Abstract
Autism is marked by overgrowth of the brain at the earliest ages but not at older ages when decreases in structural volumes and neuron numbers are observed instead. This has lead to the theory of age-specific anatomic abnormalities in autism. Here we report age-related changes in brain size in autistic and typical subjects from 12 months to 50 years of age based on analyses of 586 longitudinal and cross-sectional MRI scans. This dataset is several times larger than the largest autism study to date. Results demonstrate early brain overgrowth during infancy and the toddler years in autistic boys and girls, followed by an accelerated rate of decline in size and perhaps degeneration from adolescence to late middle age in this disorder. We theorize that underlying these age-specific changes in anatomic abnormalities in autism there may also be age-specific changes in gene expression, molecular, synaptic, cellular and circuit abnormalities. A peak age for detecting and studying the earliest fundamental biological underpinnings of autism is prenatal life and the first three postnatal years. Studies of the older autistic brain may not address original causes but are essential to discovering how best to help the older aging autistic person. Lastly, the theory of age-specific anatomic abnormalities in autism has broad implications for a wide range of work on the disorder including the design, validation and interpretation of animal model, lymphocyte gene expression, brain gene expression, and genotype/CNV-anatomic phenotype studies.
Keywords: Autism, overgrowth, magnetic resonance imaging, pathology, degeneration
1. Introduction
Recent research has lead to the theory of age-specific anatomic abnormalities in autism (Courchesne et al., 2001, 2007; Courchesne & Pierce, 2005) (see Fig. 1). At early ages there is abnormal overgrowth of the brain, but during adolescence and young adulthood there may be abnormal decline and possible degeneration (Fig. 1). Because early abnormal overgrowth occurs at the time of the first detectable behavioral and clinical signs of autism (Pierce et al. 2009, Pierce et al., in review) (Table 1), neural defects that cause overgrowth may be the neural bases of autism.
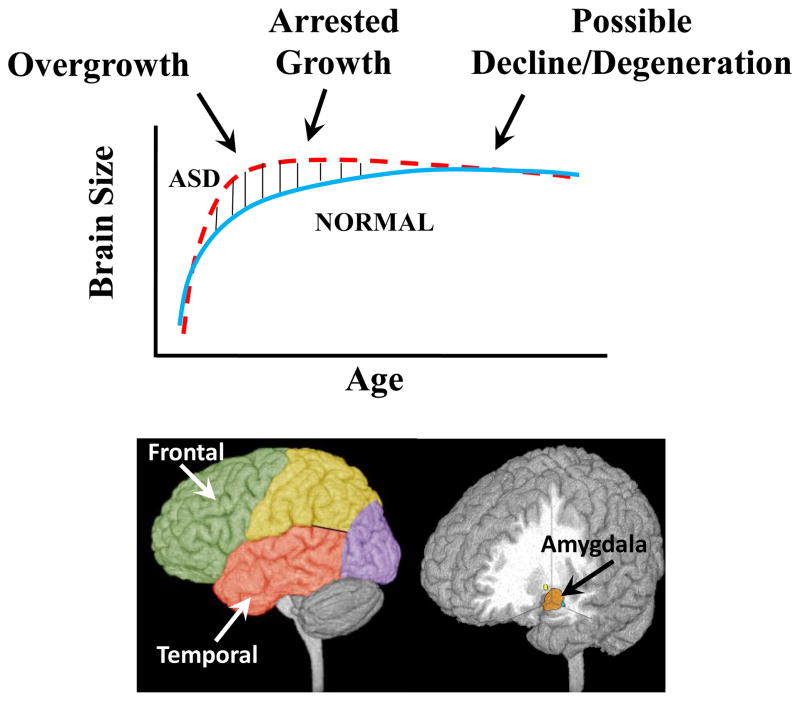
Figure 1.
Three Phases of Growth Pathology in Autism (A) Model of early brain overgrowth that is followed by arrest of growth. Red line represents ASD, while blue line represents age-matched typically developing individuals. In some regions and individuals, the arrest of growth may be followed by degeneration, indicated by the red dashes that slope slightly downward. (B) Sites of regional overgrowth in ASD include frontal and temporal cortices, and amygdala. (from Courchesne et al., 2007)
Table 1.
