Abstract
This study investigated the anti-amnesic effect of fermented Ganoderma lucidum water extracts (GW) on scopolamine-induced memory impairment in rats. GW were fermented by the lactic acid bacterium Bifidobacterium bifidum (FGWB), followed by Lactobacillus sakei LI033 (FGWBL). To induce amnesia, scopolamine (1 mg/kg) was intraperitoneally injected into rats 30 min before the behavioral tests. Step-through latencies of rats treated with primary fermented extracts (300 mg/kg, FGWB) and secondary fermented extracts (300 mg/kg, FGWBL) were significantly longer than those of rats treated with GW (300 mg/kg) in the retention trial of the multiple trial passive avoidance test. In the Morris water maze task, FGWBL significantly shortened escape latencies in training trials. Furthermore, swimming times within the target zone during the probe trial with FGWBL were significantly higher than the GW and FGWB treatments. In addition, acetylcholinesterase activities were lower in the brains of scopolamine-treated rats treated with FGWBL. These results suggest that FGWBL could be useful to enhance learning memory and cognitive function via cholinergic dysfunction.
Keywords: Ganoderma lucidum, fermentation, scopolamine, passive avoidance test, Morris water maze test
INTRODUCTION
Geriatric diseases have recently attracted much attention due to the rapid aging of society, development of medical technology, and improvement of living standards (1). In particular, greater emphasis is now placed on preventing cognitive impairment, which is the initial symptom of dementia and memory impairment due to aging (2). Geriatric diseases refer to cerebral or spinal disorders caused by abnormal neuronal death and are accompanied by impaired cognitive, walking, and motor abilities (3). Lack of acetylcholine due to reduction of hippocampal cholinergic neuronal activity is one of the most important causes of memory impairment (4). Further, although there are many choline acetyltransferase agonists and acetylcholinesterase (AChE) inhibitors for memory enhancement, they are ineffective and controversial due to their serious side effects and toxicity. For this reason, research has tried to identify memory enhancers from natural materials (5,6).
Scopolamine is a muscarinic receptor antagonist that is frequently used in memory-impaired animals. It inhibits connections between acetylcholine and muscarinic receptors, thereby temporarily blocking information transmission and causing learning and memory impairment (7).
Ganoderma lucidum has been used in traditional medicine in Korea, China, Japan, and other countries (8). It contains many physiological active substances, including nucleosides, steroids, alkaloids, proteins, amino acids, minerals, polysaccharides, and triterpenes, which are known as physiologically active substances. Of these, ganoderic acid is rarely generated from the liquid-cultured mycelium of G. lucidum and is instead concentrated on the surfaces of fruit bodies (9). In addition, G. lucidum has anti-tumor, anti-cancer, anti-inflammatory, anti-virus, anti-oxidative, hepatoprotective, and neuroprotective activities, and it has been shown to improve immunity and memory (10–14).
As traditional fermented foods have been found to possess various health effects, researchers have made efforts to confirm the various physiological activities of natural extracts fermented by lactic acid bacteria. These beneficial activities include control of intestinal pathogenic bacteria and intestinal regulation, anti-cancer activity, immune system stimulation, and lowering of blood cholesterol (15,16). In particular, fermentation by lactic acid bacteria can improve taste, flavor, texture, storability, safety, and physiological activity by improving digestion efficiency and synergy between natural substances and lactic acid bacteria (17). However, few studies have analyzed the effects of G. lucidum water extracts fermented by lactic acid bacteria in terms of cognitive ability and memory.
This study examined fermentation by lactic acid bacteria as a method to improve the memory-enhancing effects as well as maximize the efficacy of G. lucidum water extracts by two-step fermentation with Bifidobacterium bifidum and Lactobacillus sakei. Morris water maze test and passive avoidance test were performed using scopolamine-induced memory-impaired animals, and behavioral changes and hippocampal AChE activities were measured.
