Abstract
In order to investigate the thermal degradation of glucose and maltose solutions after high temperature and high pressure (HTHP) treatment, the samples were treated at temperatures of 110, 120, 130, 140, and 150°C for 1, 2, 3, 4, and 5 h in an apparatus for HTHP treatment. Glucose and maltose solutions (20% w/w) were prepared by weighing glucose and maltose and adding distilled water in the desired proportion. Chromaticity, pH, organic acids, 5-hydroxymethylfurfural (HMF), free sugar contents, electron donating ability (EDA), and ascorbic acid equivalent antioxidant capacity (AEAC) were evaluated. With increasing heating temperatures and times, the L-, a-, and b-values decreased. The pH and free sugar contents decreased, and organic acids and HMF contents increased with greater temperatures and times. EDA (%) and the AEAC of the heating sugars increased with the increases in temperatures and times.
Keywords: glucose, maltose, thermal degradation, antioxidant activity, 5-hydroxymethylfurfural
INTRODUCTION
Thermal degradation of sugars may occur by two different major reaction pathways: the Maillard reaction, which takes place in the presence of amino acids, and caramelization, that occurs when simple sugars are heated at high temperatures (1,2). Caramelization is the common name for a group of reactions that occur when carbohydrates are exposed to high temperatures with no amino groups involved. This reaction is influenced by pH and sugar concentrations (3–5). Caramelization commonly occurs when sugars are heated, dry or in concentrated solution, either alone or with certain additives (6).
When highly concentrated sugar solutions are heated at high temperatures and neutral pH, sugar degradation occurs (3,4). Such solutions are especially important in confectionery products or during sugar boiling for white sugar production. The first reaction step in the caramelization process is sucrose hydrolysis leading to glucose and fructose production (7,8). Further degradation of these products is responsible for the formation of other compounds, with special preponderance to the thermodynamically stable 5-hydroxymethylfurfural (9–11). Little information is available on the kinetics of the thermal degradation of sucrose at high concentrations. Eggleston et al. (7) modeled the reaction assuming a pseudo-first-order reaction for concentrated sucrose thermal degradation in the presence of different salts. Haghighat Khajavi et al. (12) found this type of behavior in the thermal degradation of sucrose in subcritical water. Most of such studies reported the sucrose thermal degradation, but no study has examined changes in physicochemical characteristics in a glucose and maltose model system, with varying temperature and time.
Most studies reported sucrose thermal degradation, but we reported glucose and maltose thermal degradation. Thus, the objective of this study was to investigate the thermal degradation characteristics of glucose and maltose model system at neutral pH and absence of impurities. To achieve that, experiments were carried out in 20% (w/w) glucose and maltose solutions at various heating temperatures and times. After heating, chromaticity, pH, organic acids, 5-hydroxymethylfurfural (HMF), free sugar contents, electron donating ability (EDA), and ascorbic acid equivalent antioxidant capacity (AEAC) were investigated.
MATERIALS AND METHODS
Materials and sample preparation
Glucose, maltose, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate, acetic acid, citric acid, formic acid, lactic acid, and levulinic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). HMF was purchased from Wako Pure Chemical Inc. (Osaka, Japan). Water, acetonitrile, and methanol were purchased from J.T. Baker (Phillipsburg, NJ, USA). All other reagents were of analytical grade.
Twenty percent glucose and maltose solutions were prepared by weighing glucose and maltose, and adding distilled water in the desired proportion. Glucose and maltose solutions were placed in sample bottles and sealed tightly. Sample bottles were heated using high-pressure steam generated by a temperature-controlling apparatus (Jisico, Seoul, Korea). Sample bottles were heated to 110, 120, 130, 140, or 150°C for 1, 2, 3, 4, or 5 h (13–15). Heated samples were centrifuged (1,800 g, 10 min) and then the supernatant was filtered through a 0.45 μm syringe filter (Millipore, Billerica, MA, USA). Filtered samples were kept at −20°C until analysis.