Red Flags of Autism Spectrum Disorder by 1 to 2 Years of Age.
| Reduced social interest and affect |
| lack of warm, joyful emotional expressions |
| lack of sharing emotional enjoyment or interest |
| lack of response to name |
| lack of showing and interacting |
| Abnormal language development |
| Lack of coordination of gaze, facial expression, gesture & sound during interactions |
This theory was originally based on evidence from four studies in the early 2000s. First, in an MRI study, Courchesne et al. (2001) reported evidence of an unusual brain growth trajectory in autism. They discovered abnormal brain and cerebrum enlargement in autistic 2–4 year olds, but then slightly smaller overall brain volumes by 12 to 16 years of age (Fig. 2). Some subsequent studies also reported brain or cerebral overgrowth in autistic 2 to 4 year olds (Carper et al. 2002; Sparks et al., 2002; Hazlett et al., 2005; Bloss & Courchesne 2007; Schumann et al. 2010), while autistic adolescents and adults are reported to display cortical atrophy (Hadjikhani et al., 2006) and amaygdala (Aylward et al., 1999; Pierce et al., 2004) and frontal cortex volume reduction (Kosaka et al., 2010) (reviews: Amaral et al., 2008; Courchesne et al., 2010). Moreover, meta-analyses of MRI brain volume in the autism literature (Redcay & Courchesne, 2005; Stanfield et al., 2008) and postmortem autistic brain weight (Redcay & Courchesne, 2005) also confirmed early brain overgrowth in autism by 2 to 6 years of age.
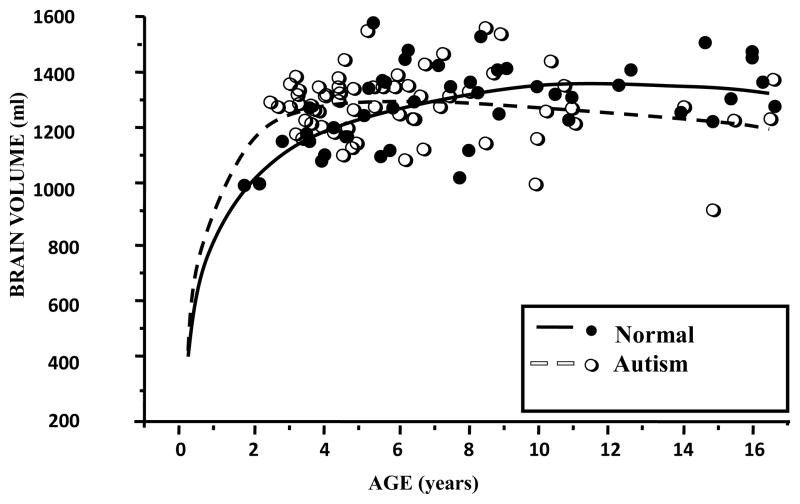
Figure 2.
Brain Growth in Autism through 16 years. Data plot shows individual MRI-based volumes in autistic 2–4 year old males as compared to the average volume in typical 2–4 year old males and smaller overall brain volumes by 8 –16 years of age (from Courchesne et al. 2001).
Second, based on analyses of head circumference (HC), it was discovered that this abnormal brain enlargement is not present at birth in most cases, but instead begins during the first two years of postnatal life (Courchesne et al., 2003). Multiple HC studies since then have also found this early pathological HC overgrowth effect in the first postnatal years (Hazlett et al., 2005, Dementieva et al., 2005, Dissanayake et al., 2006, Dawson et al., 2007, Mraz et al., 2007, Webb et al., 2007, Elder et al., 2008; Fukumoto et al., 2008).
Third, Carper et al (2002) determined that this overgrowth had an important gradient in the cerebrum: greatest in frontal and temporal cortices and least in occipital (Fig. 3). This finding has also been reported in subsequent studies (Schumann et al., 2010; reviews: Courchesne et al., 2007, 2010) (Fig. 3).
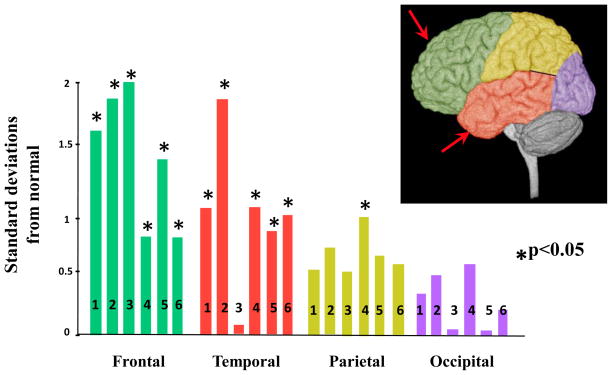
Figure 3.
Gray Matter overgrowth in Autism. The bars represent abnormalities in Different Cerebral Regions (standard deviations from normal average) in Children and Adolescents with ASD. Note the general gradient of abnormality with frontal and temporal regions most abnormally enlarged. References: 1, Carper et al., 2002 3.4 years; 2, Bloss and Courchesne, 2007 3.8 years; 3, Kates et al., 2004 (7.6 years); 4, Palmen et al., 2005 11.1 years; 5, Hazlett et al., 2005 19.1; 6. Schumann et al,. 2010 2–4 years. (From Courchesne et al, 2007)
Finally, Dawson and colleagues (Sparks et al., 2002) discovered overgrowth of the amygdala, a structure vital to emotional processing and memory, in autistic 4 year olds. Subsequent studies have verified and extended those findings showing associations between degree of overgrowth and severity clinical symptoms (Munson et al., 2006, Mosconi et al., 2009, Schumann et al., 2009). However, the underlying neural defects that cause overgrowth in these structures have yet to be identified. Whatever these underlying early defects may be, they will likely explain why autistic behavior develops and provide clues as to the genetic or non-genetic factors that trigger those overgrowth defects.