MATERIALS AND METHODS
Fermentation of G. lucidum
Fermentation of G. lucidum was carried out according to the method described by Yang et al. (18). Briefly, water extract of G. lucidum (GW) was prepared by soaking 100 g of dry-sliced G. lucidum in 2 L of water at 80°C for 3 h. After centrifugation at 15,000 g for 10 min (Beckman Coulter, Fullerton, CA, USA), GW was collected for further examination. GW was adjusted to 1°Brix using a brix meter (Kyoto Electronics Manufacturing, Tokyo, Japan) and then sterilized by autoclaving at 121°C for 15 min (Ilshin Bio Base, Gyeonggi, Korea). Sterilized GW was fermented under an anaerobic system using 2% (v/v) B. bifidum KCCM 12096 (Korean Culture Center of Microorganisms, Seoul, Korea) at 37°C for 72 h. Fermented GW was sterilized by autoclaving at 121°C for 15 min (FGWB), followed by fermentation with 2% (v/v) L. sakei LI033, which was previously isolated from kimchi (19) at 37°C for 24 h. Secondary fermented GW was sterilized by autoclaving at 121°C for 15 min (FGWBL). After centrifugation at 15,000 g for 10 min, the supernatant from each sample was freeze-dried and stored at −20°C prior to use.
Animals
Male Sprague-Dawley rats weighing 200~250 g each (age: 6 weeks) were purchased from Central Lab. Animal Inc. (Seoul, Korea). The experimental procedure was conducted in compliance with the institutional guidelines of Jeonnam Institute of Natural Resources Research for the care and use of laboratory animals. Rats were housed at 3 or 4 per cage, allowed access to water and food ad libitum, and maintained at an ambient temperature of 22±3°C under 50±20% humidity and a 12-h diurnal light cycle (lights on 08:00~20:00 h) prior to testing. The rats were habituated for 6 days before drug administration. All behavioral experiments were carried out in a room adjacent to the housing room under the same ambient conditions.
Sample group and drug administration
Male Sprague-Dawley rats (200~250 g) were randomly assigned to six groups (seven rats per group): control group; scopolamine group, scopolamine-treated (1 mg /kg) group; donepezil group, donepezil (1 mg/kg) and scopolamine (1 mg/kg) co-treated groups; GW group, GW (100 and 300 mg/kg) and scopolamine (1 mg/kg) co-treated groups; FGWB group, FGWB (100 and 300 mg/kg) and scopolamine (1 mg/kg) co-treated groups; FGWBL group, FGWBL (100 and 300 mg/kg) and scopolamine (1 mg/kg) co-treated groups. After 15 days, rats were orally treated with GW, FGWB, and FGWBL (100 and 300 mg/kg). After 16 days, rats were orally treated with GW, FGWB, FGWBL, or donepezil (1 mg/kg). To induce amnesia, scopolamine (1 mg/kg) was intraperitoneally injected into rats 30 min before starting behavioral tests (Fig. 1). Scopolamine and donepezil were obtained from Sigma-Aldrich (St. Louis, MO, USA). All the reagents used in the present study were of analytical grade.
Fig. 1.

Scheme of experimental schedule.
Morris water maze test
The Morris water maze (20) involved a circular pool (180 cm in diameter and 75 cm in height) with a featureless inner surface. The pool was filled with water maintained at 21±2°C. The tank was placed in a dimly lit, sound proof test room with various visual cues. The pool was conceptually divided into quadrants. A hidden escape platform was placed into one of the pool quadrants and submerged to 1 cm below the water surface, so it was not visible at water level. Over the next 5 days, rats underwent three trials per day with the platform in place. For each training trial, rats were placed in the water facing the pool wall in each pool quadrant in a different order each day. When a rat located the platform, it was permitted to remain on the platform for 20 s. If the rat did not locate the platform within 45 s, it was placed on the platform for 20 s. The animal was taken to its home cage and then allowed to dry under an infrared lamp after each trial. During each trial, the time required to find the hidden platform (latency) was recorded using a video tracking system (Panlab, Barcelona, Spain). Immediately after the last training session, the rats were subjected to a probe trial session in which the platform was removed from the pool, and the rats were allowed to swim for 90 s to search for it. The swimming time in the pool quadrant where the platform was placed was recorded (21).
