0.98-1.09) |
| DBP | 1.04 (1.01-1.06)b | 1.03 (0.99-1.10) | 1.03 (1.01-1.06)b | 1.02 (0.97-1.08) |
CI, confidence interval; OR, odds ratio; D-value, difference value. / a BMI D-value and WC D-value were included in the multilevel regression model as continuous variables, and the ORs were computed for each unit increase in BMI (kg/m2) and WC (cm). The ORs were adjusted for age, TC, HDL-C, LDL-C, FPG, TG, smoke and alcohol status, family history of hypertension. A low level of multicollinearity between WC D-value and BMI D-value, and among triglycerides, HDL-C, total cholesterol and LDL-C was found, because tolerance values were >0.5, VIF<10. / b Significantly greater odds, P<0.05
The participants were further stratified by WC or BMI at baseline and the first follow-up, and the incidence rate and RRs of hypertension for each group were calculated by multilevel proportional hazards regression models. Compared with those with no abdominal obesity at the first follow-up, the RRs of incident hypertension were all significantly higher for subjects with abdominal obesity at the first follow-up than for those participants who did not have abdominal obesity at the first follow-up, regardless of their abdominal obesity status at baseline (all P values less than 0.05).
Among the subjects with a normal BMI at baseline, there was a higher RR of incident hypertension for subjects with an abnormal BMI at the first follow-up than for subjects with a normal BMI at the first follow-up (P< 0.05). In the populations with an abnormal BMI at baseline, there was no significant difference between subjects with an abnormal BMI at the first follow-up and subjects with a normal BMI at the first follow-up (P> 0.05). (Table 3 and 4) Table 5 shows the incidence rate and RRs of hypertension for participants stratified by whether WC and/or BMI were categorically increasing. The lowest cumulative incidence rate of hypertension was 19.1% in males and 16.0% in females in populations in which both WC and BMI were modified; the highest hypertension incidence was 45.1% in males and 35.8% in females in populations in which WC was not modified and BMI was modified. In both sexes, the hypertension risk was higher in populations in which WC was categorically increasing but BMI was modified than that of subjects in which both WC and BMI were modified (P< 0.01). No significant difference in hypertension risk was obtained between subjects whose WC was modified but BMI was categorically increasing and subjects whose WC and BMI were both modified (P> 0.05).
Table 3.
Cumulative incidence and hazard ratio of hypertension stratified by WC at baseline and the first follow-up
| Abdominal obesity at baseline | Abdominal obesity at the first follow-up | Cumulative incidence % (n) | HR (95%CI) | |
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| yes | no | 21.9 (24) | 1.00 | 1.00 |
| yes | yes | 34.8 (153) | 1.89 (1.17-3.22)** | 4.27 (1.62-9.81) ** |
| no | no | 18.1 (329) | 1.00 | 1.00 |
| no | yes | 35.4 (154) | 2.41 (1.84-3.01) ** | 1.45 (1.02-2.41) * |
CI, confidence interval; HR, hazard ratio; N, number of cases./Model 1: Only WC was included as independent variable. Model 2: Adjusted for sex, age, TC, HDL-C, LDL-C, FPG, TG, smoke and alcohol status, family history of hypertension, SBP and DBP./No multicollinearity was found among triglycerides, HDL-C, total cholesterol and LDL-C, because tolerance values were >0.5, VIF<10./*Significantly greater odds, P < 0.05./**Significantly greater odds, P < 0.01.
Table 4.
Cumulative incidence and hazard ratio of hypertension stratified by BMI at baseline and the first follow-up
| Overall obesity at baseline | Overall obesity at the first follow-up | Cumulative incidence % (n) | HR (95%CI) | |
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| yes | no | 28.6 (47) | 1.00 | 1.00 |
| yes | yes | 33.7 (141) | 1.25 (0.79-1.99) | 1.29 (0.82-2.06) |
| no | no | 20.9 (388) | 1.00 | 1.00 |
| no | yes | 29.8 (84) | 1.58 (1.19-2.10)* | 1.67 (1.24-2.26)* |
CI, confidence interval; HR, hazard ratio; N, number of cases./Model : Only BMI was included as independent variable./Model 2: Adjusted for sex, age, TC, HDL-C, LDL-C, FPG, TG, smoke and alcohol status, family history of hypertension, SBP and DBP./No multicollinearity was found among triglycerides, HDL-C, total cholesterol and LDL-C, because tolerance values were >0.5, VIF<10./* Significantly greater odds, P<0.05
Table 5.