Color measurement
Samples were poured into a clear glass petri dish, and color parameters were determined using a tri-stimulus colorimeter (Chroma Meter CR300, Konica Minolta Holdings, Inc., Tokyo, Japan). Results were calculated by the equipment based on the Hunter Lab color scale. In this scale, the L-value (lightness) ranges from 0 (black) to 100 (white), the a-value indicates degree of greenness (for negative a values) and degree of redness (for positive a results), and the b-value ranges from negative to positive values indicating, respectively, degree of blueness to yellowness. The apparatus was calibrated with a standard white tile (L-value=98.90, a-value=−0.10, and b-value=−0.36) before use. The mean values were obtained from triplicate measurements.
pH and organic acid contents
A pH meter (Model 320, Thermo Orion, Beverly, MA, USA) was used for the determination of pH values. Organic acid contents were measured according to a modification of the method of Šturm et al. (16). The separation of organic acids was performed with a high-performance liquid chromatograph (HPLC; Thermo Separation Products, San Jose, CA, USA), using an Aminex Ion Exclusion HPX-87H (7.8×300 mm, Bio-Rad Laboratories, Hercules, CA, USA) column with a guard column (Aminex Cation-H guard column, Bio-Rad Laboratories). Elution was carried out at a solvent flow rate of 0.6 mL/min, isocratically, with sulfuric acid (0.008 N) as the mobile phase. Detection was performed with a UV detector set at 215 nm. All samples were analyzed in triplicate.
Analysis of free sugars
The free sugar content was measured according to a modification of the method of Woo et al. (17), using fructose, glucose, and maltose as standards for calibration curves. Samples were filtered through a 0.45-μm syringe filter (Millipore) and analyzed by HPLC (Waters 2695, Waters, New Castle, DE, USA). The analytical column was for carbohydrates (4.6×150 mm, Waters) and the mobile phase was water-acetonitrile (25:75, v/v) at a flow rate of 1 mL/min. The injection volume was 20 μL, and the detector was an evaporative light scattering detector (Waters 2420, Waters). All samples were analyzed in triplicate.
Analysis of HMF contents
HMF contents were measured according to a modification of the method of Woo et al. (17). Samples were filtered through a 0.45 μm syringe filter (Millipore) and analyzed by HPLC (Thermo Separation Products). The analytical column was an LC-18 (4.6×250 mm), obtained from Phenomenex (Torrance, CA, USA). Water-acetonitrile (80:20 v/v) was used at a flow rate of 0.8 mL/min. The injection volume was 20 μL, and the UV detector was set at 280 nm. The standard used was HMF, and all samples were analyzed in triplicate.
Radical scavenging activity
The scavenging activity of samples for the DPPH radical was measured according to the method of Woo et al. (15), with some modifications. An aliquot of 0.8 mL of 0.2 mM DPPH methanolic solution was mixed with 0.2 mL of sample. The mixture was shaken vigorously and left to stand for 30 min under low light. The absorbance was measured at 520 nm. The scavenging activity of the extracts for the ABTS cation radical was measured according to the method of Woo et al. (14), with some modifications. The ABTS cation radical was generated by adding 7 mM ABTS to 2.45 mM potassium persulfate solution, and leaving the mixture to stand overnight in the dark at room temperature. The ABTS cation radical solution was diluted with distilled water to obtain an absorbance of 1.4~1.5 at 735 nm (molar extinction coefficient, e=3.6×104 mol−1 · cm−1). Diluted ABTS cation radical solution (1 mL) was added to 50 μL of extract, ascorbic acid standard solution, or distilled water. After 60 min, the absorbance was measured at 735 nm. The ABTS cation radical scavenging activity was expressed in terms of AEAC, as milligrams of ascorbic acid equivalents per gram of sample (mg AA eq/g sample).
Statistical analysis
Data were expressed as mean±standard deviation (SD). The significance of differences among treatment means was determined by one-way analysis of variance (ANOVA) and Duncan’s multiple range tests using SAS version 9.2 (SAS Institute, Cary, NC, USA) with a significance level of 0.05.
RESULTS AND DISCUSSION
Chromaticity
Changes in the color of glucose solution under different heating temperatures and times are shown in Table 1. The L-, a-, and b-values of unheated glucose solution were 40.66, 0.40, and −1.24, respectively. L-value decreased with increasing heating temperatures and time from 40.49 at 110°C for 1 h to 21.02 at 140°C for 4 h, and then increased thereafter. The a-value decreased at 140°C for 3 h and increased afterward. The b-value of heated glucose solution increased at 130°C for 3 h and decreased afterward. The L-, a-, and b-value of the heated glucose solution at 130°C for 3 h were 31.74, 1.98, and 12.13, respectively.
Table 1.