Passive avoidance test
The passive avoidance test is a well-established experimental procedure used to assess short-term reference memory, which is dependent on cortical and hippocampal circuitries (22,23). The step-through passive avoidance test was performed in identical illuminated and dark chambers (Gemini Avoidance System, San Diego, CA, USA). The illuminated compartment contained a bulb, and the floor of the non-illuminated compartment was composed of stainless steel rods. The two compartments were separated by a guillotine door. For the acquisition trial, the rats were initially placed in the illuminated compartment, after which the door between the two compartments was opened 20 s later. After the rats entered the dark compartment, the door closed automatically, followed by an electrical foot shock (75 V, 0.5 mA, 50 Hz) of 5 s duration delivered through the stainless steel rods. At 8 h after the acquisition trial, the rats were again placed in the illuminated compartment for the retention trials. The time taken for each rat to enter the dark compartment after the door opened was measured as the latency time in both the acquisition and retention trials, with a maximum of 300 s.
Rotarod test
The rotarod test was carried out using a rod with a diameter of 8 cm, rotating at a constant speed of 20 rpm (five-lane accelerating rotarod; Jeung DO Bio & Plant, Seoul, Korea). For the training trials, the rats were placed on the rotarod at 20 rpm for about 10 min per day before beginning each experiment. After 2 days, the experiment measured the fall time using the same conditions.
Vertical pole test
To assess the equilibrium function, the vertical pole test was carried out according to the method of Ogawa et al. (24). Rats were placed on a vertical pole and the fall time from the pole was measured. Rats were habituated to the task over two trials per day for 2 days. On test day (third day), three measurements were taken over five trials per rat.
AChE activity in brains of rats
Samples of brains were homogenized in 100 mM phosphate buffer and centrifuged at 2,400 g for 15 min at 4°C. AChE activity was measured spectrophotometrically at 410 nm using supernatants according to the method of Ellman et al. (25).
Statistical analysis
Data were expressed as mean±SD (standard deviation), and statistical significance was analyzed using Student’s t-test and one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) post hoc test using SPSS for windows version 17.0 (SPSS Inc., Chicago, IL, USA). The values were considered to be significant when P-value was <0.05.
RESULTS AND DISCUSSION
Effectiveness of fermented G. lucidum water extracts in recovering memory in the Morris water maze test
The Morris water maze test was performed to determine the efficacy of fermented G. lucidum water extracts in improving spatial memory despite scopolamine-induced memory and cognitive impairment (Fig. 2). Of the high-concentration experimental groups (300 mg/kg), the FGWBL group (75.44±16.95 s) showed a significantly reduced escape time compared to the scopolamine group (100.82±24.29 s) (Fig. 2B, 1st trial). For short-term memory, the 300 mg/kg of FGWBL group (61.94±15.09 s) also showed a significantly reduced escape time compared to the scopolamine group (79.89±15.06 s) (Fig. 2B, 4th trial). After 5 days of training, the escape platform was removed and the probe test was performed to measure swimming time in the quadrant where the platform was placed. The scopolamine group (19.53±5.82 s) was incapable of normal learning since scopolamine administration made it difficult for the rats to remember in which quadrant the platform was placed, resulting in significantly reduced swimming time compared to the control group (48.67±5.58 s) (Fig. 3). In contrast, the 300 mg/kg of GW group (24.79±4.82 s), FGWB group (23.53±4.53 s), and FGWBL group (27.62±6.06 s) showed significantly increased swimming times in the target quadrant compared to the scopolamine group. These results suggest that fermentation of G. lucidum increased cognitive enhancing activity.
Fig. 2.
Effects of GW, FGWB, and FGWBL on scopolamine-induced memory impairment in the Morris water maze test. (A) the effects of the low-concentration experimental groups (100 mg/kg) on scopolamine-impaired memory in rats on the first and fourth trials of the Morris water maze test over 4 days. (B) the effects of the high-concentration experimental groups (300 mg/kg) on scopolamine-impaired memory in rats on the first and fourth trials of the Morris water maze test over 4 days. Data are expressed as the mean±SD (n=7). ***P<0.001, **P<0.01, *P<0.05, significantly different from the scopolamine group. Statistical significance was tested by unpaired Student’s t-test. Scopolamine, scopolamine group; Donepezil, scopolamine plus donepezil group; GW, scopolamine plus G. lucidum water extracts group; FGWB, scopolamine plus GW fermented with B. bifidum; FGWBL, scopolamine plus GW fermented with B. bifidum and L. sakei LI033.
Fig. 3.