Cumulative incidence and hazard ratio of hypertension stratified by whether WC and/or BMI were modified
| BMI was modified | WC was modified | Cumulative incidence n (%) | HR (95%CI) | |
|---|---|---|---|---|
| Model 1 | Model 2 | |||
| Males (n=1097) | ||||
| yes | yes | 166 (19.1) | 1.00 | 1.00 |
| no | yes | 28 (28.4) | 1.24 (0.78-2.12) | 1.13 (0.68-2.99) |
| yes | no | 11 (45.1) | 2.71 (1.61-4.69) ** | 3.83 (1.56-10.15) ** |
| no | no | 49 (38.5) | 2.41 (1.49-3.83) ** | 2.21 (1.11-4.89) ** |
| Females (n=1681) | ||||
| yes | yes | 147 (16.0) | 1.00 | 1.00 |
| no | yes | 12 (23.6) | 1.44 (0.91-2.35) | 1.16 (0.85-5.97) |
| yes | no | 111 (35.8) | 2.37 (1.81-3.09) ** | 1.59 (1.16-3.68) * |
| no | no | 136 (33.3) | 3.21 (2.38-4.29) ** | 2.53 (1.48-4.14) ** |
CI, confidence interval; HR, hazard ratio; N, number of cases./Model 1: Only WC and BMI were included as independent variables./Model 2: Adjusted for age, TC, HDL-C, LDL-C, FPG, TG, smoke and alcohol status, family history of hypertension, SBP and DBP./No multicollinearity was found among triglycerides, HDL-C, total cholesterol and LDL-C, because tolerance values were >0.5, VIF<10./*Significantly greater odds, P < 0.05./**Significantly greater odds, P < 0.01.
Discussion
The results of this study showed that for every 1.0 kg/m2 increase in BMI, the odds of developing hypertension increased by 9.0% and 3.0% in males and females, respectively, and that for every 1.0 cm increase in WC, the odds of developing hypertension increased by 4.0% and 3.0% in males and females, respectively. Regardless of whether the WC or BMI was abnormal at baseline, compared with subjects with a normal WC or BMI at the first follow-up, the incidence rate of hypertension was significantly higher for subjects with an abnormal WC or BMI at the first follow-up. These results indicate that the hypertension risk of obese subjects would decrease if they reduce their WC or BMI and that the hypertension risk of subjects with a normal WC or BMI would increase if their WC or BMI becomes abnormal. During follow-up in cohort studies, the WC or BMI of subjects can change due to lifestyle modification or a targeted intervention. The WC or BMI of obese subjects can return to normal, and subjects with a normal WC or BMI at baseline can become obese between baseline and the first follow-up. The impact of such a change on hypertension incidence, however have only been shown in clinical trials (10–12). The changes in WC and BMI reflect changes in the distribution and composition of body fat. In cohort populations, the WC could decrease when BMI increases and the WC could increase when BMI decreases, or both the WC and BMI could be maintained at a normal level similar to the values found at baseline. Therefore, when we evaluate the impact of obesity control on blood pressure, it should be determined whether WC or BMI is the more practical and sensitive indicator. The results from most of the cross-sectional studies have shown a stronger association of hypertension prevalence with central obesity (as measured by WC) than with general obesity (as measured by BMI) in different ethnic groups (18–21). A previous study suggested that WC was indeed a better predictor of visceral adipose tissue than BMI (22). Siani et al. (23) reached the conclusion that the relationship between WC and BP was unaffected by adjustment for BMI; in contrast, the correlation between BMI and BP was no longer significant when WC was modified. The observational study conducted by Tseng et al. (24) with 1183 type 2 diabetes patients showed that the metabolic disease risk was higher in subjects with a normal BMI but an abnormal WC than that in subjects with an abnormal BMI but a normal WC. Alberts et al. (25) reported that WC was the strongest obesity indicator related to hypertension. Janssen et al (26) indicated that there was no difference in the health risk between overweight and obese subjects with same WC, but that the health risk of subjects with the same BMI increased with WC; these studies confirmed that the impact of WC on hypertension risk factors