Abstract
In this study, we propose an organic solvent-free, one-step mechanochemistry approach to engineer water-dispersible graphene oxide/superparamagnetic iron oxide (GO/SPIOs) hybrids, for biomedical applications. Although mechanochemistry has been proposed in the graphene field for applications such as drug loading, exfoliation or polymer-composite formation, this is the first study to report mechanochemistry for preparation of GO/SPIOs hybrids. The statistical design of experiment (DoE) was employed to control the process parameters. DoE has been used to control formulation processes of other types of nanomaterials. The implementation of DoE for controlling the formulation processes of graphene-based nanomaterials is, however, novel. DoE approach could be of advantage as one can tailor GO-based hybrids of predicted yields and compositions. Hybrids were characterized by TEM, AFM FT-IR, Raman spectroscopy, and TGA. The dose–response magnetic resonance (MR) properties were confirmed by MR imaging of phantoms. The biocompatibility of the hybrids with A549 and J774 cell lines was confirmed by the modified LDH assay.
Keywords: graphene, mechanochemistry, DoE, SPIO, formulation, nanomedicine, drug delivery, toxicity
Graphene is a single layer carbon sheet (∼1 nm/layer) with sp2 hybridization.1 When oxidized to graphene oxide (GO), the material becomes water dispersible, which is a favorable property particularly in biomedicine. The nanometer-range dimension and large surface areas of GO provide great potential for targeted drug delivery and tissue engineering.2,3 GO can also be covalently or noncovalently functionalized to provide additional features.4 For example, GO hybridized with superparamagnetic iron oxide nanoparticles (SPIOs) has been reported as a theranostic platform (a single therapeutic and diagnostic system),5 as a magnetic resonance imaging (MRI)/photoacoustic (PA) multimodal imaging system,6 and as magnetically targeted nanocarriers capable of localized hyperthermia.7
Traditionally, the hybridization of GO with iron oxide nanoparticles has been reported using in situ synthesis,8 direct graphitization of oleic acid coated iron oxide nanoparticles,9 precipitation methods,10−12 solvent-thermal method by autoclaving at 180 °C for 4–16 h,13 direct mixing with heating at 98 °C,14 and microwave-heated synthesis.15 However, all of these methods involve the use of multistep processing,8 organic solvents,8,12−15 or external-heating.8−10,13−15 An organic solvent-free, one-step process for formation of GO/SPIOs is still to be found. Mechanochemistry, however, has been proposed in the graphene field to exfoliate graphite with16 or without drug loading,17−21 to synthesize GO from graphite via oxidation reactions,22,23 to functionalize graphene,24−28 or to synthesize various graphene/graphite-polymer composites.29−33 Mechanical force provides an alternative source of energy to control chemical reactivity that is usually achieved by thermal and chemical treatment.34 Herein, we propose a one-step mechanochemistry approach to produce water-dispersible GO/SPIO hybrids, using a high-speed vibrational ball-mill. The process is deemed superior to previously reported methods, as it is simple and is organic solvent-free. Furthermore, no heating step is required. It is hypothesized in this study that the mechanical force, an alternative source to heat, can enhance the interaction between oleic acid coated SPIOs and GO, forming water-dispersible hybrids. In brief, freeze-dried GO and oleic acid coated hydrophobic SPIOs were loaded into the milling jar, as dry powders, and dry milled for 1–2 h at 10–20 Hz. The control over the mechanical force and process parameters was achieved by implementation of the statistical design of experiment (DoE) (Figure 1A), as will be described later. GO or SPIOs were milled alone as controls. Oleic acid coated SPIOs, milled in absence of GO remained hydrophobic and sedimented, from aqueous dispersions, upon standing. GO remained water-dispersible before and after ball milling and did not respond to the magnet. The water-dispersible hybrids were separated from hydrophobic SPIOs and GO, by leaving the dispersion to stand for 2 min, followed by magnetic separation, respectively, as described in the methods section. Figure 1B shows the black dispersion of ball-milled GO/SPIO hybrids, which turned transparent within 30 s of exposure to the magnet, indicating the magnetic responsiveness of the hybrid.
Figure 1.