Effects of GW, FGWB, and FGWBL on scopolamine-induced impairment of memory acquisition and retention in the probe trials. (A) effects of GW, FGWB, and FGWBL on probe trial sessions of the Morris water maze test. Cumulative time in the target quadrant of the pool in the 90 s probe trial is shown. (B) typical trace of swimming patterns on probe trial sessions of the Morris water maze test. Data are expressed as the mean±SD (n=7). ***P<0.001, **P<0.01, *P<0.05, significantly different from the scopolamine-treated group. Statistical significance was tested by unpaired Student’s t-test. Groups are the same as in Fig. 2.
Effectiveness of fermented G. lucidum water extracts in recovering memory in the passive avoidance test
The passive avoidance test was performed to determine the efficacy of fermented G. lucidum water extracts in improving short-term memory despite scopolamine-induced memory and cognitive impairment (Fig. 4). The scopolamine group (131.39±41.57 s) showed reduced learning and memory and stayed in the bright space for a significantly shorter time period compared to the control group (295.29±50.28 s). In contrast, the 300 mg/kg of GW group (167.73±28.50 s), FGWB group (174.63±22.38 s), and 300 mg/kg of FGWBL group (177.73±27.50 s) stayed in the bright space for significantly longer time periods compared to the scopolamine group, suggesting improvement of scopolamine-induced memory and learning. Yoo et al. (26) previously used a senescence-accelerated mouse model to investigate the efficacy of G. lucidum water extracts in improving memory and oxidative stress. Yuan et al. (27) used an animal model of Alzheimer’s disease to examine the efficacy of G. lucidum polysaccharides in improving memory and spatial recognition ability. Their results showed that G. lucidum water extracts can be used as functional ingredients to improve memory. In this study, FGWB was secondarily fermented with lactic acid bacteria separated from kimchi in Imsil in order to resolve polymer polysaccharides from G. lucidum water extracts into less differentiated polysaccharides and obtain functional ingredients, including polysaccharides, triterpenes, alkaloid, and diverse vitamins (28). Therefore, FGWB and FGWBL improve scopolamine-induced memory and cognitive function by preventing brain cell death or by stimulating secretion of memory-related neurotransmitters such as acetylcholine and glutamate.
Fig. 4.
Effects of GW, FGWB, and FGWBL on scopolamine-induced memory impairment in the passive avoidance test. Data are expressed as the mean±SD (n=7). ***P<0.001, **P<0.01, *P<0.05, significantly different from the scopolamine-treated group. Statistical significance was tested by unpaired Student’s t-test. Groups are the same as in Fig. 2.
Effectiveness of fermented G. lucidum water extracts in improving motor ability deficit
The rotarod test and the vertical pole test were carried out to determine the efficacy of fermented G. lucidum water extracts in improving scopolamine-induced motor coordination and behavioral disorders (Fig. 5). Most degenerative diseases such as memory impairment and dementia are accompanied by reduction of motor ability, including sense of balance and coordination (29). The rotarod test measures the animal’s ability to maintain its balance on a rolling cylinder, and it is widely used to assess motor ability of an animal with degenerative brain disease (30). In the rotarod test, the scopolamine group stayed on the cylinder for a significantly shorter period of time (54.65±6.18 s) than the control group (65.27±5.64 s) (Fig. 5A). In contrast, the 300 mg/kg of GW group (43.31±6.61 s), FGWB group (43.15±6.27 s), and FGWBL group (45.99±6.20 s) stayed on the cylinder for significantly longer time periods than the scopolamine group. In the vertical pole test for measuring grip and motor coordination ability, the length of time on a slanted pole was significantly shorter in the scopolamine group (5.35±1.73 s) but significantly longer in the 300 mg/kg of GW group (6.53±1.29 s), FGWB group (6.72±1.09 s), and FGWBL group (7.34±1.31 s) compared to the control group (12.13±2.27 s) (Fig. 5B). In particular, oil from G. lucidum spores administered to an animal with Parkinson’s disease accompanied by brain neuronal death was reportedly effective in improving motor ability and neuroprotective activity (31). Based on these results, FGWBL administration can be effective in recovering sense of balance, coordination, and grip strength in an animal with scopolamine-induced memory and cognitive impairment.
Fig. 5.
