Abstract
This study was performed to investigate changes in the content and purity, as well as physical characteristics of β-glucan extracted from acid hydrolyzed whole grain barleys. Waxy and non-waxy barleys (Hordeum vulgare) were hydrolyzed with different concentrations of HCl (0.1~0.5 N) for 1 h. As the HCl concentration increased, the contents of total and soluble β-glucan from acid hydrolyzed barley decreased. However the ratio of soluble/total β-glucan content and purities of β-glucan significantly increased. The ratio of β-(1→4)/β-(1→3) linkages, molecular weight, and viscosity of soluble β-glucan of raw barleys were 2.28~2.52, 6.0~7.0×105 g/mol, and 12.8~32.8 centipoise (cP). Those of isolated soluble β-glucan were significantly decreased to 2.05~2.15, 6.6~7.8×103 g/mol, and 3.6~4.2 cP, respectively, with increasing acid concentration. The re-solubility of raw barley β-glucan was about 50%, but increased to 97% with increasing acid concentration. Acid hydrolysis was shown to be an effective method to produce β-glucan with high ratio of soluble β-glucan content, purity, water solubility, and low viscosity.
Keywords: barley, β-glucan, acid hydrolysis, molecular weight, viscosity
INTRODUCTION
Cereal β-glucan is a major structural polysaccharide composed of d-glucose with β-(1→3) and β-(1→4) glycosidic linkages in the endospermic cell walls of oats, barley, and other cereals (1). Naked barley and unhulled barley contain approximately 2~8% β-glucans (2–4). The β-glucan content of waxy barley is approximately 1.0~2.5% higher than that of non-waxy barley (3), and waxy barley has been suggested to be a major dietary source of β-glucan (5). The average soluble β-glucan content of barley has been recorded at 30%, with a range of 20 to 50% as percentage of total β-glucan but varies depending on barley variety, growing environment, milling ratio, and extraction conditions (2–5). Soluble barley β-glucan, as a cereal soluble dietary fiber, is known to reduce post-prandial blood glucose levels (6), blood cholesterol levels (7), and improves serum lipid profiles (8). However, the potential food applications of β-glucans are still limited to cereal-based products because of β-glucan’s unique physical properties, such as high viscosity and low solubility, which causes poor wort separation and difficulties in beer filtration. Problems also occur in sauces, salad-dressings, and ice-cream formulations, and formation of undesirable precipitates may occur (9–11). Since the molecular weight of a polymer is an important factor affecting viscosity, several methods have been used for β-glucan depolymerization, including enzymatic hydrolysis (8,12) and physical treatments (9,13,14). Although these methods have been effective in β-glucan depolymerization, they have their own disadvantages such as a high cost, low yield, long processing time and the need to dispose of wastes (15,16).
Acid hydrolysis using hydrochloric acid, sulphuric acid, phosphoric acid or nitric acid can quickly and efficiently degrade β-glucan without requiring pre-treatment to break down the glycosidic bond of the polysaccharide, which is required for enzymatic/physical hydrolysis (17). Müller et al. (18) reported that acid hydrolysis of yeast β-glucan using HCl, acetic acid, formic acid, or phosphoric acid decreased both the molecular weight and viscosity; among acids studied, HCl showed superior depolymerisation abilities. Acid hydrolysis using HCl or sulphuric acid is generally carried out at concentrations of 1~10% and temperatures of 100~150°C. Under these conditions, the functionality of β-glucan as soluble dietary fiber is limited because the polysaccharide is largely hydrolyzed to glucose (17). Other workers have reported that excessive acid hydrolysis produces by-products such as furfural, aldehyde and acetic acid (19). Appropriate acid-hydrolysis conditions are necessary for depolymerization because acid use results in a large amount of salt produced after neutralisation.
Research for improving the physical properties and solubility of barley β-glucan by acid hydrolysis is needed because the high molecular weight and viscosity of β-glucan currently limits its industrial use (9–11). Many researches have reported that acid hydrolysis decreases the molecular weight and viscosity of β-glucan. However, these studies performed acid hydrolysis on extracted and purified β-glucan or the β-glucan rich-fraction from barley bran. Also, few studies investigated changes in content and purity of β-glucan from whole grain barley after acid hydrolysis.
The aim of the present study was to investigate the changes in the content and purity of total and soluble β-glucan from three acid-hydrolysed whole grain barley varieties under different concentrations of hydrochloric acid and to evaluate the physical characteristics of soluble β-glucan.
MATERIALS AND METHODS
Materials
Whole grain barley was grown and harvested in 2010 (winter barleys), and obtained from the Division of Rice and Winter Cereal Crop, National Institute of Crop Science, Iksan, Korea. The varieties Saessal, Saechal, and Hinchal, recommended cultivars, were milled (Micro hammer cutter mill type-3; Culatti AG, Zurich, Switzerland) to a particle size of <180 μm and stored at −18°C before acid hydrolysis.
Acid hydrolysis
For acid hydrolysis, 5 g of barley powder was dissolved in 100 mL of HCl (Samchun Chemical, Pyeongtaek, Korea) under different concentrations of 0.1~0.5 N. Samples were subjected to hydrolysis at 50°C for 60 min using a shaking water bath (JSSB-30T; JS Research Inc., Gongju, Korea). The samples were then cooled to room temperature and neutralized with 1.0 N NaOH. The extract was centrifuged at 3,500 rpm for 15 min, and then freeze-dried (MCFD, Ilshin BioBase Co., Ltd., Dongduchun, Korea).