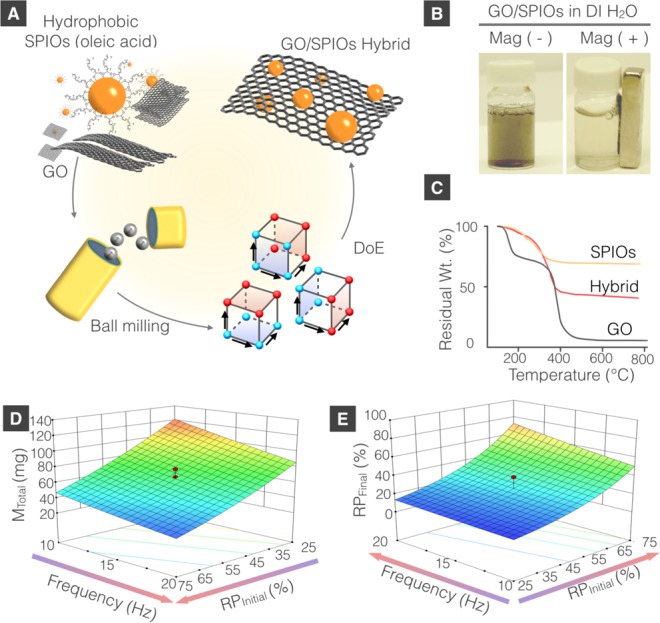
Engineering graphene-based magnetic responsive hybrids using mechanochemistry. (A) Schematic representation of GO/SPIO hybrid formulation. (B) Images of the water-dispersible GO/SPIO hybrid before and after exposure to a magnet (rated 0.42 T on its surface). (C) Representative thermogravimetric curves of GO, SPIOs, and GO/SPIO hybrid. The 3D response surface at center points (1.5 h) for (D) hybrid yield (MTotal) and (E) GO/SPIOs wt/wt % (RPFinal).
The total mass MTotal (SPIOs + GO) (mg) and the composition of the hybrid i.e. the final relative weight percentage of GO/SPIOs (RPFinal) were measured by thermogravimetric analysis (TGA). As illustrated in Figure 1C, GO decomposes completely at 800 °C, in compressed air. Oleic acid coated SPIOs demonstrated a weight loss of 31.48 ± 0.01% at 800 °C (n = 3), due to oleic acid combustion. Ball-milled GO/SPIO hybrids, however, showed a weight loss of 34–69% at 800 °C, indicating the presence of GO and SPIOs in the sample.
DoE constitutes an efficient procedure for planning experiments.35 It offers the advantage of being able to investigate the effect of two or more factors, on a system, while keeping the number of runs minimal. As a result, DoE offers advantages of obtaining the same amount of information while keeping the number of experimental conditions minimal, without losing precision. A full 2-level factorial DoE can investigate the effect of three factors, i.e., variables, 2-factor interactions (2FI), and 3-factor interactions (3FI) on the responses, from only 8 experiments. Implementation of DoE for controlling the formulation processes of other types of nanocarriers such as liposomes,36−38 nanosuspension,39 and solid lipid nanoparticle40 have been reported. There are no studies, however, which have employed this approach in processing graphene-based nanomaterials. To better understand the impact of formulation and process parameters, on GO/SPIO hybrid system formulation, DoE with a 23 (2-level 3 factors) full factorial design with replicated center points was introduced.41 Three factors were included in the design: milling frequency in Hz (A), milling time in h (B), and the initial relative weight percentage of GO/SPIOs (RPInitial), being loaded into the milling jar (C). Actual values of each factor were represented in coded pattern, i.e., +1 (high level) and −1 (low level) (Table S1 in the Supporting Information). Responses include the total weight of the hybrids in mg (MTotal) and RPFinal. MTotal represents the yield whereas RPFinal reflects the content of SPIOs within the hybrid. The design layout and response values are summarized in Table 1.
Table 1. Full Two-Level, Three-Factor Factorial Design.
| factor |
response |
||||
|---|---|---|---|---|---|
| std | Aa (Hz) | Bb (h) | Cc (%) | MTotald (mg) | RPFinale (%) |
| 1 | 10 | 1 | 25 | 107.10 | 9.77 |
| 2 | 20 | 1 | 25 | 77.53 | 10.41 |
| 3 | 10 | 2 | 25 | 125.71 | 8.63 |
| 4 | 20 | 2 | 25 | 95.83 | 14.04 |
| 5 | 10 | 1 | 75 | 44.50 | 32.60 |
| 6 | 20 | 1 | 75 | 20.46 | 61.42 |
| 7 | 10 | 2 | 75 | 50.15 | 62.64 |
| 8 | 20 | 2 | 75 | 26.82 | 81.74 |
| 9 | 15 | 1.5 | 50 | 66.98 | 24.16 |
| 10 | 15 | 1.5 | 50 | 64.13 | 38.82 |
| 11 | 15 | 1.5 | 50 | 77.19 | 25.94 |
Milling frequency in Hz.
Milling time in h.
Initial GO/SPIOs wt/wt %.
Total hybrid weight in mg.
Final GO/SPIOs wt/wt % in the hybrid.
The effect of factors, 2FI and 3FI on MTotal and RPFinal were calculated using eq S1 (Figure S1 and Table S2 in the Supporting Information). Detailed information on the effect evaluations and the establishment of predictive equations are described in the Supporting Information, methods and Figures S2–S5.