Effects of GW, FGWB, and FGWBL on scopolamine-induced behavioral deficits. (A) effects of GW, FGWB, and FGWBL on scopolamine-induced motor coordination and balance deficits in the rotarod test. (B) effects of GW, FGWB, and FGWBL on scopolamine-induced sensorimotor deficits in the vertical pole test. Data are expressed as the mean±SD (n=7). ***P<0.001, **P<0.01, *P<0.05 significantly different from scopolamine group. Statistical significance was tested by unpaired Student’s t-test. Groups are the same as in Fig. 2.
Effects of fermented G. lucidum water extracts on AChE activity in brain tissues
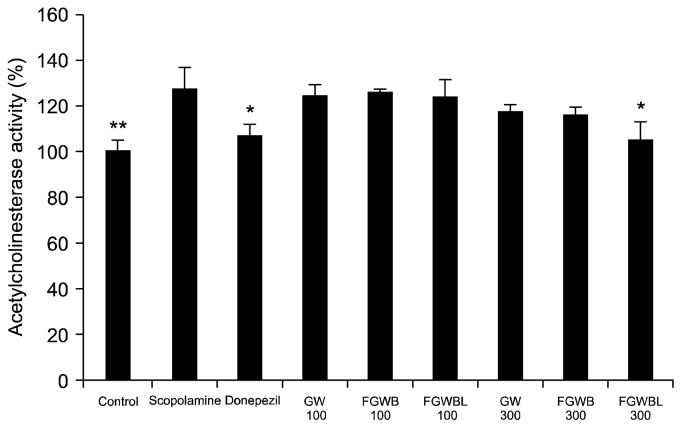
Lack of acetylcholine due to malfunction of cholinergic nervous system is known as one of the most important causes of memory impairment. Dementia patients with neuronal damage generate only a small amount of acetylcholine even though AChE, which helps break down acetylcholine, keeps functioning. This results in abnormal neurotransmission and pathological phenomena, such as learning disorders, memory deficits, and cognitive impairment (32). Research is currently being conducted on natural materials capable of AChE inhibition and improving cognitive functions (33,34). When AChE activity in brain tissues was examined to determine the efficacy of fermented G. lucidum water extracts in improving memory despite scopolamine-induced memory and cognitive impairment (Fig. 6), the scopolamine group showed significantly increased AChE activity. In contrast, the GW, FGWB, and FGWBL groups (300 mg/kg) showed reduced AChE activities in brain tissues. In particular, the FGWBL group showed significantly reduced AChE activity compared to the scopolamine group. G. lucidum contains numerous physiologically active substances such as polysaccharides, triterpenes, nucleoside, steroids, and alkaloids. Lee et al. (35) reported that lanostane triterpenes separated from fruit bodies of G. lucidum are excellent inhibitors of AChE. In particular, G. lucidum triterpenoids were shown to improve learning and memory dysfunction in a rat model of Alzheimer’s disease by increasing the acetylcholine content in the brain (36). Further, G. lucidum water extracts inhibit AChE activity in brain tissues and thus prevent reduction of acetylcholine levels, resulting in the protection of brain tissues from cerebral ischemia, vascular dementia, as well as Alzheimer’s dementia (37). Therefore, triterpenes and many other useful substances contained in G. lucidum water extracts fermented by lactic acid bacteria can be effective in inhibiting AChE activity in brain tissues via the cholinergic nervous system in scopolamine-induced rats. Further research is needed to determine the exact mechanism of AChE inhibition.
Fig. 6.
Effects of GW, FGWB, and FGWBL on AChE activity in scopolamine-injected rats. Data are expressed as the mean±SD (n=3). ***P<0.001, **P<0.01, *P<0.05, significantly different from the scopolamine-treated group. Statistical significance was tested by unpaired Student’s t-test. Groups are the same as in Fig. 2.
