Abstract
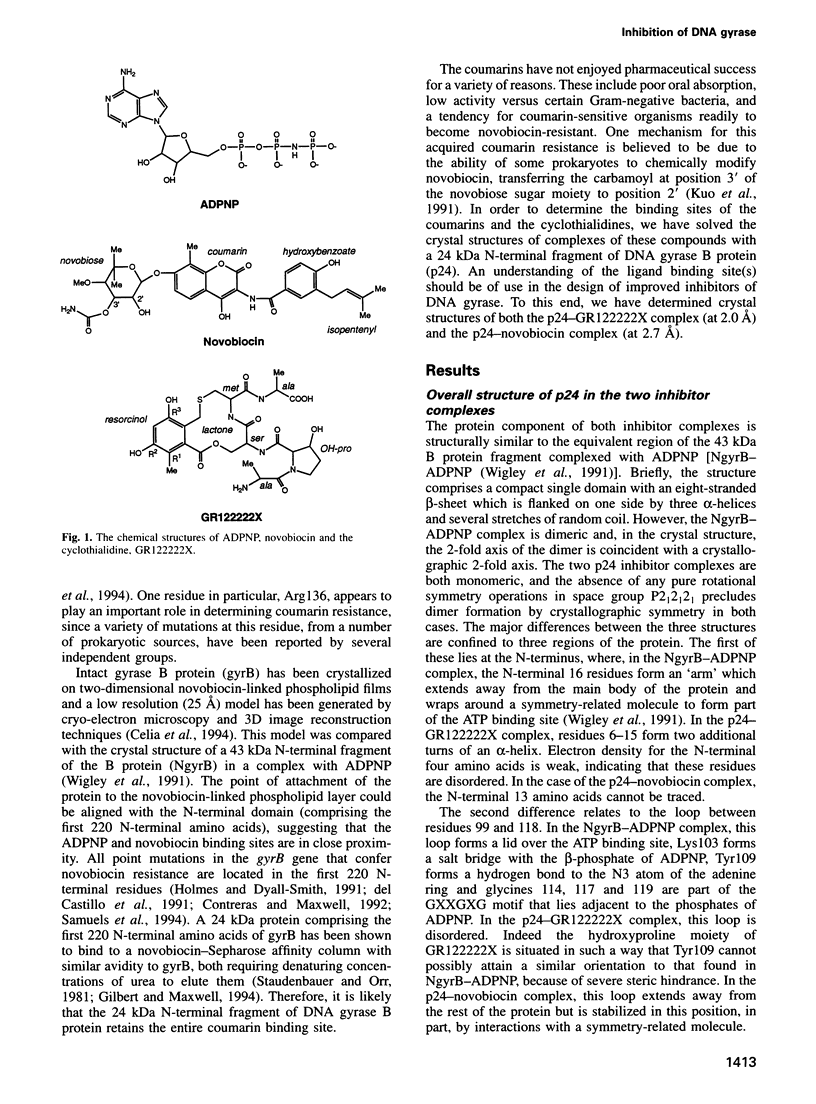
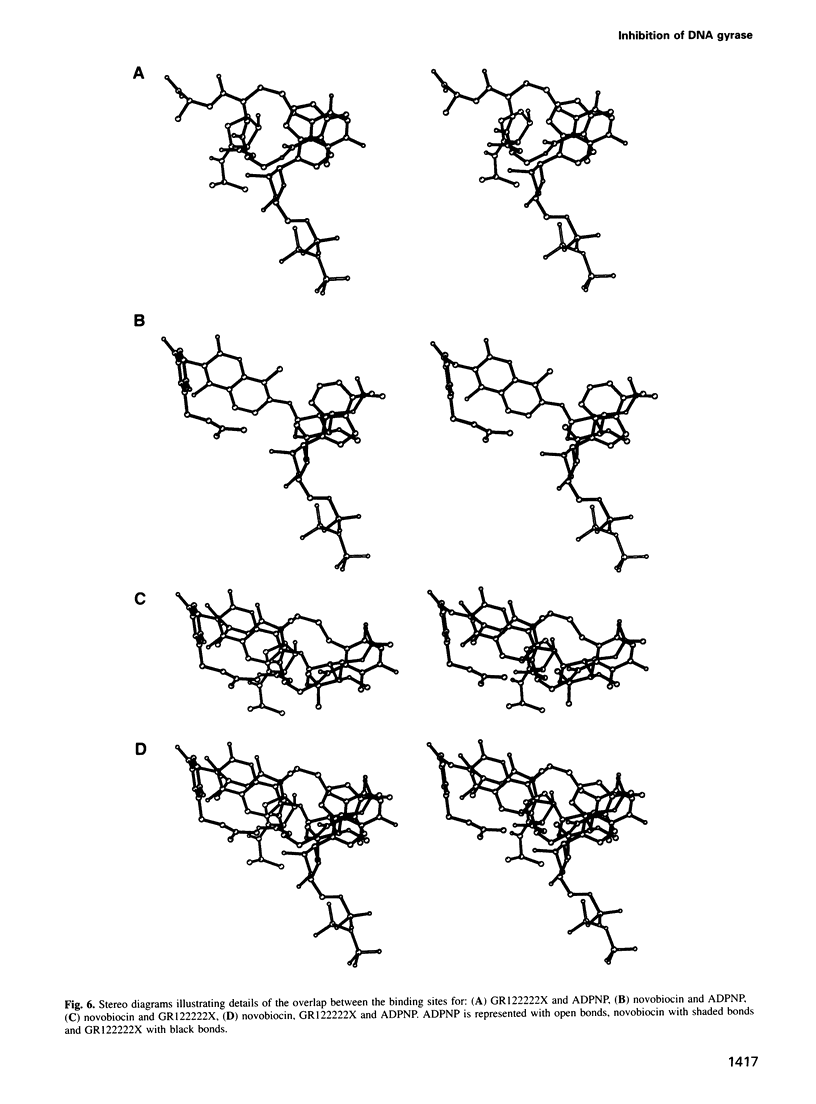
This study describes the first crystal structures of a complex between a DNA topoisomerase and a drug. We present the structures of a 24 kDa N-terminal fragment of the Escherichia coli DNA gyrase B protein in complexes with two different inhibitors of the ATPase activity of DNA gyrase, namely the coumarin antibiotic, novobiocin, and GR122222X, a member of the cyclothialidine family. These structures are compared with the crystal structure of the complex with an ATP analogue, adenylyl-beta-gamma-imidodiphosphate (ADPNP). The likely mechanism, by which mutant gyrase B proteins become resistant to inhibition by novobiocin are discussed in light of these comparisons. The three ligands are quite dissimilar in chemical structure and bind to the protein in very different ways, but their binding is competitive because of a small degree of overlap of their binding sites. These crystal structures consequently describe a chemically well characterized ligand binding surface and provide useful information to assist in the design of novel ligands.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali J. A., Jackson A. P., Howells A. J., Maxwell A. The 43-kilodalton N-terminal fragment of the DNA gyrase B protein hydrolyzes ATP and binds coumarin drugs. Biochemistry. 1993 Mar 16;32(10):2717–2724. doi: 10.1021/bi00061a033. [DOI] [PubMed] [Google Scholar]
- Althaus I. W., Dolak L., Reusser F. Coumarins as inhibitors of bacterial DNA gyrase. J Antibiot (Tokyo) 1988 Mar;41(3):373–376. doi: 10.7164/antibiotics.41.373. [DOI] [PubMed] [Google Scholar]
- Brünger A. T. Assessment of phase accuracy by cross validation: the free R value. Methods and applications. Acta Crystallogr D Biol Crystallogr. 1993 Jan 1;49(Pt 1):24–36. doi: 10.1107/S0907444992007352. [DOI] [PubMed] [Google Scholar]
- Caron P. R., Wang J. C. Appendix. II: Alignment of primary sequences of DNA topoisomerases. Adv Pharmacol. 1994;29B:271–297. doi: 10.1016/s1054-3589(08)61143-6. [DOI] [PubMed] [Google Scholar]
- Celia H., Hoermann L., Schultz P., Lebeau L., Mallouh V., Wigley D. B., Wang J. C., Mioskowski C., Oudet P. Three-dimensional model of Escherichia coli gyrase B subunit crystallized in two-dimensions on novobiocin-linked phospholipid films. J Mol Biol. 1994 Feb 18;236(2):618–628. doi: 10.1006/jmbi.1994.1171. [DOI] [PubMed] [Google Scholar]