Thus, many MRI studies have found abnormal early overgrowth in several regions that mediate the development of higher order social, emotion, language and communication abilities, namely frontal and temporal cortices and the amygdala (Reviews: Courchesne et al., 2007, Amaral et al., 2008, Courchesne et al., 2010). Nonetheless, how anatomic pathology alters with age after these early years is little studied. To gain insight into this question, two studies (Redcay & Courchesne, 2005; Stanfield et al., 2008) conducted formal statistical meta-analyses of a large number of separate reports each of which represented a different relatively narrow age window. Both meta-analyses showed statistical evidence suggestive of substantial age-related changes in the degree of deviation from normal in brain size in autism.
However, as recently pointed out (Courchesne et al., 2010), no single study has ever directly examined age-related changes across early life to adulthood in autism because of the difficulty in collecting MRI scans across such a wide age range in autistic and healthy typical subjects. Without direct evidence, age-related differences in pathology at younger versus older ages remain no more than a statistical inference from these meta-analyses.
Here, in the largest autism MRI sample ever analyzed, we report in 2 to 50 year olds the first direct longitudinal and cross-sectional MRI evidence in support of the theory of age-specific anatomical abnormality in autism. We found strikingly different pathological trajectories in brain size during early as compared to later life in autism, with overgrowth evident early one but an accelerated rate of decline marking adult life.
2. Experimental Procedure
To further test the theory of age-specific anatomic abnormalities in autism, we analyzed 586 longitudinal and cross-sectional MRI scans from N= 259 ASD subjects ages 2 to 50 years and N= 327 typical subjects ages 1 to 50 years. This is many times larger than any previous MRI autism data set and incorporates longitudinal MRI evidence from both young as well as much older autism and typically developing subjects. This large dataset encompasses MRI scans from our studies conducted across the past eighteen years. This unique sample enabled us to analyze changes in the autistic brain across much of the life span up to late middle age. Evidence from about one third of these scans has been reported in a series of publications (Courchesne et al., 2001, 2003; Carper & Courchesne 2000, 2005; Carper et al., 2002; Schumann et al., 2009, 2010). Longitudinal scans were collected from the majority of subjects, with shorter longitudinal intervals for younger subjects and longer ones for older subjects. Only a subset of the longitudinal scan data has been reported (Schumann et al., 2010). Data from 253 of these 586 total scans has not been previously reported by us. These 253 scans include 67 autistic and 83 typical control 9 to 50 year olds, and 103 scans from 1 to 4 year old typically developing subjects. As described in previous publications from our laboratory, all ASD subjects underwent state-of-the-art deep clinical phenotyping including ADI-R, ADOS, cognitive, language, family and medical history, and chromosomal analyses for fragile-X. ASD as well as typical controls with confounding medical or family history (e.g., fragile-X or other chromosomal defects, pre- or postnatal exposure to drugs, toxins or pathogens, etc) were excluded from analyses. Across our sample, less than ten percent of ASD subjects were multiplex, the rest being simplex. Three different MRI scanners, imaging protocols and anatomical measurement programs were used across time, although longitudinal collections within an individual subject were always with the same scanner, protocol and measurement program.
3. Results
Growth curves of overall brain size across the life span from 2 to 50 years of age in ASD are shown in Fig. 4. The growth curves reveal an early period of brain overgrowth in ASD boys and girls followed by slowed growth during later childhood when the normal brain catches up with that of the autistic brain volumes. Thereafter, brain volumes decrease in size in ASD at a faster rate than normal so that, in ASD males, by later adulthood the brain is slightly smaller than average.
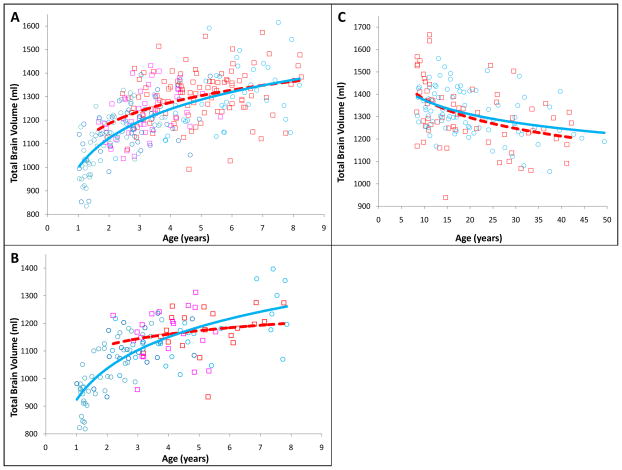
Figure 4.