The final fitted model for the MTotal with the coded factors of milling frequency (A), milling time (B), and RPInitial (C) is shown in eq 1. A good correlation was obtained between the observed and predicted values as indicated by the R2 value of 0.9795, adjusted R2 value of 0.9707 and predicted R2 value of 0.9578. In brief, milling frequency, milling time and the RPInitial significantly influenced the response MTotal (p-value <0.05). RPInitial (C) was the dominating factor toward MTotal, as it exhibited the largest coefficient compared to A and B. Higher milling frequency (A) and higher RPInitial (C) resulted in a lower MTotal, as indicated by their negative coefficients. Longer milling time (B), however, increased MTotal, as indicated by its positive coefficient. The predictive equation of coded and actual factors is represented in eq 2. A comparison of the predicted and actual (experimental) values for MTotal is shown in Figure S3 in the Supporting Information. A 3D factorial response surface was produced for MTotal according to the predictive eqs 1 and 2 and is shown in Figure 1D.
| 1 |
| 2 |
The final fitted model for the RPFinal with the coded factors of milling frequency (A), milling time (B), and RPInitial (C) is shown in eq 3. A good correlation was obtained between the observed and predicted values as indicated by the R2 value of 0.9438, adjusted R2 value of 0.9197 and predicted R2 value of 0.8662. In brief, higher milling frequency, higher RPInitial and longer milling time all resulted in a higher RPFinal as reflected by their positive coefficient. The predictive equation of coded and actual factors is represented in eq 4. A comparison of the predicted and actual (experimental) values for RPFinal is shown in Figure S5 in the Supporting Information. A 3D factorial response surface was produced for RPFinal according to the predictive eqs 3 and 4 and is shown in Figure 1E.
| 3 |
| 4 |
Using DoE, it was established that a higher initial SPIOs loading, i.e., lower RPInitial value, and longer milling time resulted in hybrids of higher SPIOs content, i.e., lower RPFinal value (Figure 1E). Higher milling frequency adversely affected both MTotal and SPIOs content in the hybrids (Figure 1D, E). From these studies, it was concluded that the DoE approach could be reliably implemented in the process of formulating GO-SPIOs hybrids.
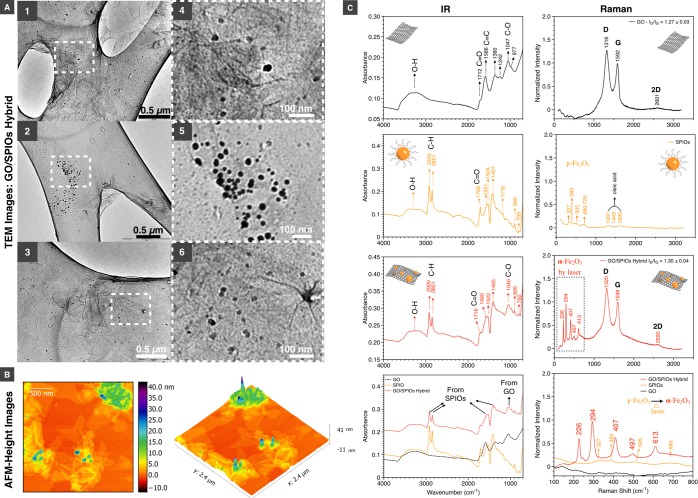
Morphological examination of GO and GO/SPIO hybrids was performed using transmission electron microscopy (TEM) and atomic force microscopy (AFM). The TEM and AFM images of GO sheets are shown in Figures S6 and S7 in the Supportng Information, respectively. The height of the GO sheets, as shown with AFM, is ∼1 nm confirming the formation of single-layer GO sheets. The size (surface area) of GO was generally small, with a medium area of 181.35 nm2 and an average of 441 nm2 (n = 438) (Figure S7 in the Supporting Information). TEM images of the GO/SPIO hybrids showed electron-dense structures, typical of SPIOs, associated with GO sheets confirming the formation of GO/SPIO hybrids (Figure 2A). AFM images showed height between 10 and 20 nm, which agreed with the theoretical size of the SPIOs (Figure 2B and Figure S8 in the Supporting Information). Some larger SPIOs aggregates could be also found (Figure S9 in the Supporting Information). This was expected as the milling processing occurred under dry conditions. The breaking of the SPIOs aggregates is dependent on the process-capability of the ball-mill, which is one of the limitations of this approach.
Figure 2.
Characterization of GO/SPIOs hybrid. (A 1–3) TEM images of GO/SPIO hybrids on lacey carbon supports are shown. SPIOs were found as scattered black spots due to their higher electron density. (A 4–6) The 4× magnification of selected areas from A 1–3. (B) 2D and 3D AFM height images are shown. The height of the SPIOs is around 10–20 nm. (C) IR and Raman spectra of starting materials and the hybrid.