ACKNOWLEDGEMENTS
This study was supported by research grants from the ministry of Trade, Industry and Energy R&D program, Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Lee GJ, Lee KL, Yang S, Jun WH. Quality of life and the associated factors in dementia. J Korean Acad Psychiatr Ment Health Nurs. 2008;17:273–280. [Google Scholar]
- 2.Anderson L, McConnell SR. Cognitive health: an emerging public health issue. Alzheimers Dement. 2007;3:S70–S73. doi: 10.1016/j.jalz.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Xu Z, Li H, Jin P. Epigenetics-based therapeutics for neurodegenerative disorders. Curr Transl Geriatr Exp Gerontol Rep. 2012;1:229–236. doi: 10.1007/s13670-012-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akaike A, Takada-Takatori Y, Kume T, Izumi Y. Mechanisms of neuroprotective effects of nicotine and acetylcholinesterase inhibitors: role of α4 and α7 receptors in neuroprotection. J Mol Neurosci. 2010;40:211–216. doi: 10.1007/s12031-009-9236-1. [DOI] [PubMed] [Google Scholar]
- 5.Lee SE, Shim IS, Kim GS, Yim SV, Park HJ, Shim HS, Ye MS, Kim SY. The neuroprotective effect of white ginseng (Panax ginseng C. A. Meyer) on the trimethyltin (TMT)-induced memory deficit rats. Korean J Medicinal Crop Sci. 2011;19:456–463. doi: 10.7783/KJMCS.2011.19.6.456. [DOI] [Google Scholar]
- 6.Shi DH, Yan ZQ, Zhang LN, Wang YR, Jiang CP, Wu JH. A novel 7-O-modified genistein derivative with acetylcholinesterase inhibitory effect, estrogenic activity and neuroprotective effect. Arch Pharm Res. 2012;35:1645–1654. doi: 10.1007/s12272-012-0916-y. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe F, Miyasaka N, Kubota T, Aso T. Estrogen and progesterone improve scopolamine-induced impairment of spatial memory. J Med Dent Sci. 2004;51:89–98. [PubMed] [Google Scholar]
- 8.Boh B, Berovic M, Zhang J, Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Bio-technol Annu Rev. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- 9.Liu GQ, Xiao HX, Wang XL, Zhao Y, Zhang YG, Ren GP. Stimulated production of triterpenoids of Ganoderma lucidum by an ether extract from the medicinal insect, Catharsius molossus, and identification of the key stimulating active components. Appl Biochem Biotechnol. 2011;165:87–97. doi: 10.1007/s12010-011-9235-x. [DOI] [PubMed] [Google Scholar]
- 10.Shiao MS. Natural products of the medicinal fungus Ganoderma lucidum: occurrence, biological activities, and pharmacological functions. Chem Rec. 2003;3:172–180. doi: 10.1002/tcr.10058. [DOI] [PubMed] [Google Scholar]
- 11.Chan GC, Chan WK, Sze DM. The effects of β-glucan on human immune and cancer cells. J Hematol Oncol. 2009;2:25. doi: 10.1186/1756-8722-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu H, Ahn NS, Yang X, Lee YS, Kang KS. Ganoderma lucidum extract induces cell cycle arrest and apoptosis in MCF-7 human breast cancer cell. Int J Cancer. 2002;102:250–253. doi: 10.1002/ijc.10707. [DOI] [PubMed] [Google Scholar]
- 13.Cheung WWM, Hui WS, Chu PWK, Chiu SW, Ip NY. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000;486:291–296. doi: 10.1016/S0014-5793(00)02317-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Y, Qu ZQ, Zeng YS, Lin YK, Li Y, Chung P, Wong R, Hägg U. Neuroprotective effect of preadministration with Ganoderma lucidum spore on rat hippocampus. Exp Toxicol Pathol. 2012;64:673–680. doi: 10.1016/j.etp.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Park YS, Chang HG. Lactic acid fermentation and biological activities of Rubus coreanus. J Korean Soc Agric Chem Biotechnol. 2003;46:367–375. [Google Scholar]
- 16.Kannan N, Aravindan R. Effect of fermentation parameters on extra cellular tannase production by Lactobacillus plantarum MTCC 1407. E-J Chem. 2009;6:979–984. doi: 10.1155/2009/505087. [DOI] [Google Scholar]
- 17.Hubert J, Berger M, Nepveu F, Paul F, Daydé J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008;109:709–721. doi: 10.1016/j.foodchem.2007.12.081. [DOI] [PubMed] [Google Scholar]
