Abstract
The purpose of this research was to investigate the inhibitory effect of jicama extract on α-glucosidase activity, α-amylase activity, and postprandial hyperglycemia in streptozotocin (STZ)-induced diabetic mice. Jicama extract showed prominent inhibitory effects against α-glucosidase and α-amylase. The IC50 values of jicama extract against α-glucosidase and α-amylase were 0.083±0.004 and 0.091±0.017 mg/mL, respectively. The increase in postprandial blood glucose levels was more significantly suppressed in the jicama extract-administered group than in the control group of both STZ-induced diabetic and normal mice. Blood glucose levels of the control group increased to 383.75±11.54 and 402.50±15.32 mg/dL at 30 and 60 min after a meal and decreased to 349.67±11.62 mg/dL at 120 min. However, postprandial blood glucose levels were significantly decreased, when diabetic mice were fed with jicama extract (342.00±15.73, 367.00±13.00, and 329.67±12.43 mg/dL at 30, 60, and 120 min, respectively). Furthermore, the area under the curve was significantly decreased with jicama extract administration in diabetic mice (P<0.05). Therefore, these results indicate that jicama extract may help decrease postprandial blood glucose level by inhibiting α-glucosidase.
Keywords: jicama extract, postprandial hyperglycemia, diabetic mice
INTRODUCTION
Diabetes is one of the most serious health concerns all over the world. More Koreans have diabetes than ever before. Diabetes can seriously shorten the life expectancy to 10 years (1). The disease is classified into type 1 and type 2 diabetes. The prevalence of type 2 diabetes is increasing worldwide (2). Postprandial hyperglycemia plays a key role in the development of type 2 diabetes (3). Postprandial hyperglycemia induces endothelial dysfunction oxidative stress (4). The postprandial phase features a rapid and large increase in blood glucose levels, and it is possible that these postprandial “hyperglycemic spikes” may be relevant to diabetes complications such as cardiovascular disease (5). When diabetic complications develop, the harm to the heart (6), kidneys (7), nerves (8), and eyes (9) may be irreversible. Therefore, the management of postprandial hyperglycemia is thought to be important in the treatment of diabetes and the prevention of cardiovascular complications (10).
Acarbose is a commonly used oral hypoglycemic agent, which blocks the degradation of starch and sucrose, and delays the absorption of glucose and fructose (11). Anti-diabetic drugs are helpful to control postprandial blood glucose levels. However, long-term use of these drugs should be restricted because of possible side effects such as drug induced hypoglycemia, abdominal discomfort, and weight gain (12,13). Therefore, investigations have been conducted on α-glucosidase and α-amlyase inhibitors based on natural plants (14,15).
Jicama (Pachyrhizus erosus) is a starchy root and one of most the popular edible roots grown in many parts of Central America, South Asia, Caribbean, and in some Andean South American regions. Jicama is also known as yam bean. Cultivating jicama in Korea has recently succeeded, and it has started to spread out in Korea (16). Many people are still unfamiliar with jicama, Table 1 explained the nutritional composition of jicama. Research on jicama is limited, although one publication has reported the chemical constituents of jicama root (17). Mussary et al. (18) studied the postharvest conservation of jicama root and there are immunomodulatory activities in jicama fiber (19). Jicama is rich in fructooligosaccharides including inulin, a soluble fiber. Inulin is sometimes called natural insulin. So jicama which contains inulin might be helpful for alleviating blood glucose levels. However, there is no experimental data on the effects of jicama improving postprandial blood glucose levels. Therefore, we investigated the α-glucosidase and α-amlyase inhibitory effect of jicama extract in vitro, and studied the effect of jicama extract on postprandial blood glucose levels after a meal in diabetic mice.
Table 1.
Nutritional composition of jicama1)
| Principle | Nutrient value |
|---|---|
| Energy | 38 kcal |
| Carbohydrates | 8.82 g |
| Protein | 0.72 g |
| Total fat | 0.19 g |
| Cholesterol | 0 mg |
| Dietary fiber | 4.9 g |
Nutritional composition of jicama is per 100 g.
