Abstract
Systematic study of how different drying methods, namely hot-air drying, vacuum-drying, and freeze-drying, affect color, browning index, degree of rehydration, water solubility, and vitamin C content is critical for utilizing pine needle powders (PNP) as a novel ingredient in functional foods. Samples prepared by vacuum-drying showed a significantly higher L*-value, whereas higher a*- and b*-values were detected in the hot-air dried samples (P<0.05). The browning index was significantly higher in samples prepared by vacuum-drying compared to samples prepared by freeze-drying (P<0.05). Freeze-dried PNP exhibited a significantly higher degree of rehydration than hot-air dried samples (P<0.05). Water solubilities of freeze-dried and hot-air dried samples were significantly higher than that of vacuum-dried sample (P<0.05). Vitamin C was less destroyed during freeze-drying compared to hot-air or vacuum-drying (P<0.05). Freeze-dried samples displayed a clear porous structure and appeared to have a bigger space, whereas hot-air dried samples showed lower porosity than vacuum and freeze-dried samples.
Keywords: pine needle, physicochemical properties, powder, drying methods
INTRODUCTION
Pinus koraiensis, a large conifer that has important economic value, is found across Korea, Japan, and the Northeastern part of China (1). P. koraiensis has long been used in oriental folk medicine due to its anti-fatigue, anti-ageing, and anti-inflammatory effects as well as its use as a food supplement (2). Antioxidant activities of seed extracts (3) as well as anti-browning and anti-microbial activities of pine needle extracts of P. koraiensis (4) have been reported. Its leaves (called pine needles) are recognized as healthy food materials with abundant proteins, vitamins, and minerals (5), and they are utilized extensively as ingredients in dietary supplements, functional beverages, and healthy wines (6). Pine needles have been accepted by consumers and are used in the manufacturing of different products, including powders, wines, and teas in East Asia (4). The development of a novel functional food based on pine needles would be beneficial due to its physiological activities and therapeutic effects (6).
Plant based food materials, which contain high moisture levels up to 90% by weight (7) hold a significant portion of the global food market (8). In order to increase its shelf-life, commercial value, and applicability as a value-added food ingredient, pine needles must be converted into a powder through drying. Different drying techniques have evolved for improving such food drying processes. Hot-air drying is a relatively simple and economic procedure (9); however, it causes the degradation of sensitive components (8). Freeze-drying produces a high-quality product, but its application is limited due to its relatively expensive processing cost (10). Vacuum drying is another alternative method, and it is especially suitable for products such as fruits and vegetables that are prone to heat damage (11).
These processing techniques cause food materials to undergo structural deformations and other changes of the physicochemical properties (8); therefore, understanding these changes as affected by different drying methods is of great importance for improving product quality as well as utilizing pine needle powder (PNP) as a novel ingredient in functional foods. To the best of our knowledge, a systematic evaluation of different drying methods is limited in the literature, and information on the physicochemical properties of pine needle powder is scarce.
Therefore, the goal of this study was to systematically investigate how different drying methods [i.e., hot-air drying (AD), vacuum-drying (VD), and freeze-drying (FD)] affect microstructure, color, browning index, degree of rehydration, water solubility, and vitamin C content.
MATERIALS AND METHODS
Materials and powder preparations
Pine needles of P. koraiensis were collected at suburban hills in the vicinity of Pocheon, Korea. Three drying methods were used to prepare the powders as follows. Hot-air drying: samples were dried at 50°C using a hotair drying oven (DMC-122SP, Daeil Engr. Co., Seoul, Korea) for 48 h to a final moisture content of approximately 5~7%, on a moisture-free basis (MFB), which was determined by the gravimetric method at 105°C, until the weight reached a constant value. Vacuum-drying: drying at 50°C and vacuum pressure of 0.1 MPa in a vacuum dryer oven (VOS-301SD, Tokyo Rikakikai Co. Ltd., Tokyo, Japan) for 24 h, at which point the final moisture content reached the value described above. Freeze-drying: samples were frozen at −40°C in a deep freezer (VLT 1450-3-D-14, Thermo Electron Corp., Asheville, NC, USA), and then freeze- dried using a freeze-dryer (PPU-1100, Tokyo Rikakikai Co. Ltd.) at a vacuum pressure of 8.5 Pa for 5 days. Dehydrated samples were milled using an analytical mill (M20, IKA, Werke Staufen, Germany) with different particle size sieves (D-55743, FRITSCH, ldar-Oberstein, Germany) to yield particle sizes of less than 425 μm. The powders were placed in a desiccator prior to measurements.