In our study, stabilization of GO-SPIOs hybrids, upon ball-milling, could be a result of enhanced hydrophobic or π–π interactions between the oleic acid coated SPIOs and aromatic rings of GO. Alternatively, GO’s oxygen-containing functional groups, i.e., COOH could interact with SPIOs, mimicking the ligand exchange process. Mechanical force has been reported to exchange the ligand on oleic acid coated iron oxide nanoparticles with poly(acrylic acid), polyethylenimine, and glutathione.42 A slight to moderate increase in temperatures inside the milling jar may have occurred. Temperatures, however, remained lower (<40 °C) than those reported in previous studies (>95 °C), used to formulate GO/SPIOs hybrids.8−10,13,14 Avoiding high-temperature is of critical importance when conjugating biomolecules i.e., targeting peptides or antibodies.
IR and Raman spectra of GO, SPIOs and the hybrids are shown in Figure 2C. The C–H stretching vibrations, at 2920 and 2851 cm–1, corresponding to the oleic acid coating can be seen in both SPIOs and GO/SPIOs. The presence of oleic acid in GO/SPIO hybrids may have facilitated flocculation overtime (Figure 1B, left panel). Future work could involve PEGylation of GO/SPIO hybrid to improve physical stability. Characteristic D and G bands can be seen in GO and GO/SPIOs hybrids with ID/IG peak-height ratios of 1.30 ± 0.04 and 1.27 ± 0.04, respectively (p > 0.05, n = 3). Both IR and Raman spectra confirmed the formation of the hybrid. In addition to mechanical forces, it is anticipated that the mild milling may result in local heat generation, at the nanointerface, which is required to enhance hydrophobic or π–π interactions forming the hybrid. The weak but characteristic Raman peaks of γ-Fe2O3 (maghemite) can be seen at 327, 383, 505, and 680–720 cm–1 (Figure 2C Raman spectra). Under the same laser power (1%), these peaks were difficult to be visualized in GO/SPIOs hybrids, as shown in Figure S10 in the Supporting Information.
When samples were preheated with higher power laser (50%) during Raman measurement, the appearance of 5 peaks, characteristic of α-Fe2O3 at 226, 294, 407, 497, 613 cm–1, could be seen in GO/SPIO hybrids (Figure 2C and the Raman spectra in FIgure S10 in the Supporting Information). It is known that Fe3O4 and γ-Fe2O3 (maghemite) can undergo phase-transformation to α-Fe2O3 (hematite), which exhibits different Raman spectrum. This transformation can be artificially triggered when heated by laser.43 Raman spectroscopy did not only confirm the formation of the hybrid but also suggested that if heat was generated during the milling process, then the amount produced was not sufficient to induce transformation of γ-Fe2O3 to α-Fe2O3. The Raman spectra of SPIOs and GO/SPIO hybrids measured using different laser power are shown in Figure S-10.
In vitro toxicity of the GO/SPIO hybrids was also evaluated using A549 cells (adenocarcinoma human alveolar basal epithelial cells) and J774 cells (murine macrophages). Optical microscopy images of A549 and J774 cells, incubated with GO/SPIO hybrids, were captured after 24 and 72 h. GO/SPIO hybrids are expressed based on SPIOs concentration. Cells incubated with GO/SPIO hybrids showed normal morphology at all concentrations tested while cells treated with dimethyl sulfoxide (DMSO), used as a positive control, appeared unhealthy and detached from the plate. The extent of GO/SPIO hybrids taken up by cells was concentration- and time-dependent (Figure S11 in the Supporting Information).
The modified lactate dehydrogenase (mLDH) assay was used to assess the cytotoxicity of GO/SPIO hybrids on A549 cells and J774 cells using the method described in the methods in the Supporting Information.44 Cells were incubated with the hybrids at 10, 25, 50, and 100 μg/mL for 24 and 72 h. DMSO resulted in viability of 2.2% and 1.80% for A549 and J774 cells, respectively. The cytotoxicity result from the mLDH assay is shown in Figure 3. No statistically significant effect on cell viability was observed in all treated groups up to 100 μg/mL for 72 h. Reported studies in the literature showed no significant toxicity for SPIOs or GO up to 100 μg/mL.45,46
Figure 3.
