Abstract
Traditional assays for secreted proteins include methods such as Western blot or ELISA detection of the protein in the cell culture media. We describe a method for the detection of a secreted protein based on fluorescent measurement of a mCherry fusion reporter. This microplate reader-based mCherry fluorescence detection method has a wide dynamic range of 4.5 orders of magnitude and a sensitivity that allows detection of 1-2 fmol of fusion protein. Comparison with the Western blot detection method indicated greater linearity, wider dynamic range, and a similar lower detection threshold for the microplate-based fluorescent detection assay of secreted fusion proteins. A mCherry fusion protein of matrix metalloproteinase-9, a secreted glycoprotein, was created and expressed by transfection of HEK293 cells. The cell culture media was assayed for the presence of the fluorescent signal up to 32 hrs after transfection. The secreted MMP-9-mCherry fusion protein was detected 6 hrs after transfection with a linear increase in signal intensity over time. Treatment with chloroquine, a drug known to inhibit the secretion of many proteins, abolished the MMP-9-mCherry secretion demonstrating the utility of this method in a biological experiment.
Keywords: secretion, fluorescence, microplate, mCherry, MMP-9
Introduction
Protein secretion from cells is a fundamental process of great importance in normal biology and pathobiology. Establishment of the secreted protein discovery initiative [1] and a web-based secreted protein database [2] have provided much new information on defining the secretome. Additionally the recent availability of many new bioinformatics tools [3] has helped in the interpretation of the biological significance of the secretome. Exogenous expression of a fusion protein between a secreted protein of interest and a fluorescent reporter such as green fluorescent protein (GFP) has been used extensively to characterize the subcellular localization and trafficking of secreted proteins since the earliest days of the discovery of the fluorescent reporter (reviewed in Presley [4]). Incorporating methods such as time lapse imaging, fluorescent recovery after photobleaching, and Forster resonance energy transfer has extended the capability of fluorescent reporter tagging to accurately report on dynamic protein movement and protein: protein interaction within a specific subcellular compartment involved in secretion. A recent report confirmed that for a majority of proteins, the addition of a fluorescent reporter does not alter the subcellular localization compared to the untagged proteins detected by immunofluorescence imaging against the native proteins [5]. Despite the use of a fluorescent reporter for intracellular spatiotemporal characterization, the use of fluorescent reporters to detect secreted proteins once the protein leaves the cells has not been explored. Experimentally, the detection of secreted and non-secreted proteins retained within cells are still accomplished by a Western blot or an enzyme-linked immunosorbent assay (ELISA) of the secreted protein in the cell culture media requiring considerable time and cost. We describe a simple method extending the utility of fluorescent reporter-tagged fusion protein to enable detection of the secreted proteins in the cell culture media and the non-secreted retained protein within the cell using a fluorescent microplate reader.
Materials and methods
Reagents
Chloroquine diphosphate (#C6628, Sigma Aldrich, St. Louis, MO); Purified mCherry (#4993-100, BioVision, Inc., Milpitas, CA); purified MMP-9 (BML-SE504-0005, Enzo Life Sciences, Inc., Farmingdale, NY).
Cell Culture
Human embryonic kidney (HEK) 293 cells (ATCC, Manassas, VA) were grown in Dulbecco’s Modification of Eagle’s Medium (Mediatech, Inc., Manassas, VA) with 4.5 g/L glucose, l-glutamine, sodium pyruvate, and supplemented with 10% heat inactivated fetal bovine serum (Mediatech Inc), 100 U/mL penicillin (Mediatech Inc), and 0.1 mg/mL streptomycin (Mediatech Inc).
mCherry Tagged Constructs
Starting with the wild-type human MMP-9 cDNA (Accession BC006093, Image Clone MGC: 12688) primers designed to remove the stop codon and incorporate an in-frame Mlu I site (MMP-9_Fwd_XhoI: cctcgagaaatgagcctctggcagcccctggtcctgg; MMP-9_Rev_MluI: acgcgtgtcctcagggcactgcaggatgtcatagg) were used for PCR amplification. The mCherry cDNA (Clontech Lab, Mountain View, CA) was PCR amplified to remove the start ATG replacing it with an in-frame Mlu I site (mCherry_Fwd_MluI: ggacgcgtgtgagcaagggcgaggaggataacatgg; mCherry_Rev_NotI: ccgcggccgcttacttgtacagctcgtccatgc). All PCR products were ligated into PCR Blunt (Invitrogen, Carlsbad, CA) and sequenced. The start and stop codons are in bold and the relevant restriction enzyme sites are underlined. Once correct sequences were confirmed the cDNAs were ligated in-frame with 5′ MMP-9 and 3′ mCherry-tag in pCI/neo (Promega, Madison, WI) at the Xho I/ Not I restriction enzyme sites within the multiple cloning site of the vector to yield MMP-9-mCherry.