The chromaticity of 20% glucose and maltose solutions treated with high temperature and high pressure
| Temperature (°C) | Time (h) | Glucose | Maltose | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| L | a | b | L | a | b | ||
| Control | 40.66±0.21a | 0.40±0.02l | −1.24±0.01s | 38.92±1.27ab | 0.05±0.05ijk | 0.01±0.01r | |
| 110 | 1 | 40.49±0.45a | −0.99±0.04q | −0.03±0.03r | 38.89±1.02ab | 0.11±0.02i | 0.46±0.02q |
| 2 | 39.89±0.37ab | −0.84±0.01p | 0.94±0.03q | 37.59±0.69bc | 0.05±0.01ijk | 0.82±0.04p | |
| 3 | 39.82±0.50ab | −0.63±0.02o | 2.60±0.07n | 38.11±0.99ab | 0.07±0.03ij | 1.14±0.17o | |
| 4 | 39.07±0.95bcd | −0.27±0.02n | 3.36±0.02l | 38.27±1.08ab | −0.03±0.06jkl | 1.36±0.02no | |
| 5 | 37.91±0.84de | −0.05±0.01m | 4.97±0.32i | 38.40±1.57ab | −0.10±0.03l | 1.44±0.08n | |
| 120 | 1 | 39.59±0.99abc | −1.36±0.05s | 3.46±0.09l | 39.20±0.70a | −0.04±0.03kl | 1.12±0.01o |
| 2 | 38.88±0.93bcd | −1.30±0.06rs | 7.32±0.16g | 38.36±0.81ab | −0.12±0.04l | 1.99±0.03m | |
| 3 | 38.46±0.57cde | −0.85±0.04p | 7.55±0.15fg | 38.22±0.27ab | −0.22±0.04m | 2.10±0.05m | |
| 4 | 37.33±0.64ef | −0.36±0.03n | 8.87±0.20e | 38.35±0.35ab | −0.70±0.04p | 4.88±0.04i | |
| 5 | 36.45±0.26f | −0.31±0.06n | 11.17±0.11b | 36.01±1.05d | −0.57±0.04o | 5.19±0.05h | |
| 130 | 1 | 36.49±0.97f | −1.23±0.09r | 10.46±0.23c | 38.96±0.24ab | −0.82±0.05q | 3.75±0.05j |
| 2 | 32.13±0.97g | 0.47±0.07kl | 11.14±0.38b | 36.53±1.06cd | −0.66±0.06op | 6.50±0.34g | |
| 3 | 31.74±0.48g | 1.98±0.08g | 12.13±0.23a | 36.11±1.13d | −0.57±0.06o | 7.54±0.12f | |
| 4 | 29.20±0.14h | 3.72±0.03d | 11.02±0.21b | 35.49±0.41de | −0.47±0.01n | 9.61±0.10d | |
| 5 | 27.10±0.21i | 5.32±0.05b | 9.32±0.17d | 34.28±0.03ef | −0.03±0.06jkl | 10.75±0.05b | |
| 140 | 1 | 30.22±0.35h | 3.42±0.04e | 11.19±0.09b | 36.71±0.43cd | −1.05±0.02r | 9.52±0.10d |
| 2 | 26.43±0.81i | 5.29±0.09b | 7.62±0.15f | 33.93±0.19f | 0.12±0.04i | 11.61±0.11a | |
| 3 | 22.61±0.38k | 5.47±0.10a | 4.53±0.21j | 31.29±0.73g | 2.18±0.05e | 11.39±0.06a | |
| 4 | 21.02±1.46l | 3.41±0.21e | 2.29±0.12o | 28.18±0.19h | 4.43±0.14c | 9.27±0.31e | |
| 5 | 21.63±0.07kl | 1.71±0.05h | 1.66±0.11p | 25.39±0.47i | 5.13±0.08a | 7.47±0.20f | |
| 150 | 1 | 21.70±0.13kl | 4.36±0.08c | 2.97±0.04m | 28.98±0.40h | 3.56±0.03d | 10.46±0.31c |
| 2 | 21.94±0.49kl | 0.61±0.02j | 2.18±0.06o | 23.41±0.72j | 4.91±0.12b | 4.82±0.24i | |
| 3 | 24.13±1.10j | 0.53±0.05jk | 3.74±0.18k | 22.11±0.40k | 1.46±0.06g | 2.61±0.09l | |
| 4 | 25.22±0.22j | 0.97±0.05i | 4.39±0.03j | 23.42±0.47j | 0.57±0.05h | 3.03±0.07k | |
| 5 | 26.38±0.39i | 2.23±0.04f | 5.96±0.03h | 24.33±0.44ij | 1.74±0.03f | 4.72±0.18i | |
Each value is mean±SD (n=3).
Means in the same column followed by the same letters (a–s) are not significantly (P<0.05) different by Duncan’s multiple range test.