Extraction of β-glucan
Extraction of β-glucan from hydrolyzed barley powders was carried out using the methods of Wood et al. (1) for the extraction of total β-glucan. An amount of 250 mL of 1.0 N NaOH was added to the hydrolyzed solution and extracted at 65°C for 60 min using a shaking water bath. After, samples were quickly cooled to room temperature and neutralised (pH 6.5) with 1 N HCl. The extract was centrifuged at 3,500 rpm for 15 min (4ºC), and then 250 μL of heat stable α-amylase (A3306; Sigma-Aldrich Co., St. Louis, MO, USA) and 175 mg of calcium chloride (C1016; Sigma-Aldrich Co.) were added to the supernatant. The supernatant was then incubated at 95°C for 60 min with shaking (150 rpm) and cooled to room temperature; then, the pH was adjusted to 4.5 with 1.0 N HCl. The supernatant was again centrifuged at 3,500 rpm for 15 min and the pellet was discarded. Ethanol was added to equal 80% of the final concentration and kept overnight at 4°C. Total β-glucan was then obtained using centrifugation at 3,500 rpm for 15 min. For the extraction of soluble β-glucan, 250 mL of deionised water was added to the hydrolysed solution and then extracted using the same method as the total β-glucan extraction procedure. Total and soluble β-glucan were re-suspended in water and washed with 100% ethanol twice and centrifuged again, after which the pellets were homogenised (20,000 rpm; IKA Laboratory, Staufen, Germany) in water and freeze-dried.
Measurement of β-glucan purity
The purity of isolated soluble β-glucan was measured according to the method of McCleary and Glennie-Holmes (4) using the β-D-glucan enzymatic assay kit (Megazyme International Ireland Ltd., Wicklow, Ireland.). A 100 mg dried soluble β-glucan powder was transferred into a test tube and followed by adding 0.2 mL of 50% (v/v) ethanol and 4.0 mL of 20 mM sodium phosphate buffer (pH 6.5). The contents were then vortexed to produce a homogenous solution. The tubes were incubated in a boiling water bath for 3 min followed by cooling to 50°C. The enzyme, lichenase (10 U), was added and tubes were incubated for 60 min at 50°C with intermittent vortexing. Then 5.0 mL of 200 mM sodium acetate buffer (pH 4.0) was added to terminate the enzyme reaction and samples was centrifuged at 4,000 rpm for 10 min. The supernatant (0.1 mL) was transferred to three test tubes. Then 0.1 mL of 50 mM sodium acetate buffer (pH 4.0) was added to one of these tubes (reaction blank), while in the other two tubes, 0.2 U β-glucosidase was added. All three were incubated at 50°C for 10 min. After incubation, 3.0 mL of glucose oxidase/peroxidase (GOPOD) reagent was added in all tubes and incubated at 50°C for 20 min. A spectrophotometer (UV-1650 PC; Shimadzu, Kyoto, Japan) was used to measure absorbance at 510 nm.
Ratio of β-(1→4)/(1→3) linkages determination
The ratio of cellulosic oligomers in the chain of β-glucans was determined by lichenase and β-glucosidase treatment (1). β-Glucan powders were dissolved in buffer solution (25 mg/mL, 20 mM sodium phosphate buffer, pH 6.5) and incubated with lichenase (10 U/mL, Megazyme International Ireland Ltd.) for 1 h at 50°C. Then 5.0 mL of 200 mM sodium acetate buffer (pH 4.0) was added to terminate the enzyme reaction and tubes were centrifuged at 13,000 rpm for 15 min. The supernatants (1.0 mL) were incubated with exo-(1→3)-glucanse (1 U/mL, Sigma-Aldrich Co.) for 1 h at 50°C. The digests were heated to 95°C (15 min) to inactivate the enzyme and the supernatants were reacted with GOPOD determining reagent and measured at 510 nm for analysis of total glucose contents from the β-glucans. In another experimental, 1.0 mL of the supernatant was transferred to three separate test tubes. Then 0.1 mL of 50 mM sodium acetate buffer (pH 4.0) was added to the first tube (reaction blank). In the other two tubes, β-glucosidase (5 U/mL) was added and tubes were incubated at 50°C for 20 min. After incubation, 3.0 mL of GOPOD determining reagent was added to all tubes and incubated at 50°C for 20 min. A spectrophotometer was used to measure absorbance at 510 nm for analysis of glucose contents from β-(1→3)-glucans.
Average molecular weight
Measurements of the average molecular weight of isolated soluble β-glucan were carried out using the method of Kim and Ryu (20). Gel permeation chromatography (GPC) was performed at room temperature using a high-performance liquid chromatography system (Acme 9000 HPLC system; Younglin Instrument Co., Anyang, Korea) with an YMC Diol-300 size exclusion column (300×4.6 mm I.D., particle size 5 μm, pore size 30 nm; YMC Co. Ltd., Kyoto, Japan) and a refractive index detector (RI 750F; Younglin Instrument Co.). The flow rate of the mobile phase (deionized water) was 0.5 mL/min and the injection volume of 20 μL at a concentration of 1.0% (w/v). Pullulan standards (Mw range, 5,900~708,000 g/mol; Showa-Denko, Tokyo, Japan) were used to calibrate the method. The regression equation of interpolation was
where y is log molecular weight and x is retention time, respectively, and coefficient determination was 0.995.