Secreted eGFP
The N-terminal signal sequence of a GPI-anchored T-cadherin was fused to the N-terminus of eGFP after removal of the endogenous start methionine (Supplemental Figure 1) [6]. The resulting T-cadherin signal sequence-eGFP chimera cDNA was subcloned into the pCI/neo expression vector. We call this construct expressing the secreted form of eGFP the signal sequence eGFP (ss-eGFP). Used as a transfection control, when transfected into cells the fusion ss-eGFP protein was secreted into the cell culture media and quantified in tandem with mCherry by a fluorescent microplate reader.
Time-Course Secretion Assay
HEK293 cells grown overnight (~75-90% confluence) were transfected with MMP-9-mCherry (750 ng) and ss-eGFP (750 ng) in a 6-well plate using 4 μL Lipofectamine 2000 (Invitrogen). 4 hours post-transfection media was replaced with 1.2 mL of OPTI-MEM. The use of OPTI-MEM avoided the auto-fluorescence interference from phenol red-containing and serum supplemented standard cell culture media. At various time points post-transfection 50 μL of cellular media was removed. 50 μL of water was added and centrifuged at 15,000 rpm for 1 minute. 90 μL was added to a black 96-well plate and dual fluorescence was recorded individually. eGFP was recorded using an excitation wavelength/bandwidth of 485/20 nm and emission wavelength/bandwidth of 528/20 nm. mCherry was recorded using an excitation wavelength/bandwidth of 590/20 nm and emission wavelength/bandwidth of 645/40 nm with a Synergy2 microplate reader (BioTek Inc, Winooski, VT).
Endpoint Secretion Assay
HEK293 cells grown overnight (~85-95% confluence) were transfected with MMP-9-mCherry (250 ng) and ss-eGFP (250 ng) in a 12-well plate using 2 μL Lipofectamine 2000 (Invitrogen). 4 hours post-transfection the media was replaced with 0.6 mL of OPTI-MEM. 24 hours post-transfection cellular media was collected and cells were harvested. The cellular media was centrifuged at 15,000 rpm for 1 minute and 100μL was added to a black 96-well plate in triplicate. Cells were harvested from the plate by physical trituration using cold phosphate buffered saline (PBS) and spun at 10,000 rpm for 5 minutes at 4°C. PBS was aspirated and cells were lysed on ice for 15 minutes using 50 μL of RIPA lysis buffer (10mM Tris-HCL, 50mM NaCl, 1mM Na-orthovanadate, 30mM Na-pyrophosphate, 50 mM NaF, 1% Nonidet P-40, 0.1% SDS, 1 mM PMSF, 1% Triton X-100, 0.5% Na-deoxycholate, and dissolved protease inhibitor cocktail (Sigma Aldrich) in water, pH 7.4). Lysed cells were spun at 15,000 rpm for 5 minutes and 20 μL of the cellular lysate was diluted 1:10 in water. 180 μL of the diluted cellular lysate was added to a black 96-well plate to eliminate cross contamination of the fluorescent signal from adjacent wells. GFP and mCherry fluorescent signals were recorded as indicated above. The mCherry standard curve was obtained using purified mCherry protein serially diluted (range of 10 pg to 1 μg) in OPTI-MEM (Invitrogen).
Western Blot
24 hours post-transfection cell culture media and cell pellets were collected for analysis. Cells were lysed in RIPA lysis buffer and 20 μg of cell lysate and equal volume cell media were loaded in 10% SDS-PAGE gels. Under the transfection condition used, this method gave a linear range of correlation between the total cell lysate and the culture cell media [7]. Samples were run and transferred onto nitrocellulose membranes that were blocked using 5% milk and washed in TBST (Tris buffered saline with 0.1% Triton X-100). Membranes were probed with appropriate antibodies, exposed using enhanced chemiluminescence reagent (Promega) or SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Rockford, IL), and imaged using the ChemiDoc XRS+ Molecular Imager (BioRad) running the Quality One software (BioRad, Hercules, CA). The densitometric quantification of Western blots was performed using Image Lab 4.1 software (BioRad). The mCherry and MMP-9 standard curves were generated using purchased purified mCherry or MMP-9 proteins. A range of 50 pg to 10 ng of input protein diluted in RIPA lysis buffer was used for the standard curve analysis.