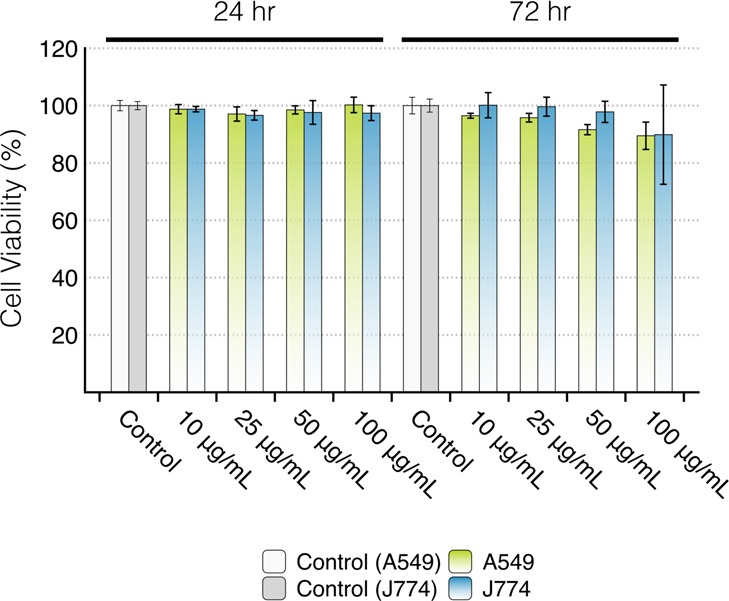
Modified LDH assay of A549 and J774 cells incubated with GO/SPIO hybrids. The A549 and J774 cells were incubated with GO/SPIO hybrids at SPIOs concentrations of 10, 25, 50, and 100 μg/mL for 24 and 72 h. The toxicity was assessed using the modified LDH assay. No significant effect on cell viability was observed.
To demonstrate the imaging potential of the GO-SPIOs hybrids, their MR contrast properties were determined by an in vitro MR phantom study using a 7T MR scanner. The GO/SPIOs hybrid samples were suspended in 1% agarose to mimic the tissue-like environment in vivo.47 Their transverse (T2) relaxation times were measured at increasing Fe concentrations and the R2 (1/T2) values were calculated. The GO/SPIOs hybrids exhibited a concentration-dependent darkening effect with an r2 relaxivity of 144 mMs–1 (Figure 4A, B). The obtained values are higher than some of those GO-based MRI imaging nanocomposites reported in literature.7,48 The obtained r2 relaxivity was comparable to values reported for the commercially approved MRI contrast agent Feridex (127.47 and 108 mM s–1)49,50 Regional signal reductions were seen with the GO/SPIO hybrids and became more obvious at higher Fe concentrations. The heterogeneous hypointense signals are likely to be due to the inhomogeneous presence of iron oxide in the hybrids as observed in TEM and AFM images. The T2-weighted contrast enhancement, combined with water-dispersibility, made the GO-SPIOs as a potential MR imaging agent in the future.
Figure 4.
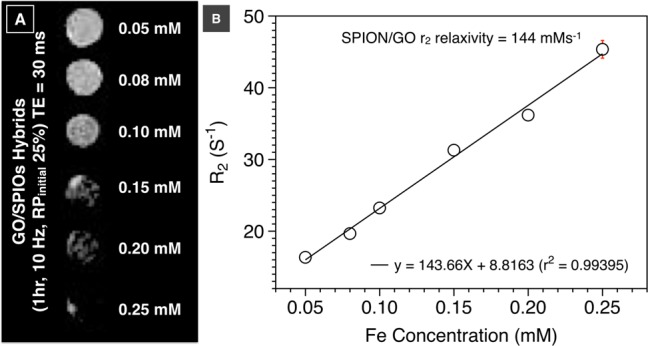
Phantom MR imaging of GO/SPIO hybrids. (A) T2-weighted phantom MR imaging of GO/SPIO hybrids at increasing Fe concentrations. (B) R2 relaxation rate analysis as a function of Fe concentration.
In conclusion, engineering GO/SPIO hybrids using organic solvent-free mechanochemistry is reported for the first time. The mechanochemistry approach replaced the traditional solvent-based and/or external heat-based treatment for GO/SPIO hybridization. The formulation process was rendered eco-friendly, i.e., organic solvent-free without significant heating being produced, as confirmed by Raman spectroscopy. The preparation time was dramatically reduced to a maximum of 1–2 h. The purification step is rapid, simple and only requires a magnet. The resulting GO/SPIO hybrids showed no significant toxicity on A549 and J774 cells at the SPIOs concentrations up to 100 μg/mL and 72 h. Hybrids produced were water-dispersible and showed dose-dependent MR properties. The mechanochemical approach was combined with the DoE, which has been used for the first time, to design GO-based hybrids of predicted yields and compositions. Simple preparative methods combined with predicted design of hybrid nanomaterial provides the foundation for future applications with quality by design (QbD), which is important for clinically relevant materials.51 This approach could be extended to prepare other types of nanohybrids after careful consideration.
Acknowledgments
K.C.M. thanks King’s College London for the Graduate School International Research Award (GSIRA) studentship. Funding from Biotechnology and Biological Sciences Research Council (BB/J008656/1) and FP7-ITN Marie-Curie Network program RADDEL (290023) are acknowledged. J.B. is sponsored by the China Scholarship Council (CSC)-King’s PhD award. H.K. is sponsored by the Atomic Energy Commission of Syria. P.M.C. is a Sir Henry Wellcome Postdoctoral fellow (WT103913).