(Source: USDA National Nutrient data base)
MATERIALS AND METHODS
Materials and preparation of jicama extract
Jicama was purchased from commercial sources in Hadong, Korea. Jicama was washed in distilled water, and then cut into 0.1~1.0 cm sized pieces. Sliced jicama was dried at 60°C and ground into powder. For extraction, dried jicama powder was soaked with water at room temperature overnight. After soaking, the extract was sonicated three times for 5 h at 60°C and then filtered through filter paper (Whatman No.1). The extract was concentrated by using a rotary evaporator under reduced pressure and freeze-dried to a powder. All chemicals and reagents used were of analytical grade and obtained from commercial sources.
Inhibitory effect of jicama extract on α-glucosidase and α-amlyase in vitro
The α-glucosidase inhibitory assay was conducted using the chromogenic method described by Watanabe et al. (14), using a readily available yeast enzyme. The inhibitory effect of jicama extract against α-glucosidase was measured using p-nitrophenyl-α-glucopyranoside (pNPG) as a substrate. Yeast α-glucosidase (0.7 U, Sigma, St. Louis, MO, USA) was dissolved in 100 mM phosphate buffer (pH 7.0) including 2 g/L bovine serum albumin and 0.2 g/L NaN3 and used as an enzyme solution. 5 mM pNPG in the same buffer (pH 7.0) was used as a substrate solution. Then 50 μL of enzyme solution and 10 μL of sample dissolved in dimethylsulfoxide at the 9.8 mM concentration were mixed in a microtiter plate and absorbance measured at 405 nm at time zero. After 5 min-incubation, substrate solution (50 μL) was added and incubated for another 5 min. The increase in the absorbance from time zero was recorded. The α-amlyase inhibitory activity was assayed using similar procedures as explained for α-glucosidase inhibitory assay, except for the use of porcine pancreatic amylase (100 U, Sigma) and blocked p-nitrophenyl-α-D-malto-pentoglycoside (Sigma) as enzyme and substrate, respectively. Percent inhibitory activity was expressed as 100 minus relative absorbance difference (%) of test compounds to absorbance change of the control, where test solution was replaced by carrier solvent.
Experimental animals
Male ICR mice (4 weeks of age: purchased from Central Lab. Animal Inc., Seoul, Korea) were used for experiments. All mice were kept individually in a light (12 h on/12 h off) and temperature controlled room with food and water available ad libitum. After an adjustment period of 2 weeks, diabetes mellitus was induced by intra-peritoneal injection of streptozotocin (STZ) (60 mg/kg i.p.) dissolved in a freshly prepared citrate buffer (0.1 M, pH 4.5). After seven days, tail bleeds were performed and animals with a blood glucose concentration above 250 mg/dL were considered to be diabetic. All procedures were approved by the animal ethics committee of our university.
Measurement of blood glucose levels
The animal experiment was a one time oral administration. Blood glucose levels after the administration of jicama extract were measured in normal mice and STZ-induced diabetic mice. For the experiment, normal and STZ-induced diabetic mice were fasted overnight, and they were randomly divided into three groups. Fasted mice were deprived of food for at least 12 h, but they were allowed free access to water. After overnight fasting, the mice were orally administrated soluble starch (2 g/kg body weight) alone or with jicama extracts (200 mg/kg body weight) or acarbose (100 mg/kg body weight). Blood samples were measured at 0, 30, 60, and 120 min (10) from tail vein blood. Blood glucose was measured using a glucometer (Roche Diagnostics Deutschland GmbH, Mannheim, Germany). Areas under the curve (AUC) were estimated using the trapezoidal rule.
Data and statistical analysis
The data were presented as mean±SD. The statistical analysis was performed using SAS software (SAS Institute Inc., Cary, NC, USA). The Student’s t-test was used for comparisons between control and sample groups. The values were evaluated by one-way analysis of variance (ANOVA) followed by post-hoc Duncan’s multiple range test.