Fluorescence Microscopy of Subcellular Localization
MMP-9-mCherry was co-transfected with the ss-eGFP construct into HEK293 cells. 24 hours post-transfection cells were imaged with a Sensicam camera (PCO-Tech Inc. Romulus, MI) mounted on an Olympus IX50 microscope (Olympus Corporations, Tokyo, Japan) using the IPLab3.6 software (Spectra Services, Ontario, NY). Pseudo-color of eGFP (green) or mCherry (red) was accomplished using Adobe Photoshop CS5.1 (Mountain View, CA).
Statistical Analysis
3-6 biological replicates were obtained with each biochemical assay consisting of 1-3 replicate measurements per assay. Statistical significant difference in the mean values were defined as P<0.05 by Student T-test.
Results
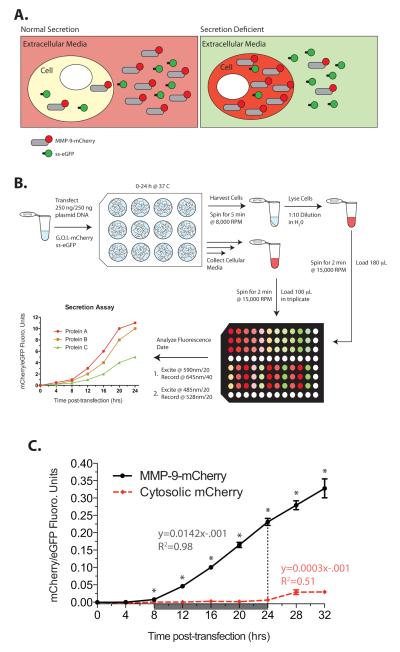
The mCherry fluorescent protein with a quick maturation time, good brightness, lack of oligomerization, and resistant to photobleaching [8] was selected as the reporter. Proteins tagged with mCherry and the secreted eGFP (ss-eGFP) transfected into cells will express and secrete the fluorescent molecules and the respective fluorescence can be detected in both the total cell lysate and cell culture media. Manipulations that decrease the secretion of the mCherry-tagged protein will result in a loss of mCherry signal in the extracellular media and an increase in the intracellular signal due to retained protein (Figure 1A). Both the cell culture media and the whole cell lysate prepared from the cell pellet can be harvested and the fluorescent signal directly measured on a fluorescent microplate reader to report on the amount of extracellularly secreted and intracellularly retained proteins (Figure 1B). We were interested in understanding the regulation of secretion of matrix metalloproteinase (MMP)-9, an N- and O-glycosylated glycoprotein secreted by many cells including the macrophage and neutrophils [9, 10]. To study the regulation of secretion of this important endopeptidase we created a C-terminally tagged MMP-9-mCherry fusion construct. Figure 1C shows the increase in mCherry fluorescence in the cell culture media over time in cells transfected with MMP-9-mCherry. The mCherry fluorescence was clearly detected by 6 hrs after transfection. Control cells transfected with the cytosolic-localized mCherry demonstrated little extracellular signal indicating that the fluorescence detected in the cell culture media was due to the secreted MMP-9-mCherry and not from the release of intracellularly restricted protein after cell death. The mCherry fluorescence of the cell culture media was stable retaining ~98% fluorescence intensity up to 36 hrs even when left at room temperature allowing collection of samples over an extended time course and a single measurement of the fluorescence at the experimental endpoint.
Figure 1.
mCherry-tagged protein secretion assay. A. Cartoon outline of the assay. The gene of interest (MMP-9) is subcloned upstream of mCherry and transfected into cells along with a plasmid encoding the secretable form of GFP (ss-eGFP). The fluorescent reporter read outs are used to quantify intracellular protein or secreted protein in the extracellular media. B. Experimental flow diagram for the 12-well secretion assay for a particular gene of interest (G.O.I.) fused to mCherry. Prototypical data are reported as ratios of mCherry/ eGFP as a function of time for three prototypical proteins (A-C) indicating fast (protein A, red line), intermediate (protein B, orange line), and slow (protein C, green line) rates of secretion. C. Cells were transfected with MMP-9-mCherry and ss-eGFP and the normalized fluorescent signals (black line) in the culture media followed over time. A control with cytosolic-restricted mCherry (red dashed line) allowed detection of dead cells releasing the fluorophore into the media. The rate of secretion (arbitrary units) and R2 value were calculated from the linear region of data from 8 to 24 hrs post-transfection (grey boxed area). Student T-test, * P-value <0.05, mean ± S.E.M., n=3.