Supporting Information Available
Experiment materials, methods, and additional figures. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.5b03577.
The authors declare no competing financial interest.
Supplementary Material
References
- Novoselov K. S. Nobel Lecture: Graphene: Materials in the Flatland. Rev. Mod. Phys. 2011, 83, 837–849. [DOI] [PubMed] [Google Scholar]
- Yang K.; Zhang S.; Zhang G.; Sun X.; Lee S.-T.; Liu Z. Graphene in Mice: Ultrahigh in Vivo Tumor Uptake and Efficient Photothermal Therapy. Nano Lett. 2010, 10, 3318–3323. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Nayak T. R.; Hong H.; Cai W. Graphene: a Versatile Nanoplatform for Biomedical Applications. Nanoscale 2012, 4, 3833–3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakilas V.; Otyepka M.; Bourlinos A. B.; Chandra V.; Kim N.; Kemp K. C.; Hobza P.; Zboril R.; Kim K. S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [DOI] [PubMed] [Google Scholar]
- Yang K.; Feng L.; Shi X.; Liu Z. Nano-Graphene in Biomedicine: Theranostic Applications. Chem. Soc. Rev. 2013, 42, 530–547. [DOI] [PubMed] [Google Scholar]
- Yang K.; Hu L.; Ma X.; Ye S.; Cheng L.; Shi X.; Li C.; Li Y.; Liu Z. Multimodal Imaging Guided Photothermal Therapy Using Functionalized Graphene Nanosheets Anchored with Magnetic Nanoparticles. Adv. Mater. 2012, 24, 1868–1872. [DOI] [PubMed] [Google Scholar]
- Ma X.; Tao H.; Yang K.; Feng L.; Cheng L.; Shi X.; Li Y.; Guo L.; Liu Z. A Functionalized Graphene Oxide-Iron Oxide Nanocomposite for Magnetically Targeted Drug Delivery, Photothermal Therapy, and Magnetic Resonance Imaging. Nano Res. 2012, 5, 199–212. [Google Scholar]
- Singh V. K.; Patra M. K.; Manoth M.; Gowd G. S.; Vadera S. R.; Kumar N. In Situ Synthesis of Graphene Oxide and Its Composites with Iron Oxide. New Carbon Materials 2009, 24, 147–152. [Google Scholar]
- Mendes R. G.; Bachmatiuk A.; El-Gendy A. A.; Melkhanova S.; Klingeler R.; Büchner B.; Rümmeli M. H. A Facile Route to Coat Iron Oxide Nanoparticles with Few-Layer Graphene. J. Phys. Chem. C 2012, 116, 23749–23756. [Google Scholar]
- Yang X.; Chen C.; Li J.; Zhao G.; Ren X.; Wang X. Graphene Oxide-Iron Oxide and Reduced Graphene Oxide-Iron Oxide Hybrid Materials for the Removal of Organic and Inorganic Pollutants. RSC Adv. 2012, 2, 8821–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubir N. A.; Yacou C.; Motuzas J.; Zhang X.; Diniz da Costa J. C.. Structural and Functional Investigation of Graphene Oxide–Fe3O4 Nanocomposites for the Heterogeneous Fenton-Like Reaction. Sci. Rep. 2014, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.; Raji A.-R. O.; Nan K.; Peng Z.; Yan Z.; Samuel E. L. G.; Natelson D.; Tour J. M. Iron Oxide Nanoparticle and Graphene Nanoribbon Composite as an Anode Material for High-Performance Li-Ion Batteries. Adv. Funct. Mater. 2013, 24, 2044–2048. [Google Scholar]
- Qian W.; Chen Z.; Cottingham S.; Merrill W. A.; Swartz N. A.; Goforth A. M.; Clare T. L.; Jiao J. Surfactant-Free Hybridization of Transition Metal Oxidenanoparticles with Conductive Graphene for High-Performance Supercapacitor. Green Chem. 2012, 14, 371–377. [Google Scholar]
- Guoxin H.; Xu Z. Monodisperse Iron Oxide Nanoparticle-Reduced Graphene Oxide Composites Formed by Self-Assembly in Aqueous Phase. Fullerenes, Nanotubes and Carbon Nanostructures 2014, 23, 283–289. [Google Scholar]
- Gollavelli G.; Ling Y.-C. Biomaterials. Biomaterials 2012, 33, 2532–2545. [DOI] [PubMed] [Google Scholar]
- Rubio N.; Serra-Maia R.; Kafa H.; Mei K.-C.; Al-Jamal K. T.; Luckhurst W.; Zloh M.; Festy F.; Richardson J. P.; Naglik J. R.; Pach E.; Ballesteros B. Production of Water-Soluble Few-Layer Graphene Mesosheets by Dry Milling with Hydrophobic Drug. Langmuir 2014, 30, 14999–15008. [DOI] [PubMed] [Google Scholar]