Scanning electron microscopy (SEM)
Structures of the freeze-dried, vacuumed, and hot-air dried samples were examined using a low vacuum scanning electron microscope (Hitachi S-4300, 5.0 kV, Hitachi Ltd., Tokyo, Japan) operated at 15.0 kV, WD 15 mm under high vacuum mode. Each sample was then mounted on specimen stubs, sputter-coated with platinum and viewed under the microscope at 3 different magnifications 500×; 1,000×; 1,500×.
Color and browning index
The color of the PNP was evaluated using a colorimeter (model CM-600d, Minolta Co., Osaka, Japan), and reported as CIE L*-value (lightness), a*-value (redness), and b*-value (yellowness). Measurements were repeated five times, and mean values were compared.
The browning index was determined by extracting the powder (1 g) with distilled water (40 mL) at room temperature for 1 h, with occasional stirring at 5 min intervals, under darkness in a cupboard. The clear supernatant was obtained through centrifugation at 8,000 rpm for 20 min at 4°C. Absorbance of the colored product was determined spectrophotometrically at 420 nm against a distilled water blank (12). Measurements were made in triplicate, and mean values were compared.
Degree of rehydration and water solubility
The degree of rehydration was determined using the modified method of Medcalf and Gilles (13). PNP was dispersed in distilled water (1 g to 20 mL distilled water) with shaking for 1 h at 120 rpm in a 20°C water bath. Samples were then centrifuged at 3,500 rpm for 15 min. The centrifuge tubes were placed upside down for 1 min, and the supernatant decanted. Measurements were made in triplicate and mean values were compared. The degree of rehydration was calculated as follows:
Water solubility was determined following the modified methods of Dubois et al. (14) and Leach et al. (15). In a capped centrifuge tube, 0.2 g of sample was suspended in 10 mL of distilled water. The samples were heated at 60°C for 30 min with stirring at 120 rpm. The heated sample was immediately and rapidly cooled in an ice water bath for 3 min and centrifuged at 1,600 rpm at 4°C for 30 min. The supernatant was then decanted, and the samples were dried for 3 h at 105°C. Measurements were made in triplicate, and mean values were compared. Percentage water solubility was determined using the following formula:
Statistical analysis
All experimental data for each treatment were analyzed by ANOVA, and Duncan’s multiple range tests (P=0.05) were performed to determine any significant difference among various treatments using SAS (ver. 9.1, SAS Institute Inc., Cary, NC, USA).
RESULTS AND DISCUSSION
Microstructure
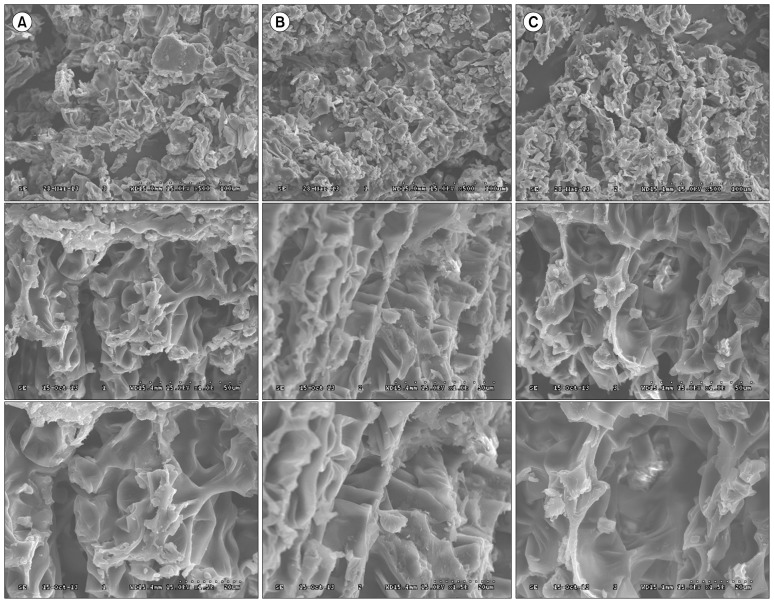
The surface structures of freeze-dried, vacuum-dried, and hot-air dried samples were shown to be distinctively different by SEM, as shown in Fig. 1. The size and density of the particles appeared to be different among the samples. Freeze-dried samples displayed a clear porous structure and appeared to occupy a bigger space. This could be due to the fact that the majority of the water content was removed by sublimation, and a “honeycomb network” was left after sublimation (16). A highly porous structure was observed for freeze-dried garlic (17), Panax notoginseng extract (18), and Inonotus obliquus mushroom powders (19). On the other hand, the hot-air dried samples showed lower porosity than the vacuum-dried and freeze-dried samples, resulting in poorer rehydration ability. Similar findings were reported for drying of sea cucumber (20).