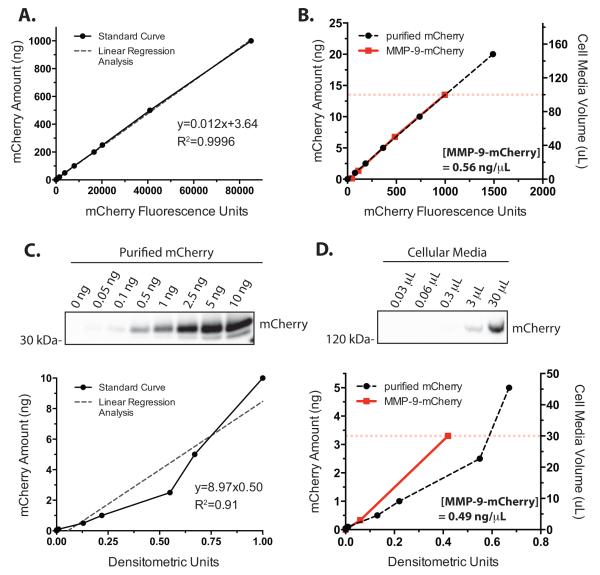
The linearity and the dynamic range of the mCherry fluorescence reported by the microplate-based method were determined by measuring the fluorescence of serially diluted purified mCherry protein and the cell culture media. The mCherry fluorescence demonstrated linear signal intensity up to 1000 ng of mCherry protein (Figure 2A) with an excellent linearity retained even for small amounts of the mCherry protein (Figure 2B, black dashed line). Linear regression analysis of the standard curve gave an R2 value of 0.9996. The lower limit of detection above background was 50 pg (1.7 fmol) of mCherry indicating a linear detection dynamic range of at least 4.5 orders of magnitude. Serial dilution of the cell culture media (Figure 2B, red line) superimposed on the calibration curve determined that 100 μL of cell culture media emitted a fluorescence intensity equal to about 13.4 ng (Figure 2B, dashed red line) of purified mCherry. Given the molecular mass of mCherry (28.8 kDa), 13.4 ng is 0.46 pmoles indicating that 100 μL cell culture media contained about 56.1 ng of the MMP-9-mCherry fusion protein (28.8 kDa mCherry+ 92 kDa mature MMP-9 = 121 kDa fusion protein) or an MMP-9-mCherry fusion protein concentration of 0.56 ng/ μL (Figure 2B, inset) assuming that the behavior of the fluorophore was the same for the free- and fusion-mCherry. By comparison, a serial dilution of purified mCherry detected by a chemiluminescent substrate and densitometric analysis of the Western blot resulted in a slightly reduced linearity with the regression analysis of the standard curve giving an R2 value of 0.91 with a dynamic range of about 2.5 orders of magnitude from 0.05-10 ng (Figure 2C). Serial dilution of the cell culture media (Figure 2D, red line) superimposed on the calibration curve determined that 30 μL of the cell culture media gave densitometric measurement, when blotting for mCherry, equal to about 3.27 ng (Figure 2D, red dashed line). Assuming equivalent anti-mCherry antibody detection between free- and fusio- mCherry, the densitometric quantitation resulted in the calculated MMP-9-mCherry concentration in the cell culture media to be 0.49 ng/ μL (Figure 2D, inset) comparable to the protein concentration estimation based on the fluorescent detection assay. In contrast to the mCherry fluorescent detection assay, a serial dilution of the purified MMP-9 protein by a densitometric quantification of the Western blot gave a limited detection range of 1.5 orders of magnitude (0.5 to 10 ng) with even a narrower linear range (Supplemental Figure 2A). The use of a high sensitivity chemiluminescent substrate increased the detection sensitivity but did not increase the dynamic range of detection due to saturation of the Western blot signal for larger amounts of MMP-9 (Supplemental Figure 2B). Nevertheless, the mCherry fluorescent detection and Western blot assays of the cell culture media correlated well with each other over a limited dynamic range and this correlation between the two assays extends to the whole cell lysate analysis of the non-secreted retained protein within the cells (Supplemental Figure 3).