Changes in Brain Growth Across the Lifespan in ASD. Total Brain Volume is shown for ASD (squares) and Control (circles) subjects for A) Males ages 1 to 9 years B) Females ages 1 to 9 years C) Males ages 9 to 50 years. Different colored markers represents the 3 different source datasets. The red, dotted lines represent growth trajectories of ASD subjects. The blue solid lines represent the growth trajectories of Control subjects. Data from 586 longitudinal and cross-sectional MRI scans.
This very large sample, longitudinal and cross-sectional MRI study of male and female brain size in autism across the life span from 2 to 50 years strongly supports the theory of age-specific anatomic abnormalities in autism. The results show there are at least three different periods of pathological brain development in ASD. Early in postnatal life in autism there is a relatively brief period lasting several years or less marked by abnormally accelerated overgrowth. This short period is followed by a period of abnormally slowed or arrested growth between young childhood and older childhood or preadolescence. This second short developmental period of growth pathology has been most carefully examined by Carper et al (2002) who showed strikingly reduced growth changes in autism between 2–4 years of age and about 9 years of age in frontal and temporal cortical regions, as compared to typical children whose growth in those regions remained steady and robust (see Table 2). Next, our life span evidence in Fig. 4 shows what may be a premature and accelerated rate of decline from adolescence to later middle age in autism.
Table 2.
After Early Overgrowth in Autism There is Abnormally Slow or Arrested Growth (From Carper et al. 2002)
| % Increase in Volumes during Childhood | |||
|---|---|---|---|
| Normal | Autism | ||
|
|
|||
| Frontal Gray | 20% | 1% | from 2–4 to 6–8 yrs |
| Frontal White | 45% | 13% | from 2–4 to 7–11 yrs |
| Dorsolateral Prefrontal | 48% | 10% | from 2 to 9 yrs |
| Temporal Gray | 17% | 0% | from 2–4 to 6–8 yrs |
| Temporal White | 22% | 2% | from 2–4 to 7–11 yrs |
4. Discussion
The genetic, molecular and cellular pathologies in autism that create the sudden and accelerated overgrowth during the first years of life must begin before that early age period, namely during either prenatal or very early postnatal life. We theorize that the abnormally accelerated rate of early growth and then premature arrest of growth in the autistic brain signal innate abnormalities of initial cortical neural and laminar organization and connectivity that are not the result of experience and learning-based activity. By contrast, the normal child’s brain grows more slowly, likely reflecting continued refinement of organization and connectivity via adaptive functional activity guided by experience and learning; indeed, some MRI-behavior studies of normal development indicate that slower growth, especially in frontal lobes, is associated with a higher ability long-term outcome (Shaw et al., 2006).
Since the first description of the unusual brain growth trajectory in autism, it has been theorized that the overgrowth might be due to excess neurons consequent to cell cycle dysregulation and/or failure of naturally occurring apoptosis (Courchesne et al., 2001; Courchesne et al., 2007). To test that possibility, we chose to quantify neuron counts, using stereological methods, in one of the regions with pathological early overgrowth, namely frontal cortex. In a pilot study, we found that a 3 year-old autistic postmortem male had 43% more neurons in dorsolateral prefrontal cortex as compared with a 2 year-old control male. In ongoing work, our laboratory has found this excess of neuron number in dorsolateral prefrontal cortex in every young autistic male we have analyzed to date. There is no known neurobiological mechanism in humans capable of generating during postnatal life the large excesses of frontal cortical neuron numbers we are finding. The great magnitude of this excess can only be due to abnormal events and processes beginning in prenatal life, and cannot be due to any known postnatal event or mechanism. With the exception of newly discovered cortical laminar abnormalities in some autistic cases (Courchesne and colleagues in progress), there are no other prenatal biological defects that are known to occur in the majority of young autistic cases (see reviews by Amaral et al., 2008; Courchesne et al., 2010; Wegiel et al., 2010). Therefore, this prenatally-based excess of neuron numbers, if verified by analyses of still further young autistic cases by us and other researchers, would be the earliest known indicator of the original biological events that cause autism.
Overgrowth and excessive neuron numbers and aberrant patterns of connectivity and functional activity could eventually trigger a belated “corrective” or remodeling phase involving processes that attempt to prune the excess aberrant axon connections, synapses and neurons to improve neural circuit function. Evidence of neuroinflammatory processes in the autistic person may reflect such secondary remodeling processes (Morgan et al., 2010). Our life span evidence up to late middle age (Fig. 4) plus a large existent literature points to such secondary, possibly degenerative, changes. Indeed, evidence from over 200 MRI, DTI and postmortem studies of the adolescent and adult autistic brain (reviews: Amaral et al., 2008; Courchesne et al., 2010; Murphy et al., this issue) collectively paint a picture of degeneration, atrophy, neuron loss, reduction in size of specific structures, and neuroinflammation at that later age. Examples of such evidence in adolescents and young adults appear in Table 3 and include reduced neuron numbers in the amygdala and cortex, reduced minicolumn size, decreased dendritic arbors, cortical thinning and atrophy, abnormally increased CSF volumes, activated microglia, increased pro-apoptotic and pro-neuroinflammatory signals. These later defects stand in contrast to what appears to be the condition in the very young autistic brain: increased cortical neuron numbers, normal minicolumn size, and greater cortical and amygdala volumes.