The L-, a-, and b-value of the heated glucose solution at 140°C for 3 h were 22.61, 5.47, and 4.53, respectively. The L-, a-, and b-value of the unheated maltose solution were 38.92, 0.05, and 0.01, respectively. With increasing heating temperature (110 to 150°C) and time (1 to 5 h), L-value of heated maltose solution decreased. The a-value of heated maltose solution decreased after treating at 140°C for 5 h and increased afterward. The b-value of heated maltose solution increased at 140°C for 3 h and decreased afterward. The L-, a-, and b-value of the heated maltose solution at 140°C for 3 h were 31.29, 2.18, and 11.39, respectively. There was a clear darkening of heated glucose and maltose solutions, evaluated by a decrease in the L-value, and a significant development of color expressed through the increase of heated glucose and maltose solution (3,5). Also, glucose and maltose thermal degradation products such as furfural, 5-methylfurfural, and HMF increase darkness (18).
pH
The pH of 20% glucose and maltose solutions under various HTHP treatment conditions is shown in Table 2. The pH of the water alone was about 5.0. The pH of the untreated glucose and maltose solutions was 4.53 and 4.04, respectively. The pH of heated glucose solution was acidic for all conditions and was generally between values of 2.89 and 4.14. The pH of heated maltose solution was acidic for all conditions and was generally between values of 3.03 and 3.78. The pH of heated 20% glucose and maltose solutions decreased with increased heating temperatures (110 to 150°C) and times (1 to 5 h). At a given heating time, higher temperatures gave lower pH values. At a given heating temperature, higher times gave lower pH values. The pH decrease with high temperature and high pressure can be attributed to the increase in yields of organic acids such as formic acid, lactic acid and levulinic acid (18–20). High temperatures, high pressures and heating times seem to promote the production of organic acids.
Table 2.
The pH and organic acid contents of 20% glucose and maltose solutions treated with high temperature and high pressure
| Variables | pH | Organic acid contents (mg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Temperature (ºC) | Time (h) | Glucose | Maltose | Glucose | Maltose | ||||
|
|
|
||||||||
| Formic acid | Lactic acid | Levulinic acid | Formic acid | Lactic acid | Levulinic acid | ||||
| Control | 4.53±0.03a | 4.04±0.02a | – | – | – | – | – | – | |
| 110 | 1 | 4.14±0.02b | 3.78±0.02b | – | – | 0.27±0.02m | – | – | 0.35±0.02ijk |
| 2 | 4.04±0.01c | 3.73±0.01cd | – | – | 0.29±0.01klm | – | – | 0.35±0.02hijk | |
| 3 | 3.90±0.01d | 3.71±0.05d | – | – | 0.29±0.01kl | – | – | 0.36±0.02fghi | |
| 4 | 3.75±0.01e | 3.65±0.01e | – | – | 0.42±0.01ef | – | – | 0.37±0.02efgh | |
| 5 | 3.71±0.01f | 3.64±0.01e | – | – | 0.56±0.02a | – | – | 0.37±0.01efg | |
| 120 | 1 | 3.70±0.02f | 3.75±0.02c | – | – | 0.30±0.01k | – | – | 0.31±0.01m |
| 2 | 3.62±0.01g | 3.63±0.01ef | – | 0.01±0.00m | 0.32±0.02j | – | – | 0.32±0.01l | |
| 3 | 3.57±0.01h | 3.61±0.01fg | – | 0.02±0.00lm | 0.34±0.02ij | – | – | 0.33±0.01kl | |
| 4 | 3.40±0.01j | 3.60±0.01g | – | 0.03±0.00kl | 0.37±0.01h | – | – | 0.33±0.02jkl | |
| 5 | 3.38±0.01k | 3.54±0.01i | 0.17±0.04i | 0.03±0.00kl | 0.47±0.01c | – | – | 0.35±0.01hijk | |
| 130 | 1 | 3.46±0.01i | 3.74±0.02c | 0.18±0.01i | 0.01±0.00m | 0.28±0.02lm | – | – | 0.38±0.02def |
| 2 | 3.38±0.01k | 3.55±0.01hi | 0.23±0.01h | 0.03±0.00kl | 0.28±0.02klm | – | – | 0.38±0.02de | |
| 3 | 3.33±0.01l | 3.42±0.01j | 0.25±0.02g | 0.04±0.00jk | 0.34±0.01ij | – | – | 0.39±0.01d | |
| 4 | 3.28±0.01m | 3.41±0.03j | 0.26±0.02g | 0.05±0.00ij | 0.34±0.01ij | – | – | 0.39±0.01d | |
| 5 | 3.26±0.01n | 3.40±0.01j | 0.33±0.01f | 0.06±0.00i | 0.35±0.01hi | – | – | 0.40±0.01d | |