Figure 2.
Characterization of the mCherry fluorescence. A. The fluorescence intensity of a serially diluted purified mCherry protein was assayed by a microplate reader. Quantification by linear regression analysis gave a sensitivity from the slope of the regression equation (in arbitrary units) and a R2 value. Data are mean ± S.E.M. from n=3. B. mCherry standard curve (black dashed line) in the 0 - 20 ng range with superimposed data from the cellular media containing secreted MMP-9-mCherry (red solid line). Circles are mean ± S.E.M. from n=3. The calculated amount of secreted MMP-9-mCherry fusion protein in 100 μL media (red dashed line) from the mCherry fluorescence standard curve was 0.56 ng/ μL (inset). C. Western blot and densitometric analysis of the purified mCherry protein titration series. Linear regression analysis and R2 value of slope (inset), n=1. D. Western blot and densitometric analysis of the titration series of cellular media contained secreted MMP-9-mCherry. mCherry standard curve (black dashed line) in the 0 - 5 ng range with cellular media containing secreted MMP-9-mCherry (red solid line). The calculated secreted MMP-9-mCherry protein amount present in 30 μL media (red dashed line) from the densitometric standard curve was 0.49 ng/ μL (inset).
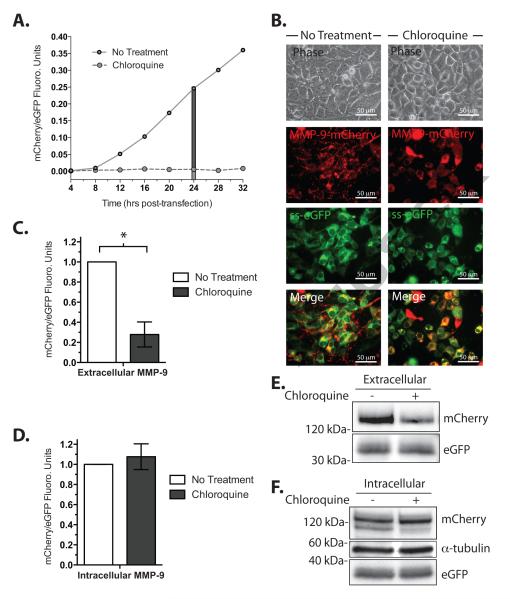
Chloroquine, a 4-aminoquinoline drug, has been used as a tool for inhibiting secretion of glycoproteins [11] possibly working by altering the intracellular pH [12]. Cell culture media from cells transfected for the expression of MMP-9-mCherry with or without chloroquine was collected and assayed for mCherry fluorescence normalized by the fluorescence intensity of the constitutively secreted ss-eGFP. The normalized mCherry/eGFP fluorescence increased with time after transfection while chloroquine essentially abolished the signal (Figure 3A). A fluorescent image of untreated cells showed punctate mCherry signal localized to what appeared to be the post-Golgi secretory vesicles as observed for untagged wild type-MMP-9 [9]. Chloroquine treatment shifted the fluorescent signal to the peri-nuclear region of the cell consistent with the secretion-inhibited MMP-9-mCherry trapped in the endoplasmic reticulum (Figure 3B). Microplate quantitation of the mCherry/eGFP signal ratio at 24 hrs after transfection showed a dramatic decrease in the cell culture media and a small increase in the fluorescence of the whole cell lysate upon chloroquine treatment consistent with the inhibition of secretion and retention of MMP-9-mCherry within cells (Figure 3C, D). A Western blot of cell culture media and whole cell lysate probed with anti-mCherry, anti-GFP, and anti-α tubulin antibodies from the above experiment confirmed a decrease in secretion and retention of intracellular MMP-9-mCherry resulting from chloroquine treatment (Figure 3E, F).
Figure 3.