was greater than that of BMI. In the current study, a low level of multicollinearity was found between the WC D-value and the BMI D-value (the tolerance value was 0.63 and the VIF was 1.58); therefore, when the WC D-value and the BMI D-value were included as continuous variables in the same regression model, the change in WC was better correlated with hypertension than the change in BMI. Indeed, the association between the BMI D-value and hypertension was no longer statistically significant when the WC D-value was modified. The hypertension risk decreased significantly if the WC changed from abnormal to normal, but a decreased risk of hypertension was not observed when the BMI changed from abnormal to normal. There was no significant difference in the hypertension risk between the group in which both WC and BMI were modified and the group in which WC was modified but BMI was categorically increasing. However, the hypertension risk in subjects whose WC was categorically increasing but whose BMI was modified was higher than that found in subjects whose WC and BMI were both modified. These results suggest that the impact of changes in the WC on hypertension risk was greater than that of changes in the BMI dynamic.
There are some limitations in our study. The ratio of men to women was not ideal: there were more women than men included in the analysis. In addition, it would have been prudent to stratify the population into ten-year age groups and to consider the length of time subjects were obese for. Thirdly, data on serum levels of uric acid and creatinine (or estimated glomerular filtration rate) are not available in our study, so we cannot adjust these variables in the multivariate analysis. In addition, the diet and physical activity are the important factors for incident hypertension, but these factors were not included in the analysis.
Conclusion
We discussed the impact of increases or decreases in WC and BMI on hypertension risk. Based on our data, there is evidence that changes in WC are better predictors of the risk for hypertension than changes in BMI. Intervention programs designed to reduce WC through lifestyle modifications including exercise and diet may have significant public health significance in reducing the incidence of hypertension.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgments
This study was supported by the Center for Disease Control and Prevention (CDC) of Jiangsu province and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The study team also thanks the investigators and staffs of the coordinating centers at the CDC of Nanjing, CDC of Xuzhou, CDC of Suzhou, CDC of Changshu, and the CDCs of Jintan, Jiangyin, Taicang, Haimen, Jurong, Suining, Si-hong and Gan Yu. The Authors declare that there is no conflict of interest.
References
- Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY (2003). Visceral Adiposity and the Prevalence of Hypertension in Japanese Americans. Circulation, 108:1718–1723. [DOI] [PubMed] [Google Scholar]
- Bosy-Westphal A, Geisler C, Onur S, Korth O, Selberg O, Schrezenmeir J (2006). Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes, 30:475–83. [DOI] [PubMed] [Google Scholar]
- de Koning L, Merchant AT, Pogue J, Anand SS (2007). Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J, 28: 850–856. [DOI] [PubMed] [Google Scholar]
- Wiklund P, Toss F, Weinehall L, Hallmans G, Franks PW, Nordström A, Nordström P (2008). Abdominal and Gynoid Fat Mass Are Associated with Cardiovascular Risk Factors in Men and Women. J Clin Endocrinol Metab, 93:4360–4366. [DOI] [PubMed] [Google Scholar]
- Ito H, Nakasuga K, Ohshima A, Maruyama T, Kaji Y, Harada M, Fukunaga M, Jingu S, Sakamoto M (2003). Detection of cardiovascular risk factors by indices of obesity obtained from anthropometry and dual-energy x-ray absorptiometry in Japanese individuals. Int J Obes Relat Metab Disord, 27:232–237. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB (1999). How useful is body mass index for comparison of body fatness across age, sex, andethnic Groups? Am J Epid, 143:228–239. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Faith MS, Allison DB, Gallagber D, Chiumello G, Heymsfield SB (1998). Body mass index as a measure of adiposity among children and adolescents: a vaildation study. J Pediatrics, 132:204–210. [DOI] [PubMed] [Google Scholar]