| 140 | 1 | 3.28±0.01m | 3.57±0.01h | 0.18±0.01i | 0.05±0.00ij | 0.39±0.01g | – | – | 0.35±0.02ghijk |
| 2 | 3.21±0.01o | 3.36±0.01k | 0.22±0.02h | 0.06±0.00i | 0.40±0.01g | – | 0.03±0.00f | 0.36±0.02fghi | |
| 3 | 3.16±0.01p | 3.30±0.02l | 0.39±0.01e | 0.08±0.00h | 0.41±0.02fg | – | 0.04±0.00ef | 0.36±0.01efghi | |
| 4 | 3.12±0.02q | 3.22±0.01m | 0.43±0.03d | 0.14±0.01f | 0.42±0.02ef | – | 0.05±0.00e | 0.39±0.03d | |
| 5 | 3.09±0.01r | 3.16±0.01n | 0.46±0.01c | 0.19±0.01e | 0.45±0.01cd | 0.18±0.03e | 0.05±0.00e | 0.39±0.03d | |
| 150 | 1 | 3.09±0.01r | 3.22±0.01m | 0.43±0.01d | 0.11±0.01g | 0.40±0.01g | 0.18±0.00e | 0.08±0.00d | 0.35±0.01fghij |
| 2 | 3.04±0.01s | 3.11±0.01o | 0.46±0.02c | 0.25±0.01d | 0.43±0.03de | 0.25±0.01d | 0.08±0.00d | 0.39±0.02d | |
| 3 | 2.96±0.01t | 3.05±0.01p | 0.50±0.03a | 0.37±0.02c | 0.44±0.03de | 0.56±0.01c | 0.24±0.00c | 0.43±0.01c | |
| 4 | 2.95±0.01t | 3.05±0.01p | 0.48±0.02b | 0.54±0.03b | 0.49±0.02b | 0.60±0.02b | 0.39±0.02b | 0.47±0.01b | |
| 5 | 2.89±0.01u | 3.03±0.02p | 0.46±0.03c | 0.85±0.02a | 0.51±0.01b | 0.65±0.02a | 0.67±0.01a | 0.51±0.03a | |
Each value is mean±SD (n=3).
Means in the same column followed by the same letters (a–u) are not significantly (P<0.05) different by Duncan’s multiple range test.
Organic acid contents
The organic acid contents of 20% glucose and maltose solutions with HTHP treatment are shown in Table 2. The organic acid of the untreated glucose and maltose solutions were not detected. The contents of organic acid such as formic acid, lactic acid and levulinic acid increased with increasing heating temperatures (110 to 150°C) and times (1 to 5 h). Formic acid content of heated glucose solution was not detected at 120°C for 4 hr. The formic acid content of heated glucose solution was 0.17 mg/mL at 120°C for 5 h and increased afterward. The maximum contents of formic acid treated at 150°C for 3 h were 0.50 mg/mL. Lactic acid content of heated glucose solution was not detected at 120°C for 1 h. The maximum contents of lactic acid treated at 150°C for 5 h were 0.85 mg/mL. Levulinic acid content of heated glucose solution increased with increasing heating temperature and time. The maximum contents of formic acid treated at 150°C for 5 h were 0.51 mg/mL. Formic acid content of heated maltose solution was not detected at 140°C for 4 h. Lactic acid content of heated maltose solution was not detected at 140°C for 1 h. Levulinic acid content of heated maltose solution increased with increasing heating temperature and time. The maximum contents of formic acid, lactic acid, and levulinic acid treated at 150°C for 5 h were 0.65, 0.67, and 0.51 mg/mL, respectively. Aida et al. (19) and Shaw et al. (21) reported strong relationships between organic acid contents and heating conditions.
Free sugar contents
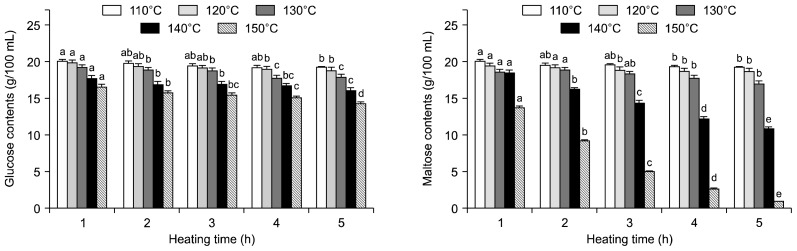
The glucose and maltose contents of 20% glucose and maltose solutions with HTHP treatment are shown in Fig. 1. The glucose and maltose contents of the untreated glucose and maltose solution were 20.07 and 20.14 g/100 mL, respectively. The glucose and maltose contents of heated glucose and maltose solutions slowly decreased with increasing heating temperature and time. However, the fructose contents of the maltose solution were suddenly decreased at 140 and 150°C (data not shown). The case of heat treatment on maltose solution was decomposed than glucose and fructose. Caramelization is the common name for a group of reactions that occur when carbohydrates are exposed to high temperatures. They often occur during the preparation of traditional sucrose syrups and caramels, which are extensively used in confectionery and pastry products (22). The case of heat treatment on fructose was formed than heat degradation products such as furfural, 5-methylfurfural, HMF, formic acid, lactic acid, and levulinic acid (18).