Table 3.
Third Phase: Evidence of Neuron Loss and Reduced Size of Anatomical Structures in Pre-Adolescence to Adulthood
| Third Phase: Pre-Adolescence to Adulthood |
|---|
DECREASED
|
THINNING
|
ACTIVATED MICROGLIA AND POSSIBLE INFLAMMATION
|
The anatomic pathology of autism changes with age. The changes are almost certainly continuous from prenatal life to old age, but rates and types of change will likely vary with brain region. Moreover, at different ages, there will likely be age-specific defects (e.g., amygdala, temporal and frontal cortex and brain overgrowth at young ages but not at older ages); that is, defects present at younger ages may not be present at older ages and conversely. Nonetheless, potentially some subset of core and early generative neural defects may be detectable across a wide age-range, perhaps even across a lifetime. In general, however, change will mark gene expression, molecular features, synaptic composition, cellular characteristics, circuit patterns and macroscopic morphology. Gene expression abnormalities in the adolescent and adult autistic brain will not be the same as those in the prenatal or toddler autistic brain. Gene expression in static, development-neutral preparations such as lymphocyte lines may not bear complete fidelity with the actual gene expression profiles in the living, developing and changing infant, toddler, child, adolescent or adult autistic person. The same would apply to identification of brain protein, synaptic, minicolumn, neuron count or other defects in the adult autistic brain. Whether a defect at that older age is an age-specific one or a core defect that is continuously present since early development would be difficult if not impossible to determine in the absence of verification in the very young brain.
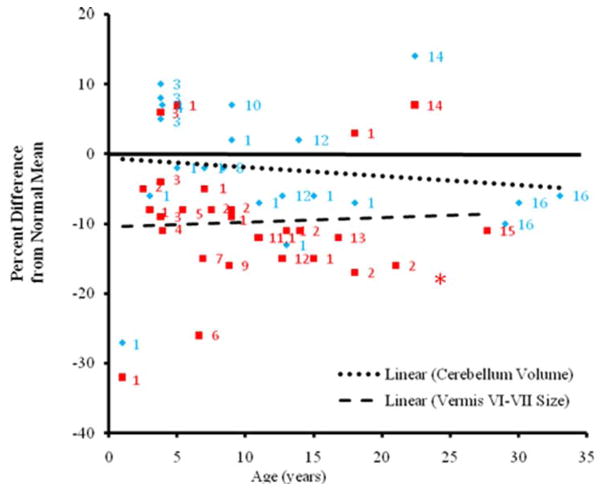
This principle of age-specific change in pathology also applies to the cerebellum in autism, even though its trajectory of growth pathology seems to differ from that of the cerebrum and amygdala (Webb et al., 2009; Hallahan et al. 2009; reviews: Stanfield et al., 2008; Courchesne et al., 2010). Subregions of the cerebellar vermis show varying degrees of abnormality with the most consistent one, vermis lobules VI–VII, showing hypoplasia by 10 months of age and throughout infancy, childhood and adulthood (Fig. 5). The cerebellar hemispheres also display complex change with age, being near normal in size at young ages to reduced in size by adulthood (Fig. 5) (review: Courchesne et al., 2010). Purkinje neuron loss in the cerebellum was not reported for the youngest autistic child examined in one study (Bailey et al., 1998), but was present in every autistic adult in that study.
Figure 5.
Cerebellum and Vermis Size from Infancy to Adulthood in Autism. Percent differences from normal average in each study for autistic subjects are plotted for cerebellar volume (blue diamonds) and vermis lobules VI–VII cross-sectional area (red squares) against age. Regression lines are shown for cerebellar volume and vermis lobules VI–VII area. The solid line represents normal average. Each number represents a separate study: 1, Hashimoto et al., 1995; 2, Courchesne et al., 2001; 3, Akshoomoff et al., 2004; 4, Webb et al., 2009; 5, Carper et al., 2000; 6, Kleiman et al., 1992; 7, Kaufmann et al., 2003; 8, Kates et al., 2004; 9, Mitchell et. al., 2009; 10, Herbert et al., 2003; 11, Elia et al., 2000; 12, Cleavinger et. al., 2008; 13, Ciesielski et al., 1997; 14, Hardan et al., 2001; 15, Piven et al., 1992; 16, Hallahan et. al, 2009. Data point from first report of hypoplasia of vermis lobules VI–VII Courchesne et al., 1988 shown as red asterisk.