Apparent viscosity and resolubility
For apparent viscosity measurements, 0.25~2.00% (w/v, dry basis) β-glucan solution was prepared by heating at 95°C for 30 min. Viscosity was measured at room temperature using a viscometer (DV-II; Brookfield Engineering Laboratories, Middleborough, MA, USA) with the S01 spindle at 100 rpm (21). Re-solubility of isolated soluble β-glucan was carried out using a centrifugal method (22). An amount of 0.5 g of the β-glucan was put into a centrifuge tube with a cap, then 50 mL of deionized water added followed by incubating at 50°C for 12 h and centrifugation at 12,000 rpm for 10 min. The pellet was dried at 105°C for 2 h. The weight of the dried soluble β-glucan was obtained in the form of a pellet.
Statistical analysis
The results are reported as the mean±standard deviation (n=3). The significance of differences among treatment means was determined using one-way analysis of variance (ANOVA), calculated by SPSS version 12 (SPSS Institute, Chicago, IL, USA), with a significance level of P<0.05 by Duncan’s multiple range test.
RESULTS AND DISCUSSION
Total, soluble, and insoluble β-glucan contents
The contents of total, soluble and insoluble β-glucan from acid hydrolyzed barley using different concentrations of HCl are shown in Table 1. Total β-glucan content of raw Saessal was 8.41%, and the content significantly decreased to 1.21% as the HCl concentration increased from 0.1 N to 0.5 N. The total β-glucan contents of raw Saechal and Hinchal were initially 7.77% and 8.24%, respectively; however, these contents decreased to 2.19% and 2.24%, respectively, after acid hydrolysis (P<0.05). In similar studies, the total β-glucan content of barley ranged from 2% to 8% and varied by cultivar, growing environment, milling ratio and extraction conditions (2–4,23,24). The soluble β-glucan contents before hydorlysis of raw Saessal, Saechal, and Hinchal were 4.83%, 4.18%, and 4.61%, respectively; however, these values decreased significantly to 1.08%, 1.77%, and 1.72% when the HCl concentration used was 0.5 N. The soluble β-glucan content of barley ranged from 1.68% to 3.13% (24), and on average, 54% of the total β-glucan in barley was soluble (2). In other studies, acid hydrolysis has been noted to decrease the degree of polymerisation of oat β-glucan by destroying the glycosidic bond of the polysaccharide, resulting in increased proportions of glucose, cellobiose, laminaribiose, and cellobiose (17). When whole grain oats were acid-hydrolysed, the total and soluble β-glucan contents decreased with a decreasing degree of β-glucan polymerization (25). In the case of solubility, the ratio of soluble to insoluble β-glucan was higher for Saessal (1.35) than for Saechal (1.16) or Hinchal (1.27). Furthermore, solubility increased to 6.75, 4.21, and 3.31, respectively, when the HCl concentration increased to 0.5 N.
Table 1.
The content of total, soluble, and insoluble β-glucan from three acid-hydrolyzed barley varieties with different concentrations of HCl
| Varieties | HCl concentration (N) | Total β-glucan (%) | Soluble β-glucan | Insoluble β-glucan | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| (%) | (% of total) | (%) | (% of total) | |||
| Saessal | Control | 8.41±0.12a | 4.83±0.13a | 57.43d | 3.58a | 42.57a |
| 0.1 | 6.21±0.21b | 3.57±0.11b | 57.49d | 2.64b | 42.51a | |
| 0.2 | 5.45±0.17c | 3.44±0.09b | 63.13c | 2.01c | 36.87b | |
| 0.3 | 2.22±0.11d | 1.57±0.05c | 70.76b | 0.65d | 29.24c | |
| 0.4 | 1.78±0.08e | 1.54±0.07c | 86.52a | 0.24e | 13.48d | |
| 0.5 | 1.21±0.05f | 1.08±0.08d | 89.18a | 0.13e | 10.82d | |
| Saechal | Control | 7.77±0.29a | 4.18±0.09a | 53.81e | 3.59a | 46.18a |
| 0.1 | 7.24±0.17b | 3.98±0.12b | 54.97e | 3.26b | 45.03a | |
| 0.2 | 5.78±0.08c | 3.27±0.11c | 56.56d | 2.51c | 43.44b | |
| 0.3 | 4.12±0.11d | 2.87±0.07d | 69.66c | 1.25d | 30.34c | |
| 0.4 | 2.84±0.08e | 2.14±0.09e | 75.33b | 0.70e | 24.67d | |
| 0.5 | 2.19±0.09f | 1.77±0.08f | 80.81a | 0.42f | 19.19e | |
| Hinchal | Control | 8.24±0.11a | 4.61±0.24a | 55.93e | 3.63a | 44.07a |
| 0.1 | 7.44±0.12b | 4.24±0.13b | 56.98e | 3.20b | 43.02a | |
| 0.2 | 6.17±0.10c | 3.78±0.11c | 61.25d | 2.39c | 38.75b | |
| 0.3 | 4.45±0.12d | 2.89±0.08d | 64.94c | 1.56d | 35.06c | |
| 0.4 | 2.81±0.09e | 1.94±0.11e | 69.00b | 0.87e | 30.99d | |
| 0.5 | 2.24±0.10f | 1.72±0.05e | 76.82a | 0.52f | 23.18e | |
Saessal as non-waxy barley, Saechal and Hinchal as waxy-barley.