Fig. 1.
Glucose and maltose contents of glucose and maltose solution treated with high temperature and high pressure treatment. Glucose content of untreated glucose solution was 20.07 g/100 mL. Maltose content of untreated maltose solution was 20.14 g/100 mL. Means within the same temperature followed by the same letter (a–e) are not significantly (P<0.05) different by Duncan’s multiple range test.
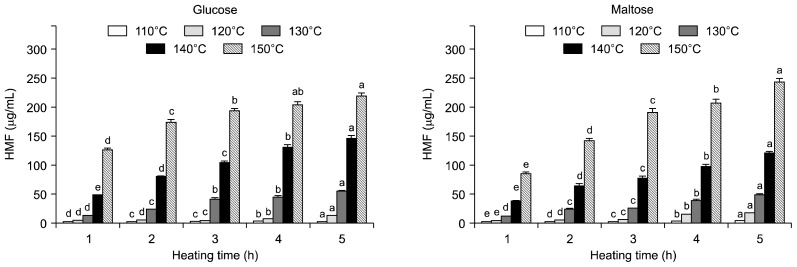
HMF contents
HMF contents of 20% glucose and maltose solutions with HTHP treatment are shown in Fig. 2. HMF is a key furan derivative readily accessible from renewable resources like carbohydrates, in particular through acid-catalyzed dehydration of fructose or fructose-precursors. HMF is a particularly suitable starting material for the preparation of further furanic monomers required for the preparation of non-petroleum-derived polymeric materials such as polyesters, polyamides and polyurethanes (23). The contents of HMF increased with increasing heating temperatures (110 to 150°C) and times (1 to 5 h). The HMF content of the untreated glucose and maltose solution was 0.40 and 0.16 μg/mL, respectively. The HMF contents of glucose and maltose solution increased with increasing heating temperatures (110 to 150°C) and times (1 to 5 h). The maximum contents of HMF of heated glucose and maltose solution treated at 150°C for 5 h were 217.99 and 243.02 μg/mL, respectively.
Fig. 2.
5-Hydroxymethylfurfural (HMF) contents of glucose and maltose solution treated with high temperature and high pressure treatment. HMF contents of untreated glucose and maltose solution were 0.04 and 0.02 mg/mL, respectively. Means within the same temperature followed by the same letter (a–e) are not significantly (P<0.05) different by Duncan’s multiple range test.
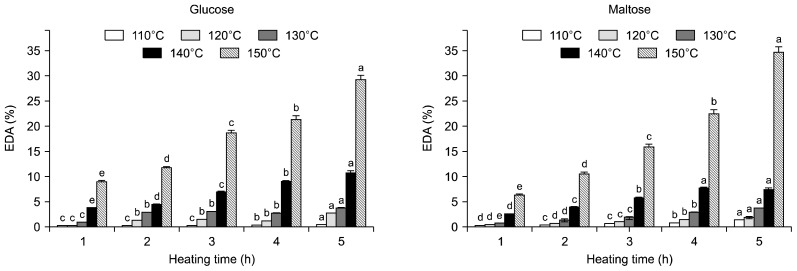
Radical scavenging activity
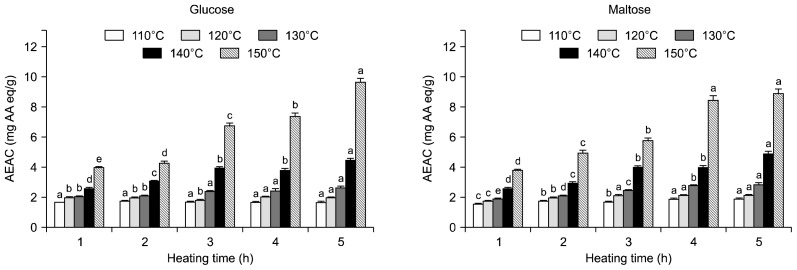
DPPH radical scavenging activities (EDA) of 20% glucose and maltose solution with HTHP treatment are show in Fig. 3. The EDA increased with increasing heating temperatures (110 to 150°C) and times (1 to 5 h). EDA of the untreated glucose solution and heated glucose solution at 110°C for 3 h and the untreated maltose solution and heated maltose solution at 110°C for 1 h at concentration of 1 g/mL were zero. The EDA of heated glucose and maltose solutions increased at 150°C for 5 h and the maximum EDA observed at 150°C for 5 h were 29.04 and 34.72%, respectively. ABTS cation radical scavenging activity (AEAC) of 20% glucose and maltose solutions with HTHP treatment are shown in Fig. 4. AEAC of the untreated glucose and maltose solution were 1.72 and 1.40 mg AA eq/g. The AEAC increased with increasing heating temperatures (110 to 150°C) and times (1 to 5 h). The AEAC of heated glucose and maltose solutions increased at 150°C for 5 h and the maximum AEAC observed at 150°C for 5 h were 9.59 and 2.96 mg AA eq/g, respectively. AEAC results appear identical to the EDA. Several researchers indicate that heating causes enhanced antioxidant activity in fruits and vegetables because of the enhancement of the antioxidant properties of naturally occurring compounds or the formation of novel compounds such as Maillard reaction products that have antioxidant activity (24,25). Francisco and Salvio (26) reported that there exists a proportional relationship among the degree of browning of melanodin, radical scavenging effect, and antioxidation. Also, Yilmaz and Toledo (27) reported that Maillard reaction products (MRPs) had antioxidant activity. MRPs, especially melanoidins, have been reported to have antioxidant activity through scavenging oxygen radicals or chelating metals.