There is currently much interest in brain-gene mapping but this effort will necessarily run headlong into the problem of age-specific brain effects. Therefore, brain-SNP, brain-CNV (copy number variation) or brain-“deep phenotype” studies of older children, adolescents and adults with autism will be prone to reflect outcome associations and not original causal ones. In general, discovery of the causes of autism will be more difficult via studies of the older as compared to the very young autistic brain. Inferences about causes and early processes derived from data on older cases should be made with caution.
On the other hand, the importance of studies of the adolescent, adult and aging autistic brain is very high, but for reasons having less to do with the search for original early developmental causes and defects. Instead, as delineated by a companion paper in this Special Issue (Murphy et al.), studies of the older, including aging, autistic brain are vital because there are presently no proven methods for successfully improving clinical symptoms and neural functioning at these ages. Since the autistic brain is continuously changing in abnormal ways even across these older ages, as demonstrated by Murphy and colleagues (Raznahan et al. 2010), there may be ample opportunity to intervene pharmacologically as well as behaviorally to improve the very long-term clinical course. In recent publications (Raznahan et al., 2010, Ecker et al., 2010), the ratio of cerebral lobar volume to overall total brain volume follows an abnormal trajectory across adolescence and adulthood suggesting that in autism abnormal neural and molecular processes continue to occur during adulthood. Murphy also reports that greater symptom severity is correlated with greater deviation from normal brain maturation (Ecker et al., 2010). Therefore studying the autistic adolescent and adult brain could be important in guiding treatment throughout later life. This realization should re-invigorate research focused on understanding age-specific neural pathologies in the older autistic brain.
In conclusion, across the life span autism displays complex age-specific anatomic changes, and underlying these changes must necessarily be age-related and potentially age-specific changes in gene expression, molecular, synaptic, cellular and circuit abnormalities. A peak age for detecting and studying the earliest fundamental biological underpinnings of autism would be prenatal life and the first three postnatal years. This theory of age-specific anatomic abnormalities in autism has significant broad implications for a wide range of work on the disorder including the design, validation and interpretation of animal model, lymphocyte gene expression, brain gene expression, and genotype/CNV-anatomic phenotype studies. Lastly, the search for signals of original, prenatal and early postnatal causal defects is massively complicated, but not nullified, by this principle.
Acknowledgments
The writing of this article was made possible by grants from UCSD-NIH Autism Center of Excellence (E. Courchesne, 1-P50-MH081755, 2-R01-MH036840), the National Institute of Neurological Disorders and Stroke (Grant 2-ROI-NS19855), National Institute of Mental Health (K. Pierce, R01-MH080134), Autism Speaks, and the Simons Foundation. We send many thanks to all of the children and parents who participate in autism research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akshoomoff N, Lord C, Lincoln AJ, Courchesne RY, Carper RA, Townsend J, Courchesne E. Outcome classification of preschool children with autism spectrum disorders using MRI brain measures. J Am Acad Child Adolesc Psychiatry. 2004;43:349–357. doi: 10.1097/00004583-200403000-00018. [DOI] [PubMed] [Google Scholar]
- Aylward E, Minshew MD, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD. MRI volumes of amygdale and hippocampus in non-mentally retarded autistic adolescence and adults. Neurology. 1999;53:2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- Araghi-Niknam M, Fatemi SH. Levels of Bcl-2 and P53 are altered in superior frontal and cerebellar cortices of autistic subjects. Cell Mol Neurobiol. 2003;23:945–952. doi: 10.1023/B:CEMN.0000005322.27203.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends in Neurosciences. 2008;31:137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain Research. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Courchesne E. MRI neuroanatomy in young girls with autism: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2007;46:515–523. doi: 10.1097/chi.0b013e318030e28b. [DOI] [PubMed] [Google Scholar]
- Buxhoeveden DP, Semendeferi K, Buckwalter J, Schenker N, Switzer R, Courchesne E. Reduced minicolumns in the frontal cortex of patients with autism. Neuropathol Appl Neurobiol. 2007;33:597. doi: 10.1111/j.1365-2990.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Inverse correlation between frontal lobe and cerebellm sizes in children with autism. Brain. 2000;123:836–844. doi: 10.1093/brain/123.4.836. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar Pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Steinbusch HW, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Harris RJ, Hart BL, Pabst HF. Cerebellar hypoplasia and frontal lobe cognitive deficits in disorders of early childhood. Neuropsychologia. 1997;35:643–655. doi: 10.1016/s0028-3932(96)00119-4. [DOI] [PubMed] [Google Scholar]