- Antisari M.; Montone A.; Jovic N.; Piscopiello E.; Alvani C.; PilloniI L. Low Energy Pure Shear Milling: a Method for the Preparation of Graphite Nano-Sheets. Scripta Materialia 2006, 55, 1047–1050. [Google Scholar]
- Zhao W.; Fang M.; Wu F.; Wu H.; Wang L.; Chen G. Preparation of Graphene by Exfoliation of Graphite Using Wet Ball Milling. J. Mater. Chem. 2010, 20, 5817–5819. [Google Scholar]
- Knieke C.; Berger A.; Voigt M.; Taylor R. N. K.; Röhrl J.; Peukert W. Scalable Production of Graphene Sheets by Mechanical Delamination. Carbon 2010, 48, 3196–3204. [Google Scholar]
- León V.; Quintana M.; Herrero M. A.; Fierro J. L. G.; Hoz A.; de L.; Prato M.; Vázquez E. Few-Layer Graphenes From Ball-Milling of Graphite with Melamine. Chem. Commun. 2011, 47, 10936–10938. [DOI] [PubMed] [Google Scholar]
- Yao Y.; Lin Z.; Li Z.; Song X.; Moon K.-S.; Wong C.-P. Large-Scale Production of Two-Dimensional Nanosheets. J. Mater. Chem. 2012, 22, 13494–13499. [Google Scholar]
- Posudievsky O. Y.; Khazieieva O. A.; Koshechko V. G.; Pokhodenko V. D. Preparation of Graphene Oxide by Solvent-Free Mechanochemical Oxidation of Graphite. J. Mater. Chem. 2012, 22, 12465–12467. [Google Scholar]
- Posudievsky O. Y.; Kozarenko O. A.; Khazieieva O. A.; Koshechko V. G.; Pokhodenko V. D. Ultrasound-Free Preparation of Graphene Oxide From Mechanochemically Oxidized Graphite. J. Mater. Chem. A 2013, 1, 6658–6663. [Google Scholar]
- Yan L.; Lin M.; Zeng C.; Chen Z.; Zhang S.; Zhao X.; Wu A.; Wang Y.; Dai L.; Qu J.; Guo M.; Liu Y. Electroactive and Biocompatible Hydroxyl- Functionalized Graphene by Ball Milling. J. Mater. Chem. 2012, 22, 8367–8371. [Google Scholar]
- Jeon I. Y.; Shin Y. R.; Sohn G. J.; Choi H. J.; Bae S. Y.; Mahmood J.; Jung S. M.; Seo J. M.; Kim M. J.; Wook Chang D.; Dai L.; Baek J. B. Edge-Carboxylated Graphene Nanosheets via Ball Milling. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 5588–5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J.-M.; Jeon I.-Y.; Baek J.-B. Mechanochemically Driven Solid-State Diels–Alder Reaction of Graphite Into Graphene Nanoplatelets. Chem. Sci. 2013, 4, 4273–4277. [Google Scholar]
- Jeon I.-Y.; Choi H.-J.; Jung S.-M.; Seo J.-M.; Kim M.-J.; Dai L.; Baek J.-B. Large-Scale Production of Edge-Selectively Functionalized Graphene Nanoplatelets via Ball Milling and Their Use as Metal-Free Electrocatalysts for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2013, 135, 1386–1393. [DOI] [PubMed] [Google Scholar]
- Jeon I.-Y.; Choi H.-J.; Choi M.; Seo J.-M.; Jung S.-M.; Kim M.-J.; Zhang S.; Zhang L.; Xia Z.; Dai L.; Park N.; Baek J.-B.. Facile, Scalable Synthesis of Edge-Halogenated Graphene Nanoplatelets as Efficient Metal-Free Eletrocatalysts for Oxygen Reduction Reaction. Sci. Rep. 2013, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho D.; Lee S.; Yang G.; Fukushima H.; Drzal L. T. Dynamic Mechanical and Thermal Properties of Phenylethynyl-Terminated Polyimide Composites Reinforced with Expanded Graphite Nanoplatelets. Macromol. Mater. Eng. 2005, 290, 179–187. [Google Scholar]
- Wu H.; Zhao W.; Hu H.; Chen G. One-Step in Situ Ball Milling Synthesis of Polymer-Functionalized Graphene Nanocomposites. J. Mater. Chem. 2011, 21, 8626–8632. [Google Scholar]
- Hubert P. J.; Kathiresan K.; Wakabayashi K. Filler Exfoliation and Dispersion in Polypropylene/as-Received Graphite Nanocomposites via Cryogenic Milling. Polym. Eng. Sci. 2011, 51, 2273–2281. [Google Scholar]
- Raza M. A.; Westwood A. V. K.; Brown A. P.; Stirling C. Composites Science and Technology. Compos. Sci. Technol. 2012, 72, 467–475. [Google Scholar]
- Chatterjee S.; Wang J. W.; Kuo W. S.; Tai N. H.; Salzmann C.; Li W. L.; Hollertz R.; Nüesch F. A.; Chu B. T. T. Chemical Physics Letters. Chem. Phys. Lett. 2012, 531, 6–10. [Google Scholar]