Fig. 3.
DPPH radical scavenging activity of glucose and maltose solution treated with high temperature and high pressure treatment. DPPH radical scavenging activity of untreated glucose and maltose solution were 0.00% all. Means within the same temperature followed by the same letter (a–e) are not significantly (P<0.05) different by Duncan’s multiple range test.
Fig. 4.
ABTS cation radical scavenging activity of glucose and maltose solution treated with high temperature and high pressure treatment. ABTS cation radical scavenging activity of untreated glucose and maltose solution were 1.72 and 1.40 mg AA eq/g sample, respectively. Means within the same temperature followed by the same letter (a–e) are not significantly (P<0.05) different by Duncan’s multiple range test.
ACKNOWLEDGEMENTS
This study was supported by a grant (PJ010155) from the AGENDA Program, Rural Development Administration, Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.BeMiller JN, Whistler RL. Carbohydrates. In: Fennema OR, editor. Food Chemistry. 3rd ed. Marcel Dekker, Inc; New York, NY, USA: 1996. pp. 157–224. [Google Scholar]
- 2.Quintas M, Brandão TRS, Silva CLM. Modelling autocatalytic behaviour of a food model system–sucrose thermal degradation at high concentrations. J Food Eng. 2007;78:537–545. doi: 10.1016/j.jfoodeng.2005.10.031. [DOI] [Google Scholar]
- 3.Clarke MA, Edye LA, Eggleston G. Sucrose decomposition in aqueous solution, and losses in sugar manufacture and refining. Advan Carbohyd Chem Biochem. 1997;52:441–470. doi: 10.1016/S0065-2318(08)60095-5. [DOI] [Google Scholar]
- 4.Lowary TL, Richards GN. Effects of impurities on hydrolysis of sucrose in concentrated aqueous solution. Int Sugar J. 1988;90:164–167. [Google Scholar]
- 5.Eggleston G, Vercellotti JR. Degradation of sucrose, glucose and fructose in concentrated aqueous solutions under constant pH conditions at elevated temperature. J Carbohydr Chem. 2000;19:1305–1318. doi: 10.1080/07328300008544153. [DOI] [Google Scholar]
- 6.Ratsimba V, García Fernández JM, Defaye J, Nigay H, Voilley A. Qualitative and quantitative evaluation of mono- and disaccharides in D-fructose, D-glucose and sucrose caramels by gas-liquid chromatography-mass spectrometry. Di-D-fructose dianhydrides as tracers of caramel authenticity. J Chromatogr A. 1999;844:283–293. doi: 10.1016/S0021-9673(99)00322-2. [DOI] [PubMed] [Google Scholar]
- 7.Eggleston G, Trask-Morrel BJ, Vercellotti JR. Use of differential scanning calorimetry and thermogravimetric analysis to characterize the thermal degradation of crystalline sucrose and dried sucrose–salt residues. J Agric Food Chem. 1996;44:3319–3325. doi: 10.1021/jf950836z. [DOI] [Google Scholar]
- 8.Richards GN. Initial steps in thermal degradation of sucrose. Int Sugar J. 1986;88:145–148. [Google Scholar]
- 9.Kroh LW. Caramelisation in food and beverages. Food Chem. 1994;51:373–379. doi: 10.1016/0308-8146(94)90188-0. [DOI] [Google Scholar]
- 10.Mauch W. Chemical properties of sucrose. Sugar Technol Rev. 1971;1:239–290. [Google Scholar]