Results are expressed as the average of triplicate samples with mean±SD (n=3).
Any means within the same barley varieties followed by the different letters (a–f) are significantly different (P<0.05) by Duncan’s multiple range test
Purity of β-glucan
The purity of acid hydrolyzed barley, total and soluble β-glucan with different concentrations of HCl is shown in Table 2. The initial purity of raw Saessal β-glucan was 35.79%, which significantly increased to 74.37% at 0.5 N HCl. It was also noted that the purities of raw Saechal and Hinchal β-glucan were 30.68% and 34.95%, respectively, before hydrolysis, but significantly increased to 82.35% and 76.21%, respectively, after acid hydrolysis. The main factors affecting β-glucan purity have been reported to be protein and starch, along with proteins originating in the storage protein that are mainly and strongly associated with polysaccharides such as β-glucan and arabinoxylan (1,26,27). Wood et al. (1) reported that crude β-glucan contained 5~20% of protein and 2~3% of starch, while Kim et al. (26) reported that the purities of crude β-glucan of non-waxy and waxy barley were 59.15% and 62.91%, respectively, in samples that contained considerable amounts of protein and starch. However, the purity of secondarily purified β-glucan increased to 99.60% and 99.70%, respectively, by ammonium sulphate/acetone precipitation and enzymatic hydrolysis. By increasing the acid concentration of hydrolysis, the purity of the β-glucan increased because starch was decomposed into mono- and disaccharides, and protein was precipitated and removed by acid denaturation (27).
Table 2.
Purity and content of β-glucan from three acid-hydrolyzed barley varieties with different concentrations of HCl
| Varieties | HCl concentration (N) | Purity of β-glucan | Content of purified β-glucan | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Total (%) | Soluble (%) | Total β-glucan (%) | Soluble β-glucan | |||
|
| ||||||
| (%) | (% of total) | |||||
| Saessal | Control | 45.26f | 35.79f | 3.81a | 1.93a | 45.40e |
| 0.1 | 47.42e | 42.50e | 3.07b | 1.73b | 51.52d | |
| 0.2 | 56.31d | 55.96d | 2.95b | 1.52c | 62.73c | |
| 0.3 | 62.48c | 64.23c | 1.39c | 1.11d | 72.74b | |
| 0.4 | 72.45b | 72.10b | 1.29c | 1.01d | 86.11a | |
| 0.5 | 74.46a | 74.37a | 0.90d | 0.80e | 89.10a | |
| Saechal | Control | 42.69f | 30.68f | 3.78a | 2.09a | 38.68f |
| 0.1 | 52.41e | 47.00e | 3.62b | 1.91b | 49.31e | |
| 0.2 | 62.72d | 63.86d | 3.32c | 1.87b | 57.62d | |
| 0.3 | 68.40c | 66.63c | 2.82d | 1.64c | 67.86c | |
| 0.4 | 76.76b | 76.58b | 2.18e | 1.46d | 75.15b | |
| 0.5 | 85.47a | 82.35a | 1.87f | 1.28e | 77.87a | |
| Hinchal | Control | 44.15f | 34.95f | 3.64a | 2.38a | 44.26a |
| 0.1 | 46.35e | 48.45e | 3.45b | 2.06b | 59.55b | |
| 0.2 | 53.38d | 63.08d | 3.30c | 1.89c | 68.95c | |
| 0.3 | 61.64c | 65.44c | 2.74d | 1.61d | 72.39d | |
| 0.4 | 71.19b | 75.37b | 2.00e | 1.46e | 73.04d | |
| 0.5 | 75.09a | 76.21a | 1.68f | 1.31f | 77.96e | |
Saessal as non-waxy barley, Saechal and Hinchal as waxy-barley.
Any means within the same barley varieties followed by the different letters (a–f) are significantly different (P<0.05) by Duncan’s multiple range test.
Ratio of β-(1→4)/(1→3) linkages
Changes in the ratio of β-(1→4)/(1→3) linkages of soluble β-glucan from acid hydrolysis of the three barley varieties with different concentrations of HCl are shown in Table 3. The ratio of β-(1→4)/(1→3) linkages of soluble β-glucan of raw Saessal was initially at 2.28, but significantly decreased to 2.05 with 0.5 N HCl. The ratio of β-(1→4)/(1→3) linkages of raw Saechal and Hinchal β-glucan were 2.52 and 2.48, respectively, before acid-hydrolysis; however, those significantly decreased to 2.15 and 2.13 after acid hydrolysis. The ratio of β-(1→4)/(1→3) linkages of waxy barley β-glucan was higher than that of non-waxy barley β-glucan. In several studies, the ratio of β-(1→4)/(1→3) linkages of soluble β-glucan from barley were 2.1~2.6 and were shown similar value between barley and oats (1), and varied by cultivar, extraction conditions, content and molecular weight of β-glucan. Similar values were also reported by Choi et al. (5) who found that the ratio of β-(1→4)/(1→3) linkages of waxy barley was higher than that of non-waxy barley at 2.74 and 2.85, respectively. The ratio of β-(1→4)/(1→3) linkages of oat β-glucan decreased from 2.20 to 2.07 with increasing the acid-hydrolysis time (2). The irregularly-spaced (1→3)-linkages interrupt the relatively rigid, ribbon-like (1→4)-β-glucan conformation and confer a flexibility and irregular-shape on the barley (1→3), (1→4)-β-D-glucan, consistent with its solubility in water (28).