- Contreras A., Maxwell A. gyrB mutations which confer coumarin resistance also affect DNA supercoiling and ATP hydrolysis by Escherichia coli DNA gyrase. Mol Microbiol. 1992 Jun;6(12):1617–1624. doi: 10.1111/j.1365-2958.1992.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert E. J., Maxwell A. The 24 kDa N-terminal sub-domain of the DNA gyrase B protein binds coumarin drugs. Mol Microbiol. 1994 May;12(3):365–373. doi: 10.1111/j.1365-2958.1994.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Goetschi E., Angehrn P., Gmuender H., Hebeisen P., Link H., Masciadri R., Nielsen J. Cyclothialidine and its congeners: a new class of DNA gyrase inhibitors. Pharmacol Ther. 1993 Nov;60(2):367–380. doi: 10.1016/0163-7258(93)90017-8. [DOI] [PubMed] [Google Scholar]
- Holmes M. L., Dyall-Smith M. L. Mutations in DNA gyrase result in novobiocin resistance in halophilic archaebacteria. J Bacteriol. 1991 Jan;173(2):642–648. doi: 10.1128/jb.173.2.642-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T., Wang J. C., Harrison S. C. DNA gyrase and its complexes with DNA: direct observation by electron microscopy. Cell. 1985 Jul;41(3):933–943. doi: 10.1016/s0092-8674(85)80074-x. [DOI] [PubMed] [Google Scholar]
- Klevan L., Wang J. C. Deoxyribonucleic acid gyrase-deoxyribonucleic acid complex containing 140 base pairs of deoxyribonucleic acid and an alpha 2 beta 2 protein core. Biochemistry. 1980 Nov 11;19(23):5229–5234. doi: 10.1021/bi00564a012. [DOI] [PubMed] [Google Scholar]
- Krueger S., Zaccai G., Wlodawer A., Langowski J., O'Dea M., Maxwell A., Gellert M. Neutron and light-scattering studies of DNA gyrase and its complex with DNA. J Mol Biol. 1990 Jan 5;211(1):211–220. doi: 10.1016/0022-2836(90)90021-D. [DOI] [PubMed] [Google Scholar]
- Kuo M. S., Yurek D. A., Chirby D. G., Cialdella J. I., Marshall V. P. Microbial O-carbamylation of novobiocin. J Antibiot (Tokyo) 1991 Oct;44(10):1096–1100. doi: 10.7164/antibiotics.44.1096. [DOI] [PubMed] [Google Scholar]