- 11.Antal MJ, Jr, Mok WSL, Richards GN. Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohydr Res. 1990;199:91–109. doi: 10.1016/0008-6215(90)84096-D. [DOI] [PubMed] [Google Scholar]
- 12.Haghighat Khajavi S, Kimura Y, Oomori T, Matsuno R, Adachi S. Kinetics on sucrose decomposition in sub-critical water. LWT-Food Sci Technol. 2005;38:297–302. doi: 10.1016/j.lwt.2004.06.005. [DOI] [Google Scholar]
- 13.Hwang IG, Woo KS, Kim DJ, Hong JT, Hwang BY, Lee YR, Jeong HS. Isolation and identification of an antioxidant substance from heated garlic (Allium sativum L.) Food Sci Biotechnol. 2007;16:963–966. [Google Scholar]
- 14.Woo KS, Hwang IG, Kim TM, Kim DJ, Hong JT, Jeong HS. Changes in the antioxidant activity of onion (Allium cepa) extracts with heat treatment. Food Sci Biotechnol. 2007;16:828–831. [Google Scholar]
- 15.Woo KS, Yoon HS, Lee YR, Lee JS, Kim DJ, Hong JT, Jeong HS. Characteristics and antioxidative activity of volatile compounds in heated garlic (Allium sativum) Food Sci Biotechnol. 2007;16:822–827. [Google Scholar]
- 16.Sturm K, Koron D, Stampar F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003;83:417–422. doi: 10.1016/S0308-8146(03)00124-9. [DOI] [Google Scholar]
- 17.Woo KS, Hwang IG, Lee YR, Lee JS, Jeong HS. Characteristics of sucrose thermal degradation with high temperature and high pressure treatment. Food Sci Biotechnol. 2009;18:717–723. [Google Scholar]
- 18.Kim HJ, Taub IA. Intrinsic chemical markers for aseptic processing of particulate foods. Food Technol. 1993;47:91–99. doi: 10.1111/j.1365-2621.2011.02811.x. [DOI] [Google Scholar]
- 19.Aida TM, Saito Y, Watanabe M, Tajima K, Nonaka T, Hattori H, Arai K. Dehydration of D-glucose in high temperature water at pressures up to 80 MPa. J Supercrit Fluids. 2007;40:381–388. doi: 10.1016/j.supflu.2006.07.027. [DOI] [Google Scholar]
- 20.Aida TM, Tajima K, Watanabe M, Saito Y, Kuroda K, Nonaka T, Hattori H, Smith RL, Jr, Arai K. Reactions of D-fructose in water at temperatures up to 400°C and pressures up to 100 MPa. J Supercrit Fluids. 2007;42:110–119. doi: 10.1016/j.supflu.2006.12.017. [DOI] [Google Scholar]
- 21.Shaw PE, Tatum JH, Berry RE. 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, a degradation product of a hexose. Carbohydr Res. 1971;16:207–211. doi: 10.1016/S0008-6215(00)86115-7. [DOI] [Google Scholar]
- 22.Quintas MAC, Guimarães C, Baylina J, Brandão TRS, Silva CLM. Multiresponse modelling of the caramelisation reaction. Innovative Food Sci Emerging Technol. 2007;8:306–315. doi: 10.1016/j.ifset.2007.02.002. [DOI] [Google Scholar]
- 23.Lansalot-Matras C, Moreau C. Dehydration of fructose into 5-hydroxymethylfurfural in the presence of ionic liquids. Catal Commun. 2003;4:517–520. doi: 10.1016/S1566-7367(03)00133-X. [DOI] [Google Scholar]
- 24.Manzocco L, Calligaris S, Mastrocola D, Nicoli MC, Lerici CR. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Technol. 2001;11:340–346. doi: 10.1016/S0924-2244(01)00014-0. [DOI] [Google Scholar]
- 25.Kim MJ, Kim CY, Park IS. Prevention of enzymatic browning of pear by onion extract. Food Chem. 2005;89:181–184. doi: 10.1016/j.foodchem.2004.02.018. [DOI] [Google Scholar]
- 26.Morales FJ, Jiménez-Pérez S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001;72:119–125. doi: 10.1016/S0308-8146(00)00239-9. [DOI] [Google Scholar]
- 27.Yilmaz Y, Toledo R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005;93:273–278. doi: 10.1016/j.foodchem.2004.09.043. [DOI] [Google Scholar]