Fig. 1.
Scanning electron microscope photographs (15.0 kV 500×, 15.0 kV 1,000×, and 15.0 kV 1,500×) of PNP prepared by (A) hot-air, (B) vacuum, and (C) freeze-drying methods, respectively.
Color
Table 1 illustrates color differences in the PNP. Since the enzymes that caused quality degradation were destroyed during processing by high temperature at 50°C or freezing, non-enzymatic browning was considered a major cause of color changes in PNP. The lightness (L*-value) ranged from 46.56~49.01 depending upon the drying method used to prepare the powder samples. The L*-values increased by 83.89, 93.56, and 85.35% for the hot-air, vacuum, and freezing-drying samples, respectively, in comparison with the initial color of the fresh sample. L*-value expresses the lightness of the sample, and a higher L*-value indicates a lighter color. Among the dried samples, the hot-air and freeze-dried samples showed significantly lower L*-values than the vacuum-dried sample (P<0.05); nevertheless, no real considerable differences were detected.
Table 1.
Selected physical characteristics of PNP as affected by drying methods
| Sample | Color | Browning index | Degree of rehydration (%) | Water solubility (%) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| L* | a* | b* | ||||
| Fresh | 25.32±1.26c | 1.77±0.22d | 8.88±0.57c | - | - | - |
| Hot-air drying | 46.56±0.52b | 4.43±0.03a | 30.66±0.13a | 1.411±0.010b | 286.75±25.59b | 42.37±3.98a |
| Vacuum-drying | 49.01±0.20a | 1.11±0.09b | 29.66±0.39b | 1.506±0.004a | 354.00±34.35a | 34.72±1.25b |
| Freeze-drying | 46.93±0.24b | −0.27±0.06c | 29.56±0.25b | 0.346±0.005c | 401.50±47.85a | 46.43±3.77a |
Means with different letters (a-d) in the same column are significantly different according to Duncan’s multiple range test (P <0.05).
The redness (a*-values) of the freeze-dried samples was significantly lower than that of the other dried samples, excluding the fresh sample (P<0.05). The lower a*-value might be attributed to less browning since the drying process was done at a relatively low temperature compared to the hot-air and vacuum-drying. The b*-value of the dried samples increased by 245.27, 234.01, and 232.88% for hot-air, vacuum, and freezing-drying, respectively, in comparison to the initial color of the fresh sample. Similar to L*-values, no real considerable differences in the values were found.
Browning index
The effects of the drying methods on the browning index, degree of rehydration, water solubility, and swelling power of PNP are also shown in Table 1. The freeze-dried sample had the lowest value of 0.346, whereas the hot-air and vacuum-dried samples had values of 1.506 and 1.411, respectively. The browning index values of the hot-air and vacuum-dried samples were significantly higher than that of the freeze-dried samples (P<0.05). Since the browning reaction in food during storage is heavily temperature and moisture-dependent, this result was probably due to a higher drying temperature during hot-air drying, which is in agreement with the findings of Kim et al. (21) and Son and Lee (22). The higher degree of non-enzymatic browning occurring during hot-air and vacuum-drying might be due to both, Maillard reaction and ascorbic acid oxidation (19).
Degree of rehydration and water solubility
Degree of rehydration and water solubility of PNP dried by different methods differed significantly (P<0.05) (Table 1). Rehydration, textural changes, and loss of water-holding capacity that might occur during drying process are irreversible (19). Especially, heat reduces the degree of hydration of starch, elasticity of cell walls, and coagulates proteins to reduce their water-holding capacity. In other words, the rate and extent of rehydration may be used as an indicator of food quality. Foods that are dried under optimum conditions, such as freeze-drying suffer less damage and rehydrate more rapidly and completely than poorly dried foods (23).
These effects were due to the porous structure of PNP with more space as visualized by SEM, which increases water accessibility. Similar results were reported for ginkgo nut (24), I. obliquus (19), and S. herbacea powders (25). In the case of hot-air and vacuum-drying, samples underwent relatively severe temperature treatment during drying, resulting in a lower degree of rehydration. Kim (26) reported that the rehydration rate of freeze-dried Lycium chinense Miller was three times higher than that of the hot-air dried sample.