- Cleavinger HB, Bigler ED, Johnson JL, Lu J, McMahon W, Lainhart JE. Quantitative magnetic resonance image analysis of the cerebellum in macrocephalic and normocephalic children and adults with autism. J Int Neuropsychol Soc. 2008;14:401–13. doi: 10.1017/S1355617708080594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318:1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Press GA, Yeung-Courchesne R. Parietal lobe abnormalities detected with MR in patients with infantile autism. Am J Roentgenol. 1993;160:387–393. doi: 10.2214/ajr.160.2.8424359. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Karns C, Davis HR, Ziccardi R, Carper R, Tigue Z, Pierce K, Moses P, Chisum HJ, Lord C, Lincoln AJ, Pizzo S, Schreibman L, Haas RH, Akshoomoff N, Courchesne RY. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Webb SJ, Schumann CM. From toddlers to adults: The changing landscape of the brain in autism. In: Amaral DG, Dawson G, Geschwind DH, editors. Autism Spectrum Disorders. Oxford University Press; 2010. in press. [Google Scholar]
- Dawson G, Munson J, Webb SJ, Nalty T, Abbott R, Toth K. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biol Psychiatry. 2007;61:458–464. doi: 10.1016/j.biopsych.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dementieva YA, Vance DD, Donnelly SL, Elston LA, Wolpert CM, Ravan SA, DeLong GR, Abramson RK, Wright HH, Cuccaro ML. Accelerated head growth in early development of individuals with autism. Pdeiatr Neurol. 2005;32:102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Dissanayke C, Bui QM, Huggins R, Loesch DZ. Growth in stature and head circumference in high-functioning autism and Asperger disorder during the first 3 years of life. Dev Psychopathol. 2006;18:381–393. doi: 10.1017/S0954579406060202. [DOI] [PubMed] [Google Scholar]
- Ecker C, Marquand A, Maurao-Miranda J, Johnston P, Daly E, Brammer M, Maltezos S, Murphy C, Robertson D, Williams S, Murphy D. Describing the Brain in Autism in Five Demensions-Magnetic Resonance Imaging- Assisted Diagnosis of Autism Spectrum Disorder Using a Multiparameter Classification Approach. The Journal of Neuroscience. 2010;30:10612–10623. doi: 10.1523/JNEUROSCI.5413-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Redcuded size of the corpus callosum in autism. Arch Neurol. 1995;52:794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- Elder LM, Dawson G, Toth K, Fein D, Munson J. Head circumference as an early predictor of autism symptoms in younger siblings of children with autism spectrum disorder. J Autism Dev Disord. 2008;38:1104–1111. doi: 10.1007/s10803-007-0495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia M, Ferri R, Musumeci S, Panerai S, Bottitta M, Scuderi C. Cilinical correlates of brain morphometric features of subjects with low-functioning autistic disorder. J Child Neurol. 2000;15:504–510. doi: 10.1177/088307380001500802. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, Thuras P, Merz A. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002;22:171–175. doi: 10.1023/A:1019861721160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto A, Hashimoto T, Ito H, Nishimura M, Tsuda Y, Miyazaki M, Mori K, Arisawa K, Kagami S. Growth of head circumference in autistic infants during the first year of life. J Autism Dev Disord. 2008;38:411–418. doi: 10.1007/s10803-007-0405-1. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex. 2006;16:1276–1282. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hallahan B, Daly EM, McAlonan G, Loth E, Toal F, O’Brien F, Robertson D, Hales S, Murphy C, Murphy KC, Murphy DG. Brain morphometry volume in autistic spectrum disorder: a magnetic resonance imaging study of adults. Psychol Med. 2009;39:337–346. doi: 10.1017/S0033291708003383. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Mallikarjuhn M, Keshavan MS. Brain volume in autism. J Child Neurol. 2001;16:421–424. doi: 10.1177/088307380101600607. [DOI] [PubMed] [Google Scholar]
- Hardan A, Muddasami S, Vemulapalli M, Keshavan M, Minshew J. An MRI study of increased cortical thickness in autism. Am J Psychiatry. 2006;163:1290–1292. doi: 10.1176/appi.ajp.163.7.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Murakawa K, Yoshimoto T, Miyazaki M, Harada M, Kuroda Y. Development of the brainstem and cerebellum in autistic patients. J Autism Dev Disord. 1995;25:1–18. doi: 10.1007/BF02178163. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J. Cortical gray and white brain tissue volume in adolescents and adults with autism. Biol Psychiatry. 2006;59:1–6. doi: 10.1016/j.biopsych.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien L, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness VS. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Eliez S, Strunge LA, Kaplan D, Landa R, Reiss AL, Pearlson GD. Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism. Am J Psychiatry. 2004;161:539–546. doi: 10.1176/appi.ajp.161.3.539. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cooper KL, Mostofsky SH, Capone GT, Kates WR, Newschaffer CJ, Bukelis I, Stump MH, Jann AE, Lanham DC. Specificity of cerebellar vermian abnormalities in autism: a quantitative magnetic resonance imaging study. J Child Neurol. 2003;18:463–70. doi: 10.1177/08830738030180070501. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman M. Neuropathology of infantile autism. J Neuropathol Exp Neurol. 1998;57:645–52. doi: 10.1097/00005072-199807000-00001. [DOI] [PubMed] [Google Scholar]