Table 3.
Ratio of β-(1→3) to β-(1→4) linkages in soluble β-glucans from three acid-hydrolyzed barley varieties with different concentrations of HCl
| HCl concentration (N) | Ratio of β-(1→3) to β-(1→ 4) linkages | ||
|---|---|---|---|
|
| |||
| Saessal | Saechal | Hinchal | |
| Control | 2.28±0.01aB | 2.52±0.01aA | 2.48±0.01aA |
| 0.1 | 2.22±0.01bB | 2.43±0.02bA | 2.41±0.01bA |
| 0.2 | 2.19±0.02bcB | 2.35±0.01cA | 2.32±0.01cA |
| 0.3 | 2.17±0.01bcB | 2.26±0.01dA | 2.21±0.00dAB |
| 0.4 | 2.12±0.01cB | 2.21±0.00dA | 2.18±0.01dA |
| 0.5 | 2.05±0.01dB | 2.15±0.01eA | 2.13±0.01eA |
Saessal as non-waxy barley, Saechal and Hinchal as waxy-barley.
Results are expressed as the average of triplicate samples with mean±SD (n=3).
Different capital letters (A,B) and small letters (a–e) in the same items indicate significant differences (P<0.05) among different barley varieties and concentrations of hydrochloric acid by Duncan’s multiple range test, respectively.
Average molecular weight of soluble β-glucan
The average molecular weight of soluble β-glucan of raw Saessal was 603,628 g/mol, but significantly decreased to 6,647 g/mol as concentrations of HCl increased to 0.5 N (Table 4). The initial average molecular weight of raw Saechal and Hinchal β-glucan were 696,996 g/mol and 669,169 g/mol, respectively, however, those significantly decreased to 7,751 g/mol and 7,351 g/mol after acid hydrolysis. Waxy barley β-glucan had higher molecular weight than non-waxy barley. In several studies, the average molecular weight of soluble β-glucan from barley was 1.0~5.7×105 g/mol (29), 1.3~2.5×105 g/mol (30), 2.1~2.9×105 g/mol (31) and 4.0~7.7×105 g/mol (20), and varied by cultivar, β-glucan content, growing environment and extraction conditions. Similar values were also reported by Choi et al. (5) who found that the molecular weight of waxy barley was higher than that of non-waxy barley at 625,900 g/mol and 798,600 g/mol, respectively. Acid hydrolysis accelerated the decrease in the molecular weight of barley and oat β-glucan because the glycosidic bond of β-glucane was destroyed (17). When alginate and chitosan were treated with acid, high-molecular-weight substances were converted to lower molecular weights, and the polymer distribution was increased with acid hydrolysis (31,32).
Table 4.
Average molecular weights of soluble β-glucan from three acid-hydrolyzed barley varieties with different concentrations of hydrochloric acid (HCl) (unit: g/mol)
| HCl concentration (N) | Average molecular weight | ||
|---|---|---|---|
|
| |||
| Saessal | Saechal | Hinchal | |
| Control | 603,628±4,341Ca | 696,996±5,012Aa | 669,169±5,543Ba |
| 0.1 | 182,530±3,017Ab | 173,334±10,141Ab | 171,498±2,466Ab |
| 0.2 | 90,221±1,989Bc | 97,873±1,408Ac | 99,331±3,744Ac |
| 0.3 | 43,749±1,258Bd | 48,843±1,069Ad | 48,853±1,607Ad |
| 0.4 | 15,906±132Be | 19,497±1,619Ae | 17,590±146Ae |
| 0.5 | 6,647±96Cf | 7,751±234Af | 7,351±106Bf |
Saessal as non-waxy barley, Saechal and Hinchal as waxy-barley.
Results are expressed as the average of triplicate samples with mean±SD (n=3).
Different capital letters (A–C) and small letters (a–f) in the same items indicate significant differences (P<0.05) among different barley varieties and concentrations of hydrochloric acid by Duncan’s multiple range test, respectively.
Viscosity of soluble β-glucan
The viscosity of soluble β-glucan solution significantly decreased as concentrations of HCl increased (Fig. 1). The viscosity of soluble β-glucan solution for raw Saessal was 12.8 centipoise (cP), and significantly decreased to 3.6 cP at 0.5 N HCl concentration (P<0.05). The initial viscosities of soluble β-glucan solution from raw Saechal and Hinchal were 32.8 and 26.8 cP, respectively, which significantly decreased respectively to 4.2 and 3.8 cP after acid hydrolysis (P<0.05). Viscosity declined rapidly with high concentrations of β-glucan solution. Viscosity of soluble β-glucan from waxy barley was higher than that of non-waxy barley, and similarly, the viscosity of Saechal was higher than that of Hinchal (5). β-Glucan is a high-molecular-weight substance, and the viscosity of β-glucan is affected by various factors, such as concentration of the solution, molecular weight and molecular distribution (1). Other studies have shown that as oat β-glucan was hydrolysed with acid, both its molecular weight and viscosity decreased (17,33), which is consistent with other studies on alginate and chitosan (31,32).