Inhibition of MMP-9-mCherry secretion by chloroquine. A. Time-course of MMP-9-mCherry secretion in the presence (dashed line) or absence (solid line) of 100 μg/ mL chloroquine 24 hours post-transfection, n=1. A 24-hour time point (grey boxed area) was chosen for further analysis. B. Fluorescence microscopy of cells transfected with MMP-9-mCherry (red) and ss-eGFP (green) in the absence (left column) or presence (right column) of 50 μg/ mL chloroquine. The images are (from top to bottom) views in phase contrast, mCherry, eGFP, and merged. Merged analysis (white, bottom row) displaying overlapping MMP-9-mCherry and ss-eGFP signals. C. Amount of secreted MMP-9-mCherry and ss-eGFP 24 hours post-transfection in the absence (open) or presence (solid) of 50 μg/ mL chloroquine. Mean ± Student T-test, * P<0.05, mean± S.E.M., n=6. D. Amount of intracellular MMP-9-mCherry and ss-eGFP protein 24 hours post-transfection in the absence (open) or presence (solid) of 50 μg/mL chloroquine. Student T-test, P>0.05, mean ± S.E.M., n=3. E. Western blot analysis of the secreted protein in the cell culture media for absence (−) or presence (+) of 50 μg/ mL chloroquine. F. Same as E but analyzed for the intracellular protein in total cell lysate in the absence (−) or presence (+) of 50 μg/ mL chloroquine.
Discussion
Our results demonstrate that a fluorescent reporter tagging of secreted proteins extends the utility of epitope-tagging beyond probing of subcellular trafficking but to a direct detection of secreted protein in the cell culture media. While the same information could be obtained by the traditional methods of quantifying the secreted protein by Western blot or ELISA assays, the direct measurement of fluorescent signal in the cell culture media with a microplate reader is superior, much easier, quicker, and less costly. The dynamic range of the assay was 4.5 orders of magnitude or greater with an excellent sensitivity and linearity allowing detection of pmole amounts of the mCherry fusion protein. MMP-9 Western blot (current study) or ELISA assay (insert brochure for ELISA kit, #KHC3061, Invitrogen and Supplemental Figure 2 in Duellman et al. [13]) had, at best, 2 – 2.5 orders of magnitude dynamic range and an even narrower linear range of assay. The utility of the fluorescent microplate assay method was demonstrated by a biological experiment probing the effect of chloroquine on the secretion of MMP-9-mCherry protein. The direct fluorescence measurements indicated inhibition of secretion and retention of this fusion protein within the cells by chloroquine consistent with the slight increase in the intracellular mCherry signal observed under a fluorescent microscope. A Western blot of intracellular lysate and culture media probed for mCherry confirmed the microplate assay results. This method enabling easy detection of secreted protein and also amenable to high throughput screening should help with future characterization of drugs and gene products altering the secretory process.
We chose the mCherry fluorophore for this study because of the reasonable quantum yield, brightness, and stability of the fluorescent signal. mCherry exists as a monomer adding to the likelihood that the fluorescent signal accurately reports on the molar amounts of protein under study. Moreover, the mCherry with an optimal excitation wavelength of 587 nm and an emission at 610 nm allowed adequate detection with filter sets provided as a standard in most microplate readers and fluorescent microscopes. The sensitivity and the dynamic range of the mCherry-tagged secreted protein assay were comparable, if not better, than the Western blot. However, there are numerous fluorophores available derived from different organisms or engineered for specific biophysical properties (http://nic.ucsf.edu/FPvisualization) [8] and selection of a fluorophore with a greater quantum yield, brightness, and stability may extend the sensitivity and the dynamic range of this assay. Co-expression of different secreted proteins each tagged with different fluorophores should allow a multiplexed concurrent detection of multiple secreted proteins. Co-expression of an intracellularly confined reporter could serve as a control for the detection of cell death or lysis but this will require a 3rd fluorescence detection channel in addition to the two channels required for the detection of the target protein (mCherry) and secretion control (ss-eGFP). Furthermore, a proper control experiment with cytosolic-localizing mCherry should confirm the lack of cell death or lysis, as shown in Figure 1C, without the need for a simultaneous detection of another reporter. Possible effects on the secretory events due to overexpression of the protein could be reduced by creating a stable cell-line expressing a more physiological level of the target protein. Lastly, fluorescent epitope-tagging of endogenous genes using genomic editing methods should allow detection of secretion as well as subcellular trafficking of endogenous proteins eliminating potential problems due to exogenous overexpression of the protein of interest.
Supplementary Material
Acknowledgements
This work was supported by the Translational Cardiovascular Science Training Grant from the Cardiovascular Research Center at UW-Madison, NIH T32 HL07936-12 (TD); Bamforth Endowment Fund from the Department of Anesthesiology (JY), UW-Madison; NIH RO1 GM107054 (JY).