Vitamin C
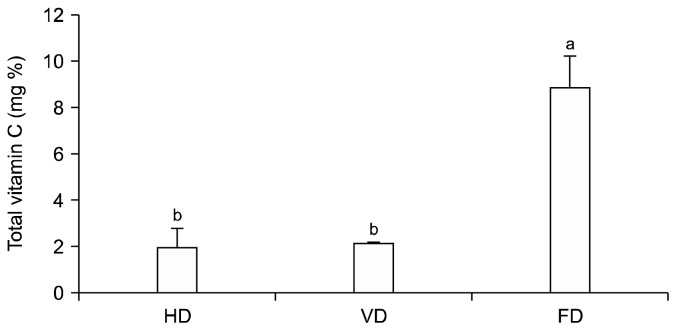
Vitamin C, one of the most important water-soluble antioxidants (27), is naturally present in fruits and vegetables and is widely used as a food additive (28). Despite its crucial role in several biochemical processes in the human body, it is extremely heat labile (29); thus, its content variation due to different drying processes is of importance to improve powder quality. Vitamin C content of P. koraiensis powders dried by different methods differed significantly as well (P<0.05) (Fig. 2). Hot-air as well as vacuum-drying resulted in greater loss of vitamin C in the samples than in the freeze-dried samples. Freeze-dried samples retained significantly higher amounts of vitamin C than the hot-air or vacuum-dried samples (P<0.05). This is due to the fact that vitamin C is easily destroyed by heat, and more heat was applied to the samples during hot-air and vacuum-drying processing.
Fig. 2.
Vitamin C content as affected by different drying methods. Means with different letters (a,b) are significantly different according to Duncan’s multiple range test (P<0.05).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Choi HD, Koh YJ, Choi IW, Kim YS, Park YK. Anticariogenic activity and glucosyltransferase inhibitory effects of extracts from pine needle and twig. Korean J Food Sci Technol. 2007;39:336–341. [Google Scholar]
- 2.Watanabe K, Momose F, Handa H, Nagata K. Interaction between influenza virus proteins and pine cone anti-tumor substance that inhibits the virus multiplication. Biochem Biophys Res Commun. 1995;214:318–323. doi: 10.1006/bbrc.1995.2290. [DOI] [PubMed] [Google Scholar]
- 3.Su XY, Wang ZY, Liu JR. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009;117:681–686. doi: 10.1016/j.foodchem.2009.04.076. [DOI] [Google Scholar]
- 4.Zeng WC, Jia LR, Zhang Y, Cen JQ, Chen X, Gao H, Feng S, Huang YN. Antibrowning and antimicrobial activities of the water-soluble extract from pine needles of Cedrus deodara. J Food Sci. 2011;76:C318–C323. doi: 10.1111/j.1750-3841.2010.02023.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang JM, Shi XF, Fan B. Chemical composition and pharmacological activities of Cedrus deodara. Chinese Trad Plant Med. 2009;31:928–933. [Google Scholar]
- 6.Chen YH, Hsieh PC, Mau JL, Sheu SC. Antioxidant properties and mutagenicity of Pinus morrisonicola and its vinegar preparation. LWT-Food Sci Technol. 2011;44:1477–1481. doi: 10.1016/j.lwt.2011.01.016. [DOI] [Google Scholar]
- 7.Jangam SV. An overview of recent developments and some R&D challenges related to drying of foods. Drying Technol. 2011;29:1343–1357. doi: 10.1080/07373937.2011.594378. [DOI] [Google Scholar]
- 8.Karunasena HCP, Brown RJ, Gu YT, Senadeera W. Application of meshfree methods to numerically simulate microscale deformations of different plant food materials during drying. J Food Eng. 2015;146:209–226. doi: 10.1016/j.jfoodeng.2014.09.011. [DOI] [Google Scholar]
- 9.Diamante LM, Ihns R, Savage GP, Leo Vanhanen L. A new mathematical model for thin layer drying of fruits. Int J Food Sci Technol. 2010;45:1956–1962. doi: 10.1111/j.1365-2621.2010.02345.x. [DOI] [Google Scholar]
- 10.Lee JH, Zuo L. Mathematical modeling on vacuum drying of Zizyphus jujuba Miller slices. J Food Sci Technol. 2013;50:115–121. doi: 10.1007/s13197-011-0312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giri SK, Prasad S. Drying kinetics and rehydration characteristics of microwave-vacuum and convective hot-air dried mushrooms. J Food Eng. 2007;78:512–521. doi: 10.1016/j.jfoodeng.2005.10.021. [DOI] [Google Scholar]