Fig. 1.
Changes in apparent viscosity of acid-hydrolysed barley soluble β-glucan with different concentrations of hydrochloric acid. Saessas is a non-waxy barley, Saechal and Hinchal are waxy-barleys.
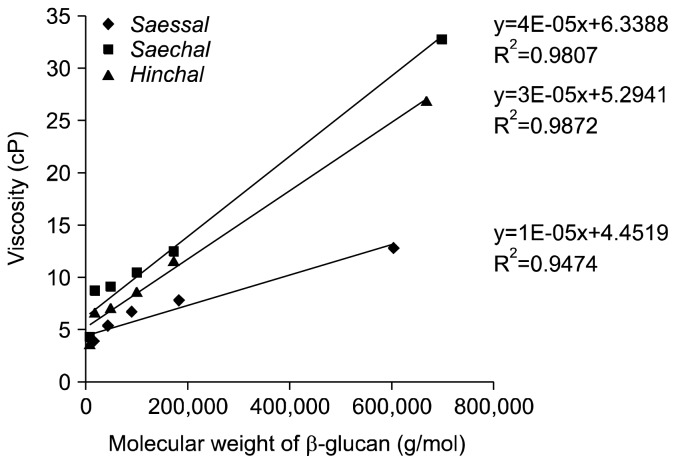
The relationship between molecular weight and viscosity of acid-hydrolysed soluble β-glucan using different concentrations of HCl were shown in Fig. 2. The coefficient of determination (R2) of Saessal, Saechal, and Hinchal were 0.947, 0.987, and 0.980, respectively. In many studies, positive correlation between molecular weight and viscosity of β-glucan has been reported, and Tosh et al. (33) reported that the coefficient of determination (R2) between molecular weight and viscosity of β-glucan was 0.991. The viscosities of acid-hydrolysed soluble β-glucan were highly concentration dependent (Table 5). The regression slopes decreased with increasing HCl concentrations for hydrolysis. These results indicate that viscosities decrease with increasing HCl concentrations because of the lower molecular weights of β-glucan (17). Also, the coefficient of determination (R2) for this relationship for Saessal, Saechal, and Hinchal were 0.989, 0.985, and 0.912, respectively. This was expected because content and viscosity of soluble β-glucan are related. These results are agreement with Bhatty (34) who reported a correlation of 0.91 between viscosity and soluble β-glucan content in 15 genotypes of barley.
Fig. 2.
Relationship between molecular weight and viscosity of acid-hydrolysed soluble β-glucan with different concentrations of hydrochloric acid. Saessal is a non-waxy barley, Saechal and Hinchal are waxy-barleys.
Table 5.
Regression equation and coefficient of determination between log viscosity and concentration of soluble β-glucan from three acid-hydrolyzed barley varieties with different concentrations of hydrochloric acid (HCl)
| Varieties | HCl concentration (N) | Regression equation1) | Coefficient of determination (R2) |
|---|---|---|---|
| Saessal | Control | y=0.471x+0.569 | 0.989 |
| 0.1 | y=0.468x−0.078 | 0.984 | |
| 0.2 | y=0.430x−0.092 | 0.966 | |
| 0.3 | y=0.368x−0.078 | 0.940 | |
| 0.4 | y=0.288x−0.049 | 0.909 | |
| 0.5 | y=0.294x−0.000 | 0.931 | |
| Saechal | Control | y=0.468x+0.477 | 0.985 |
| 0.1 | y=0.403x+0.327 | 0.944 | |
| 0.2 | y=0.359x+0.341 | 0.966 | |
| 0.3 | y=0.380x+0.259 | 0.927 | |
| 0.4 | y=0.437x+0.144 | 0.860 | |
| 0.5 | y=0.245x+0.080 | 0.744 | |
| Hinchal | Control | y=0.478x+0.197 | 0.912 |
| 0.1 | y=0.349x+0.324 | 0.958 | |
| 0.2 | y=0.351x+0.188 | 0.953 | |
| 0.3 | y=0.345x+0.095 | 0.914 | |
| 0.4 | y=0.421x−0.096 | 0.945 | |
| 0.5 | y=0.280x−0.071 | 0.826 |
Saessal as non-waxy barley, Saechal and Hinchal as waxy-barley.
y, Log viscosity (cP); x, concentrations of β-glucan (%, w/v).
Re-solubility of soluble β-glucan
The re-solubility of hydrolyzed β-glucan solution using different concentrations of HCl is shown in Fig. 3. The re-solubility of soluble β-glucan from raw Saessal was 50.00%, which increased (significantly) to 84.59% (P< 0.05). The re-solubilities of soluble β-glucan from raw Saechal and Hinchal were 51.04% and 55.90%, respectively, before hydrolysis; however, these figures increased significantly to 96.84% and 95.17%, respectively, after acid hydrolysis (P<0.05; Fig. 3). Varum and Simidsrod (35) and Beer et al. (30) reported that purified high-molecular-weight β-glucan did not dissolve completely at 60°C for 3 h or at 80°C for 0.5~1 h and that β-glucan obtained by ethanol precipitation had difficulties in re-dissolving. However, in this study, the re-solubility of β-glucan increased with an increasing of concentration of HCl. This occurred because the breakdown and depolymerisation of a polysaccharide such as β-glucan by acid hydrolysis may induce the production of low-molecular-weight substances, thereby increasing purity and decreasing viscosity, ultimately leading to an increase in the re-solubility (31,32).