- Kleiman MD, Neff S, Rosman NP. The brain in infantile autism: are posterior fossa structures abnormal? Neurology. 1992;42:753–760. doi: 10.1212/wnl.42.4.753. [DOI] [PubMed] [Google Scholar]
- Kosaka H, Omori M, Munesue T, Ishitobi M, Matsumura Y, Takahashi T, Narita K, Murata T, Saito DN, Uchiyama H, Morita T, Kikuchi M, Mizukami K, Okazawa H, Sadato N, Wada Y. Smaller insula and inferior frontal volumes in young adults with pervasive developmental disorders. NeuroImage. 2010;50:1357–1363. doi: 10.1016/j.neuroimage.2010.01.085. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz CM, Lee M, Perry RH, Baumann M, Court JA, Perry EK. Molecular analysis of nicotinic receptor expression in autism. Brain Res Mol Brain Res. 2004;123:81–90. doi: 10.1016/j.molbrainres.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Mitchell SR, Reiss AL, Tatusko DH, Ikuta I, Kazmerski DB, Botti JA, Burnette CP, Kates WR. Neuroanatomic alterations and social and communication deficits in monozygotic twins discordant for autismdisorder. Am J Psychiatry. 2009;166:917–925. doi: 10.1176/appi.ajp.2009.08101538. [DOI] [PubMed] [Google Scholar]
- Morgan J, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP. Microglial Activation and Increased Microglial Density Observed in the Dorsolateral Prefrontal Cortex in Autism. Biol Psychiatry. 2010;68:368–376. doi: 10.1016/j.biopsych.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Cody-Hazlett H, Poe MD, Gerig G, Gimple-Smith R, Piven J. Longitudinal study of amygdala volume and joint attention in 2-to- 4-year-old children with autism. Arch Gen Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz KD, Green J, Dumont-Mathieu T, Makin S, Fein D. Correlates of head circumference growth in infants later diagnosed with autism spectrum disorders. J Child Neurol. 2007;22:700–713. doi: 10.1177/0883073807304005. [DOI] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, Shaw D, Artru A, Dager SR. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry. 2006;63:993–1005. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- Murphy D, Ecker C, Craig M. Brain Research. Autism in adults. New biological findings and their translational implications. 2010 doi: 10.1016/j.brainres.2010.10.042. This Issue. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, Hulshoff Pol HE, Kemner C, Schnack HG, Durston S, Lahuis BE, Kahn RS, Van Engeland H. Increased gray-matter volume in medication-naïve high-functioning children with autism spectrum disorder. Psychol Med. 2005;35:561–570. doi: 10.1017/s0033291704003496. [DOI] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Pierce K, Glatt S, Liptak GS, McIntyre LL. The power and promise of identifying autism early: insights from the search for clinical and biological markers. Annals of Clinical Psychiatry. 2009;21:132–147. [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Carter C, Weinfeld M, Desmond J, Hazin R, Bjork R, Gallagher N. Catching, studying and treating autism early: The 1-year well-baby check-up approach. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Nehme E, Simon J, Barta P, Pearlson G, Folstein SE. Magnetic resonance imaging in autism: measurement of the cerebellum, pons and fourth ventricle. Biol Psychiatry. 1992;31:491–504. doi: 10.1016/0006-3223(92)90260-7. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, Bolton P, Paus T, Murphy D. Cortical Anatomy in Autism Spectrum Disorder: An In Vivo MRI Study on the Effect of Age. Cereb Cortex. 2010;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Barnes CC, Lord C, Courchesene E. Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biol Psychiatry. 2009;66:942–949. doi: 10.1016/j.biopsych.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, Pierce K, Hagler D, Schork N, Lord C, Courchesne E. Longitudinal magnetic resonance image study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward E, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Stanfield AC, Mcintosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry. 2008;23:289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- van Kooten IA, Palmen SJ, von Cappeln P, Steinbusch HW, Korr H, Heinsen H, Hof PR, van Engeland H, Schmitz C. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131:987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Nalty T, Munson J, Brock C, Abbott R, Dawson G. Rate of head circumference growth as a function of autism diagnosis and history of autistic regression. J Child Neurol. 2007;22:1182–1190. doi: 10.1177/0883073807306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb S, Sparks BF, Friedman SD, Shaw DW, Giedd J, Dawson G, Dager SR. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. 2009;172:61–67. doi: 10.1016/j.pscychresns.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Marchi E, Ma SY, Chauhan A, Chauhan V, Bobrowicz TW, de Leon M, Louis LA, Cohen IL, London E, Brown WT, Wisniewski T. The Neuropathology of autism: Defects of neurogenisis and neuronal migration and dysplastic changes. Acta Neuropathol. 2010;119:755–770. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]