- 12.Krishnan JG, Padmaja G, Moorthy SN, Suja G, Sajeev MS. Effect of pre-soaking treatments on the nutritional profile and browning index of sweet potato and yam flours. Innovative Food Sci Emerging Technol. 2010;11:387–393. doi: 10.1016/j.ifset.2010.01.010. [DOI] [Google Scholar]
- 13.Medcalf DG, Gilles KA. Wheat starches. I. Comparison of physicochemical properties. Cereal Chem. 1965;42:558–568. [Google Scholar]
- 14.DuBois M, Giles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 15.Leach HW, McCowen LD, Schoh T. Structure of the starch granule. I. Swelling and solubility patterns of various starches. Cereal Chem. 1959;36:534–544. [Google Scholar]
- 16.Huang LL, Zhang M, Mujumdar AS, Lim RX. Comparison of four drying methods for re-structured mixed potato with apple chips. J Food Eng. 2011;103:279–284. doi: 10.1016/j.jfoodeng.2010.10.025. [DOI] [Google Scholar]
- 17.Chung SK, Choi JU. The effects of drying methods on the quality of the garlic powder. Korean J Food Sci Technol. 1990;22:44–49. [Google Scholar]
- 18.Liu X, Qiu Z, Wang L, Chen Y. Quality evaluation of Panax notoginseng extract dried by different drying methods. Food Bioprod Process. 2011;89:10–14. doi: 10.1016/j.fbp.2010.03.008. [DOI] [Google Scholar]
- 19.Lee MJ, Seog EJ, Lee JH. Physicochemical properties of chaga (Inonotus obliquus) mushroom powder as influenced by drying methods. J Food Sci Nutr. 2007;12:40–45. doi: 10.3746/jfn.2007.12.1.040. [DOI] [Google Scholar]
- 20.Duan X, Zhang M, Mujumdar AS, Wang S. Microwave freeze drying of sea cucumber (Stichopus japonicus) J Food Eng. 2010;96:491–497. doi: 10.1016/j.jfoodeng.2009.08.031. [DOI] [Google Scholar]
- 21.Kim HR, Seog EJ, Lee JH, Rhim JW. Physicochemical properties of onion powder as influenced by drying methods. J Korean Soc Food Sci Nutr. 2007;36:342–347. doi: 10.3746/jkfn.2007.36.3.342. [DOI] [Google Scholar]
- 22.Son SM, Lee JH. Physicochemical and SEM studies on Capsosiphon fulvescens powders prepared using different drying methods. Res J BioTechnol. 2011;6:50–54. [Google Scholar]
- 23.Lee JH, Kwak EJ, Kim JS, Lee YS. Quality characteristics of sponge cake added with mesangi (Capsosiphon fulvescens) powder. Korean J Food Cookery Sci. 2007;23:83–89. [Google Scholar]
- 24.Kim JM, Lee YC, Kim KO. Effects of convection oven dehydration conditions on the physicochemical and sensory properties of ginkgo nut powder. Korean J Food Sci Technol. 2003;35:393–398. [Google Scholar]
- 25.Kim HJ, Lee JH. Physicochemical properties of Salicornia herbacea powder as influenced by drying methods. Food Eng Prog. 2009;13:105–109. [Google Scholar]
- 26.Kim BJ. MS Thesis. Korea University; Seoul, Korea: 2004. Effects of drying and extraction conditions on the chemical composition of water extract in Lycium chinense Miller. [Google Scholar]
- 27.Klimczak I, Gliszczyńska-Świgło A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015;175:100–105. doi: 10.1016/j.foodchem.2014.11.104. [DOI] [PubMed] [Google Scholar]
- 28.Özyürek M, Güçlü K, Bektaşoğlu B, Apak R. Spectrophotometric determination of ascorbic acid by the modified CUPRAC method with extractive separation of flavonoids-La(III) complexes. Anal Chim Acta. 2007;588:88–95. doi: 10.1016/j.aca.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 29.Valente A, Sanches-Silva A, Albuquerque TG, Costa HS. Development of an orange juice in-house reference material and its application to guarantee the quality of vitamin C determination in fruits, juices and fruit pulps. Food Chem. 2014;154:71–77. doi: 10.1016/j.foodchem.2013.12.053. [DOI] [PubMed] [Google Scholar]