Fig. 3.
Changes in re-solubility of acid-hydrolysed barley solubleβ-glucan with different concentrations of hydrochloric acid. Saessal is a non-waxy barley, Saechal and Hinchal are waxy-barleys. 1)Any means followed by the different letters (a–e) are significantly different (P<0.05) by Duncan’s multiple range test.
ACKNOWLEDGEMENTS
This study was supported by a grant (PJ011143022015) from the AGENDA Program, Rural Development Administration, Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Wood PJ, Paton D, Siddiqui IR. Determination of β-glucan in oats and barley. Cereal Chem. 1977;54:524–533. [Google Scholar]
- 2.Aman P, Graham H. Analysis of total and insoluble mixed-linked (1→3), (1→4)-β-D-glucans in barley and oats. J Agric Food Chem. 1987;35:704–709. doi: 10.1021/jf00077a016. [DOI] [Google Scholar]
- 3.Lee YT, Lee CK. Effects of varietal variation in barley on β-glucan and malting quality characteristics. Korean J Food Sci Technol. 1994;26:172–177. [Google Scholar]
- 4.McCleary BV, Glennie-Holmes M. Enzymic quantification of (1→3) (1→4)-β-D-glucan in barley and malt. J Inst Brew. 1985;91:285–295. doi: 10.1002/j.2050-0416.1985.tb04345.x. [DOI] [Google Scholar]
- 5.Choi HD, Seog HM, Choi IW, Park YK, Lee CH, Shin KS. Molecular structure of β-glucan isolated from non- waxy and waxy barley. Food Sci Biotechnol. 2004;13:744–748. [Google Scholar]
- 6.Wood PJ, Braaten JT, Scott FW, Riedel KD, Wolynetz MS, Collins MW. Effect of dose and modification of viscous properties of oat gum on plasma glucose and insulin following an oral glucose load. Br J Nutr. 1994;72:731–743. doi: 10.1079/BJN19940075. [DOI] [PubMed] [Google Scholar]
- 7.Rimm EB, Ascherio A, Giovannucci E, Spiegelman D, Stampfer MJ, Willett WC. Vegetable, fruit, and cereal fiber intake and risk of coronary heart disease among men. JAMA. 1996;275:447–451. doi: 10.1001/jama.1996.03530300031036. [DOI] [PubMed] [Google Scholar]
- 8.Bae IY, Lee SY, Kim SM, Lee HG. Effect of partially hydrolyzed oat β-glucan on the weight gain and lipid profile of mice. Food Hydrocolloid. 2009;23:2016–2021. doi: 10.1016/j.foodhyd.2009.03.016. [DOI] [Google Scholar]
- 9.Byun EH, Kim JH, Sung NY, Choi JI, Lim ST, Kim KH, Yool HS, Byun MW, Lee JW. Effects of gamma irradiation on the physical and structural properties of β-glucan. Radiat Phys Chem. 2008;77:781–786. doi: 10.1016/j.radphyschem.2007.12.008. [DOI] [Google Scholar]
- 10.Carr JM, Glatter S, Jeraci JL, Lweis BA. Enzymic determination of β-glucan in cereal-based food products. Cereal Chem. 1990;67:226–229. [Google Scholar]
- 11.Sayar S, White PJ, Peterson DM, Lehtinen P, Kaukovirta-Norja A. Oat β-glucan: structure, properties and health claims. In: Webster FH, Wood PJ, editors. Oats: Chemistry and Technology. American Association of Cereal Chemists International; St Paul, MN, USA: 1986. pp. 121–152. [Google Scholar]
- 12.Roubroeks JP, Andersson R, Mastromauro DI, Christensen BE, Aman P. Molecular weight, structure and shape of oat (1→3),(1→4)-β-D-glucan fractions obtained by enzymatic degradation with (1→4)-β-D-glucan 4-glucanohydrolase from Trichoderma reesei. Carbohydr Polym. 2001;46:275–285. doi: 10.1016/S0144-8617(00)00329-5. [DOI] [Google Scholar]
- 13.Sandula J, Kogan G, Kacurakova M, Machova E. Microbial (1→3)-β-D-glucans, their preparation, physicochemical characterization and immunomodulatory activity. Carbohydr Polym. 1999;38:247–253. doi: 10.1016/S0144-8617(98)00099-X. [DOI] [Google Scholar]
- 14.Machová E, Kogan G, Chorvatovicová D, Sandula J. Ultrasonic depolymerization of the chitinglucan complex from Aspergillus niger and antimutagenic activity of its product. Ultrason Sonochem. 1999;6:111–114. doi: 10.1016/S1350-4177(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 15.Jeon YJ, Kim SK. Production of chitooligosaccharides using an ultrafiltration membrane reactor and their antibacterial activity. Carbohydr Polym. 2000;41:133–141. doi: 10.1016/S0144-8617(99)00084-3. [DOI] [Google Scholar]
- 16.Ralet MC, Axelos MAV, Thibault JF. Gelation properties of extruded lemon cell walls and their water-soluble pectins. Carbohydr Res. 1994;260:271–282. doi: 10.1016/0008-6215(94)84045-8. [DOI] [Google Scholar]