Footnotes
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- [1].Clark HF, Gurney AL, Abaya E, Baker K, Baldwin D, Brush J, Chen J, Chow B, Chui C, Crowley C, Currell B, Deuel B, Dowd P, Eaton D, Foster J, Grimaldi C, Gu Q, Hass PE, Heldens S, Huang A, Kim HS, Klimowski L, Jin Y, Johnson S, Lee J, Lewis L, Liao D, Mark M, Robbie E, Sanchez C, Schoenfeld J, Seshagiri S, Simmons L, Singh J, Smith V, Stinson J, Vagts A, Vandlen R, Watanabe C, Wieand D, Woods K, Xie M-H, Yansura D, Yi S, Yu G, Yuan J, Zhang M, Zhang Z, Goddard A, Wood WI, Godowski P, Gray A. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome research. 2003;13:2265–70. doi: 10.1101/gr.1293003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen Y, Zhang Y, Yin Y, Gao G, Li S, Jiang Y, Gu X, Luo J. SPD--a web-based secreted protein database. Nucleic acids research. 2005;33:D169–73. doi: 10.1093/nar/gki093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Caccia D, Dugo M, Callari M, Bongarzone I. Bioinformatics tools for secretome analysis. Biochimica et biophysica acta. 2013;1834:2442–53. doi: 10.1016/j.bbapap.2013.01.039. [DOI] [PubMed] [Google Scholar]
- [4].Presley JF. Imaging the secretory pathway: the past and future impact of live cell optical techniques. Biochimica et biophysica acta. 2005;1744:259–72. doi: 10.1016/j.bbamcr.2005.04.010. [DOI] [PubMed] [Google Scholar]
- [5].Stadler C, Rexhepaj E, Singan VR, Murphy RF, Pepperkok R, Uhlen M, Simpson JC, Lundberg E. Immunofluorescence and fluorescent-protein tagging show high correlation for protein localization in mammalian cells. Nature methods. 2013;10:315–23. doi: 10.1038/nmeth.2377. [DOI] [PubMed] [Google Scholar]
- [6].Goubaeva F, Giardina S, Yiu K, Parfyonova Y, Tkachuk VA, Yang J. T-cadherin GPI-anchor is insufficient for apical targeting in MDCK cells. Biochemical and biophysical research communications. 2005;329:624–31. doi: 10.1016/j.bbrc.2005.02.020. [DOI] [PubMed] [Google Scholar]
- [7].Duellman T, Warren C, Yang J. Single nucleotide polymorphism-specific regulation of matrix metalloproteinase-9 by multiple miRNAs targeting the coding exon. Nucleic acids research. 2014;42:5518–31. doi: 10.1093/nar/gku197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nature methods. 2005;2:905–9. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- [9].Hanania R, Sun HS, Xu K, Pustylnik S, Jeganathan S, Harrison RE. Classically activated macrophages use stable microtubules for matrix metalloproteinase-9 (MMP-9) secretion. The Journal of biological chemistry. 2012;287:8468–83. doi: 10.1074/jbc.M111.290676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Masure S, Proost P, Van Damme J, Opdenakker G. Purification and identification of 91-kDa neutrophil gelatinase. Release by the activating peptide interleukin-8. European journal of biochemistry / FEBS. 1991;198:391–8. doi: 10.1111/j.1432-1033.1991.tb16027.x. [DOI] [PubMed] [Google Scholar]
- [11].Appenzeller-Herzog C, Roche A-C, Nufer O, Hauri H-P. pH-induced conversion of the transport lectin ERGIC-53 triggers glycoprotein release. The Journal of biological chemistry. 2004;279:12943–50. doi: 10.1074/jbc.M313245200. [DOI] [PubMed] [Google Scholar]
- [12].Weisz OA. Acidification and protein traffic. International review of cytology. 2003;226:259–319. doi: 10.1016/s0074-7696(03)01005-2. [DOI] [PubMed] [Google Scholar]
- [13].Duellman T, Warren CL, Peissig P, Wynn M, Yang J. Matrix metalloproteinase-9 genotype as a potential genetic marker for abdominal aortic aneurysm. Circ Cardiovasc Genet. 2012;5:529–537. doi: 10.1161/CIRCGENETICS.112.963082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.