- 18.Yang HS, Choi YJ, Oh HH, Moon JS, Jung HK, Kim KJ, Choi BS, Lee JW, Huh CK. Antioxidative activity of mushroom water extracts fermented by lactic acid bacteria. J Korean Soc Food Sci Nutr. 2014;43:80–85. doi: 10.3746/jkfn.2014.43.1.080. [DOI] [Google Scholar]
- 19.Choi HN, Oh HH, Yang HS, Huh CK, Bae IH, Lee JS, Jeong YS, Jeong EJ, Jung HK. Antifungal activity against cheese fungi by lactic acid bacteria isolated from kimchi. Korean J Food Preserv. 2013;20:727–734. doi: 10.11002/kjfp.2013.20.5.727. [DOI] [Google Scholar]
- 20.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Kang SJ, Woo JH, Kim AJ. The effects of Korean ginseng on memory loss in a rat models. J Korean Soc Food Sci Nutr. 2013;42:1190–1196. doi: 10.3746/jkfn.2013.42.8.1190. [DOI] [Google Scholar]
- 22.Kim MJ, Choi SJ, Lim ST, Kim HK, Kim YJ, Yoon HG, Shin DH. Zeatin supplement improves scopolamine-induced memory impairment in mice. Biosci Biotechnol Biochem. 2008;72:577–581. doi: 10.1271/bbb.70480. [DOI] [PubMed] [Google Scholar]
- 23.LeDoux JE. Emotional memory: in search of systems and synapses. Ann NY Acad Sci. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa N, Hirose Y, Ohara S, Ono T, Watanabe Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res Commun Chem Pathol Pharmacol. 1985;50:435–441. [PubMed] [Google Scholar]
- 25.Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 26.Yoo JK, Choi SJ, Kang JK, Han SS. Effects of Ganoderma lucidum extract on memory and oxidative stress of senescence-accelerated mouse. Korean J Life Science. 1999;9:548–555. [Google Scholar]
- 27.Yuan DJ, Zhang YF, Yao CX. Effects of Ganoderma lucidum polysaccharides on synapsis and hippocampal synaptophysin expression in Alzheimer’s rats model. Chin J Exp Tradit Med Formulae. 2011;17:151–155. [Google Scholar]
- 28.Lee SC, Im NK, Jeong HY, Choi EH, Jeon SM, Jeong GS. Neuroprotective effects of ethanol extract of Ganoderma lucidum L. on murine hippocampal cells. Kor J Pharmacogn. 2014;45:161–167. [Google Scholar]
- 29.Poorheidari G, Stanhope KJ, Pratt JA. Effects of the potassium channel blockers, apamin and 4-aminopyridine, on scopolamine-induced deficits in the delayed matching to position task in rats: a comparison with the cholinesterase inhibitor E2020. Psychopharmacology (Berl) 1998;135:242–255. doi: 10.1007/s002130050506. [DOI] [PubMed] [Google Scholar]
- 30.Whishaw IQ, Li K, Whishw PA, Gorny B, Metz GA. Use of rotorod as a method for the qualitative analysis of walking in rat. J Vis Exp. 2008;22:e1030. doi: 10.3791/1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu WW, Liu Z, Xu HW, Chu WZ, Ye QY, Xie AM, Chen L, Li JR. Effect of the oil from ganoderma lucidum spores on pathological changes in the substantia nigra and behaviors of MPTP-treated mice. Di Yi Jun Yi Da Xue Xue Bao. 2005;25:667–671. [PubMed] [Google Scholar]
- 32.Talesa VN. Acetylcholinesterase in Alzheimer’s disease. Mech Ageing Dev. 2001;122:1961–1969. doi: 10.1016/S0047-6374(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 33.Terry AV, Jr, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer’s disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- 34.Wang R, Yan H, Tang XC. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol Sin. 2006;27:1–26. doi: 10.1111/j.1745-7254.2006.00255.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee IS, Ahn BR, Choi JS, Hattori M, Min BS, Bae KH. Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum. Bioorg Med Chem Lett. 2011;21:6603–6607. doi: 10.1016/j.bmcl.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Luo J, Huang NH, Zhang XY. Influence of Ganoderma lucidum triterpenoids on learning memory function and ACh content in Alzheimer disease model rat. Chin J Exp Tradit Med Formulae. 2012;16:172–175. [Google Scholar]
- 37.Zhang W, Zhang Q, Deng W, Li Y, Xing G, Shi X, Du Y. Neuroprotective effect of pretreatment with Ganoderma lucidum in cerebral ischemia/reperfusion injury in rat hippocampus. Neural Regen Res. 2014;9:1446–1452. doi: 10.4103/1673-5374.139461. [DOI] [PMC free article] [PubMed] [Google Scholar]