- 17.Tosh SM, Wood PJ, Wang Q, Weisz J. Structural characteristics and rheological properties of partially hydrolyzed oat β-glucan: the effects of molecular weight and hydrolysis method. Carbohydr Polym. 2004;55:425–436. doi: 10.1016/j.carbpol.2003.11.004. [DOI] [Google Scholar]
- 18.Müller A, Ensley H, Pretus H, McNamee R, Jones E, McLaughlin E, Chandley W, Browder W, Lowman D, Williams D. The application of various protic acids in the extraction of (1→3)-β-D-glucan from Saccharomyces cerevisiae. Carbohydr Res. 1997;299:203–208. doi: 10.1016/S0008-6215(97)00004-9. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa M, Isogai A, Onabe F. Preparation of low-molecular-weight chitosan using phosphoric acid. Carbohydr Polym. 1993;20:279–283. doi: 10.1016/0144-8617(93)90100-I. [DOI] [Google Scholar]
- 20.Kim SY, Ryu CH. Extraction and physicochemical characterization of barley bran β-glucan. Korean J Food Cookery Sci. 2003;19:616–623. [Google Scholar]
- 21.Jeong HS, Kang TS, Park HJ, Jung IS, Lee HY. Characteristics of viscosity and components of soluble extract in oats. Food Engineering Progress. 2004;8:40–46. [Google Scholar]
- 22.Lee SH, Jang GY, Kim HY, Woo KS, Hwang IG, Kim KJ, Lee MJ, Kim TJ, Lee JS, Jeong HS. Physicochemical properties of barley β-glucan with different heating temperatures. J Korean Soc Food Sci Nutr. 2012;41:1764–1770. doi: 10.3746/jkfn.2012.41.12.1764. [DOI] [Google Scholar]
- 23.Lee YT. β-Glucans in barley and oats and their changes in solubility by processing. Agric Chem Biotechnol. 1996;39:482–487. [Google Scholar]
- 24.Johansson L, Virkki L, Anttila H, Esselström H, Tuomainen P, Sontag-Strohm T. Hydrolysis of β-glucan. Food Chem. 2006;97:71–79. doi: 10.1016/j.foodchem.2005.03.031. [DOI] [Google Scholar]
- 25.Forrest IS, Wainwright T. The mode of binding of β-glucans extracted from barley at different temperatures. Carbohydr Res. 1977;83:279–286. [Google Scholar]
- 26.Kim SR, Choi HD, Seog HM, Kim SS, Lee YT. Physicochemical characteristics of β-glucan isolated from barley. Korean J Food Sci Technol. 1999;31:1164–1170. [Google Scholar]
- 27.You S, Izydorczyk MS. Comparison of the physicochemical properties of barley starches after partial α-amylolysis and acid/alcohol hydrolysis. Carbohydr Polym. 2007;69:489–502. doi: 10.1016/j.carbpol.2007.01.002. [DOI] [Google Scholar]
- 28.Woodward JR, Fincher GB, Stone BA. Water-soluble (1→3), (1→4)-β-D-glucans from barley (Hordeum vulgare) endosperm. II. Fine structure. Carbohydr Polym. 1983;3:207–225. doi: 10.1016/0144-8617(83)90019-X. [DOI] [Google Scholar]
- 29.Gómez C, Navarro A, Manzanares P, Horta A, Carbonell JV. Physical and structural properties of barley (1→3),(1→4)-β-D-glucan. Part I. Determination of molecular weight and macromolecular radius by light scattering. Carbohydr Polym. 1997;32:7–15. doi: 10.1016/S0144-8617(96)00126-9. [DOI] [Google Scholar]
- 30.Beer MU, Wood PJ, Weisz J. Molecular weight distribution and (1→3), (1→4)-β-D-glucan content of consecutive extracts of various oat and barley cultivars. Cereal Chem. 1997;74:476–480. doi: 10.1094/CCHEM.1997.74.4.476. [DOI] [Google Scholar]
- 31.Lee DS, Kim HR, Pyeun JH. Effect of low-molecularization on rheological properties of alginate. J Korean Fish Soc. 1998;31:82–89. [Google Scholar]
- 32.Yan X, Evenocheck HM. Chitosan analysis using acid hydrolysis and HPLC/UV. Carbohydr Polym. 2012;87:1774–1778. doi: 10.1016/j.carbpol.2011.09.091. [DOI] [Google Scholar]
- 33.Tosh SM, Wood PJ, Wang Q. Gelation characteristics of acid-hydrolyzed oat beta-glucan solutions solubilized at a range of temperatures. Food Hydrocolloid. 2003;17:523–527. doi: 10.1016/S0268-005X(03)00017-1. [DOI] [Google Scholar]
- 34.Bhatty RS. Relationship between acid extract viscosity and total soluble and insoluble β-glucan contents of hulled and hulless barley. Can J Plant Sci. 1987;67:997–1008. doi: 10.4141/cjps87-136. [DOI] [Google Scholar]
- 35.Varum KM, Smidsrød O. Partial chemical and physical characterisation of (1→3),(1→4)-β-D-glucans from oat (Avena sativa L.) aleurone. Carbohydr Polym. 1988;9:103–117. doi: 10.1016/0144-8617(88)90008-2. [DOI] [Google Scholar]