- Rankinen T, Kim SY, Perusse L, Despres JP, Bouchard C (1999). The prediction of abdominal visceral fat level from body composition and anthropometry: ROC analysis. Int J Obes Relat Metab Disord, 23:801–9. [DOI] [PubMed] [Google Scholar]
- Molarius A, Seidell JC (1998). Selection of anthropometric indicators for classification of abdominal fatness-a critical review. Int J Obes Relat Metab Disord, 22:719–27. [DOI] [PubMed] [Google Scholar]
- Han TS, Richmond P, Avenel A, Lean MEJ (1997). Waist circumference reduction and cardiovascular benefits during weight loss in women. Int J Obesity, 21:127–134. [DOI] [PubMed] [Google Scholar]
- Villareal DT, Miller BV III, Banks M, Fontana L, Sinacore DR, Klein S (2006). Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr, 84:1317–1323. [DOI] [PubMed] [Google Scholar]
- Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J (2005). Effects of Weight Loss in Overweight or Obese Individuals and Long Term Hypertension Outcomes. A Systematic Review. Hypertension, 45:1035–1041. [DOI] [PubMed] [Google Scholar]
- Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee (1995). World Health Organ Tech Rep Ser, 854:1–452. [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem, 18:499–502. [PubMed] [Google Scholar]
- International Diabetes Federation. Worldwide definitions of the metabolic syndrome. Available: http://www.idf.org/webdata/docs/MetS_def_update2006.pdf (accessed 02.June.07).
- Report of a WHO Consultation (2000). Obesity: Preventing and managing the global epidemic. WHO Technical Report Series, Geneva, 894. [PubMed] [Google Scholar]
- World Health Organization (1999). International Society of Hypertension guidelines for the management of hypertension. GuidelinesSub-committee. J Hypertens, 17: 151–183. [PubMed] [Google Scholar]
- Hsieh SD, Muto T (2006). Metabolic syndrome in Japanese men and women with special reference to the anthropometric criteria for the assessment of obesity: proposal to use the waist-to-height ratio. Prev Med, 42:135–139. [DOI] [PubMed] [Google Scholar]
- Aekplakorn W, Kosulwat V (2006), Suriyawongpaisal P. Obesity indices and cardiovascular risk factors in Thai adults. Int J Obes, 30:1782–1790. [DOI] [PubMed] [Google Scholar]
- Dobbelsteyn CJ, Joffres MR, MacLean DR, Flowerdew G (2001). A comparative evaluation of waist circumference, waist-to-hip ratio and body mass index as indicators of cardiovascular risk factors. The Canadian Heart Health Surveys. Int J Obes Relat Metab Disord, 25:652–661. [DOI] [PubMed] [Google Scholar]
- Dalton M, Cameron AJ, Zimmet PZ, Shaw JE, Jolley D, Dunstan DW, Welborn TA, AusDiab Steering Committee (2003). Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med, 254:555–563. [DOI] [PubMed] [Google Scholar]
- Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ (1994). Waist circumference and abdominal sagittal diameter: best simple an-thropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol, 73:460–468. [DOI] [PubMed] [Google Scholar]
- Siani A, Cappuccio FP, Barba G, Trevisan M, Farinaro E, Lacone R, Russo O, Russo P, Mancini M, Strazzullo P (2002). The Relationship of Waist Circumference to Blood Pressure: The Olivetti Heart Study. Am J Hypertens, 15:780–786. [DOI] [PubMed] [Google Scholar]
- Tseng CH (2006). Body mass index and waist circumference as determinants of coronary artery disease in Taiwanese adults with type 2 diabetes mellitus. Int J Obes, 30:816–821. [DOI] [PubMed] [Google Scholar]
- Grievink L, Alberts JF, O'Niel J, Gerstenbluth I (2004). Waist circumference as a measurement of obesity in the Netherlands Antilles: associations with hypertension and diabetes mellitus. Eur J Clin Nutr, 58:1159–1165. [DOI] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R (2004). Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr, 79: 379–384. [DOI] [PubMed] [Google Scholar]