- Lebeau L., Regnier E., Schultz P., Wang J. C., Mioskowski C., Oudet P. Two-dimensional crystallization of DNA gyrase B subunit on specifically designed lipid monolayers. FEBS Lett. 1990 Jul 2;267(1):38–42. doi: 10.1016/0014-5793(90)80282-n. [DOI] [PubMed] [Google Scholar]
- Lewis R. J., Singh O. M., Smith C. V., Maxwell A., Skarzynski T., Wonacott A. J., Wigley D. B. Crystallization of inhibitor complexes of an N-terminal 24 kDa fragment of the DNA gyrase B protein. J Mol Biol. 1994 Aug 5;241(1):128–130. doi: 10.1006/jmbi.1994.1480. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Maxwell A., Gellert M. The DNA dependence of the ATPase activity of DNA gyrase. J Biol Chem. 1984 Dec 10;259(23):14472–14480. [PubMed] [Google Scholar]
- Maxwell A. The interaction between coumarin drugs and DNA gyrase. Mol Microbiol. 1993 Aug;9(4):681–686. doi: 10.1111/j.1365-2958.1993.tb01728.x. [DOI] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983 Aug;34(1):105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., O'Dea M. H., Gellert M. DNA gyrase: subunit structure and ATPase activity of the purified enzyme. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5960–5963. doi: 10.1073/pnas.75.12.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada N., Gmünder H., Hirata T., Arisawa M. Mechanism of inhibition of DNA gyrase by cyclothialidine, a novel DNA gyrase inhibitor. Antimicrob Agents Chemother. 1994 Sep;38(9):1966–1973. doi: 10.1128/aac.38.9.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakada N., Shimada H., Hirata T., Aoki Y., Kamiyama T., Watanabe J., Arisawa M. Biological characterization of cyclothialidine, a new DNA gyrase inhibitor. Antimicrob Agents Chemother. 1993 Dec;37(12):2656–2661. doi: 10.1128/aac.37.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr E., Fairweather N. F., Holland I. B., Pritchard R. H. Isolation and characterisation of a strain carrying a conditional lethal mutation in the cou gene of Escherichia coli K12. Mol Gen Genet. 1979;177(1):103–112. doi: 10.1007/BF00267259. [DOI] [PubMed] [Google Scholar]
- Rau D. C., Gellert M., Thoma F., Maxwell A. Structure of the DNA gyrase-DNA complex as revealed by transient electric dichroism. J Mol Biol. 1987 Feb 5;193(3):555–569. doi: 10.1016/0022-2836(87)90266-x. [DOI] [PubMed] [Google Scholar]