- Piermattei A.; Karthikeyan S.; Sijbesma R. P. Activating Catalysts with Mechanical Force. Nature Chem. 2009, 1, 133–137. [DOI] [PubMed] [Google Scholar]
- Czitrom V. One-Factor-at-a-Time Versus Designed Experiments. American Statistician 1999, 53, 126–126. [Google Scholar]
- Xu X.; Khan M. A.; Burgess D. J. A Quality by Design (QbD) Case Study on Liposomes Containing Hydrophilic API: I. Formulation, Processing Design and Risk Assessment. Int. J. Pharm. 2011, 419, 52–59. [DOI] [PubMed] [Google Scholar]
- Xu X.; Khan M. A.; Burgess D. J. A Quality by Design (QbD) Case Study on Liposomes Containing Hydrophilic API: II. Screening of Critical Variables, and Establishment of Design Space at Laboratory Scale. Int. J. Pharm. 2012, 423, 543–553. [DOI] [PubMed] [Google Scholar]
- Kamal N.; Cutie A. J.; Habib M. J.; Zidan A. S. QbD Approach to Investigate Product and Process Variabilities for Brain Targeting Liposomes. J. Liposome Res. 2014, 1–16. [DOI] [PubMed] [Google Scholar]
- Verma S.; Lan Y.; Gokhale R.; Burgess D. J. Quality by Design Approach to Understand the Process of Nanosuspension Preparation. Int. J. Pharm. 2009, 377, 185–198. [DOI] [PubMed] [Google Scholar]
- Rahman Z.; Zidan A. S.; Khan M. A. Non-Destructive Methods of Characterization of Risperidone Solid Lipid Nanoparticles. Eur. J. Pharm. Biopharm. 2010, 76, 127–137. [DOI] [PubMed] [Google Scholar]
- Box G. E. P.; Hunter J. S.; Hunter W. G.. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; John Wiley & Sons: New York, 2005. [Google Scholar]
- Xu Y.; Qin Y.; Palchoudhury S.; Bao Y. Water-Soluble Iron Oxide Nanoparticles with High Stability and Selective Surface Functionality. Langmuir 2011, 27, 8990–8997. [DOI] [PubMed] [Google Scholar]
- Li Y.-S.; Church J. S.; Woodhead A. L. Infrared and Raman Spectroscopic Studies on Iron Oxide Magnetic Nano-Particles and Their Surface Modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar]
- Ali-Boucetta H.; Al-Jamal K. T.; Kostarelos K.. Cytotoxic Assessment of Carbon Nanotube Interaction with Cell Cultures. In Biomedical Nanotechnology; Hurst S. J., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, 2011; Vol. 726, pp 299–312. [DOI] [PubMed] [Google Scholar]
- Singh N.; Jenkins G. J. S.; Asadi R.; Doak S. H. Potential Toxicity of Superparamagnetic Iron Oxide Nanoparticles (SPION). Nano Rev. 2010, 1, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra A. B.; Paula A. J.; de Lima R.; Alves O. L.; Durán N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [DOI] [PubMed] [Google Scholar]
- Gossuin Y.; Gillis P.; Hocq A.; Vuong Q. L.; Roch A. Magnetic Resonance Relaxation Properties of Superparamagnetic Particles. WIREs Nanomed. Nanobiotechnol. 2009, 1, 299–310. [DOI] [PubMed] [Google Scholar]
- Shen J.-M.; Huang G.; Zhou X.; Zou J.; Yang Y.; Chen Y.-F.; Men S.-K. Safety Evaluation of Graphene Oxide-Based Magnetic Nanocomposites as MRI Contrast Agents and Drug Delivery Vehicles. RSC Adv. 2014, 4, 50464–50477. [Google Scholar]
- Huang J.; Bu L.; Xie J.; Chen K.; Cheng Z.; Li X.; Chen X. Effects of Nanoparticle Size on Cellular Uptake and Liver MRI with Polyvinylpyrrolidone-Coated Iron Oxide Nanoparticles. ACS Nano 2010, 4, 7151–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.-S.; Lee J.-H.; Shin T.-H.; Song H.-T.; Kim E. Y.; Cheon J. Self-Confirming “and” Logic Nanoparticles for Fault-Free MRI. J. Am. Chem. Soc. 2010, 132, 11015–11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. X. Pharmaceutical Quality by Design: Product and Process Development, Understanding, and Control. Pharm. Res. 2008, 25, 781–791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.