- Reece R. J., Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol. 1991;26(3-4):335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- Reece R. J., Maxwell A. Probing the limits of the DNA breakage-reunion domain of the Escherichia coli DNA gyrase A protein. J Biol Chem. 1991 Feb 25;266(6):3540–3546. [PubMed] [Google Scholar]
- Reece R. J., Maxwell A. The C-terminal domain of the Escherichia coli DNA gyrase A subunit is a DNA-binding protein. Nucleic Acids Res. 1991 Apr 11;19(7):1399–1405. doi: 10.1093/nar/19.7.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusser F., Dolak L. A. Novenamine is the active moiety in novobiocin. J Antibiot (Tokyo) 1986 Feb;39(2):272–274. [PubMed] [Google Scholar]
- Samuels D. S., Marconi R. T., Huang W. M., Garon C. F. gyrB mutations in coumermycin A1-resistant Borrelia burgdorferi. J Bacteriol. 1994 May;176(10):3072–3075. doi: 10.1128/jb.176.10.3072-3075.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Cozzarelli N. R. The intrinsic ATPase of DNA gyrase. J Biol Chem. 1980 Jul 10;255(13):6299–6306. [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S. C. Effect of novobiocin on DNA-dependent DNA polymerases from developing rat brain. Biochim Biophys Acta. 1974 Aug 15;361(1):115–117. doi: 10.1016/0005-2787(74)90214-7. [DOI] [PubMed] [Google Scholar]
- Tamura J. K., Bates A. D., Gellert M. Slow interaction of 5'-adenylyl-beta,gamma-imidodiphosphate with Escherichia coli DNA gyrase. Evidence for cooperativity in nucleotide binding. J Biol Chem. 1992 May 5;267(13):9214–9222. [PubMed] [Google Scholar]
- Tamura J. K., Gellert M. Characterization of the ATP binding site on Escherichia coli DNA gyrase. Affinity labeling of Lys-103 and Lys-110 of the B subunit by pyridoxal 5'-diphospho-5'-adenosine. J Biol Chem. 1990 Dec 5;265(34):21342–21349. [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wigley D. B., Davies G. J., Dodson E. J., Maxwell A., Dodson G. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature. 1991 Jun 20;351(6328):624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- del Castillo I., Vizán J. L., Rodríguez-Sáinz M. C., Moreno F. An unusual mechanism for resistance to the antibiotic coumermycin A1. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8860–8864. doi: 10.1073/pnas.88.19.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]