RESULTS AND DISCUSSION
Inhibitory effect of jicama extract on α-glucosidase and α-amlyase activities in vitro
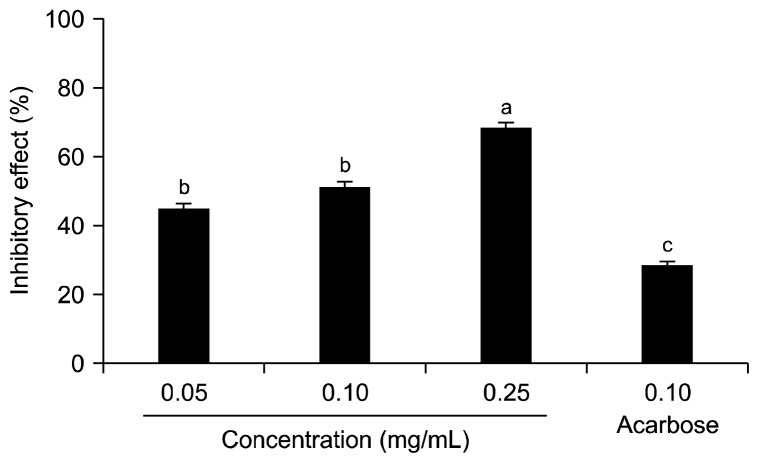
The inhibitory effect of jicama extract against α-glucosidase is shown in Fig. 1. Jicama extract inhibited α-glucosidase activity in a dose-dependent manner, as 36.1± 1.8%, 57.1±1.9%, and 72.3±1.3% at concentrations of 0.05, 0.10, and 0.25 mg/mL. The 0.10 mg/mL concentration of acarbose, which is used as an anti diabetic pharmaceutical agent, inhibited α-glucosidase activity by 64.8±1.6%. The α-amlyase inhibitory effect of jicama extract was also determined using pNPG as a substrate. The inhibitory effect of jicama extract against α-amylase is shown in Fig. 2. The inhibitory effect of jicama extract against α-amlyase was dose-dependent, and the effect was 44.6±2.0, 51.0±1.7, and 68.3±1.9% at concentrations of 0.05, 0.10, and 0.25 mg/mL, respectively. The α-amylase inhibitory activity of jicama extract (0.10 mg/mL) was higher than that of the same concentration of acarbose. The IC50 values of jicama extract against α-glucosidase and α-amlyase were 0.083±0.004 and 0.091±0.017 mg/mL, respectively (Table 2).
Fig. 1.
Inhibitory effects of jicama extract on α-glucosidase. Values are expressed as means±SD in triplicate experiments. Values with different letters are significantly different at P<0.05 as analyzed by Duncan’s multiple range test. Acarbose (0.10 mg/mL) was used as the positive control.
Fig. 2.
Inhibitory effects of jicama extract on α-amylase. Values are expressed as means±SD in triplicate experiments. Values with different letters are significantly different at P<0.05 as analyzed by Duncan’s multiple range test. Acarbose (0.10 mg/mL) was used as the positive control.
Table 2.
IC50 values of inhibitory effect of jicama extract on α-glucosidase and α-amylase (unit: mg/mL)
| Sample | IC501) | |
|---|---|---|
|
| ||
| α-glucosidase | α-amylase | |
| Jicama | 0.083±0.004NS | 0.091±0.017* |
| Acarbose | 0.075±0.006 | 0.164±0.033 |
IC50 value is the concentration of sample required for 50% inhibition. Each value is expressed as mean±SD in triplicate experiments.
P<0.05 vs positive control, acarbose.
not significant.
The roles of α-amylase and α-glucosidase are important for carbohydrate digestion. α-Amylase catalyzes the hydrolysis of α-1,4-glycosidic linkages of starch, glycogen, and diverse oligosaccharides (20). The inhibition of α-amylase activity or α-glucosidase activity is an effective solution for the management of blood glucose levels, as it delays the absorption of carbohydrate in the gastrointestinal tract thereby controlling the postprandial hyperglycemia and providing unquestioned cardiovascular benefit (21). Moorthy et al. (22) reported that diverse α-glucosidase inhibitors extracted from natural products are used for the remedy of carbohydrate mediated diseases.
Our data showed that jicama extract had significant inhibitory activities on α-glucosidase and α-amlyase, suggesting the jicama extract may be useful for diabetic control. Nieto (23) reported that starch from jicama contained soluble dietary fiber such as inulin and oligofructose. Inulin is a carbohydrate made from β(1,2)-linked fructosyl residues, mostly ending with a glucose residue (24). It can also be chemically modified to make shorter chain oligomers, called fructooligosaccharides (25). The 1,2-β-linkages making up inulin are resistant to digestive enzymes, such as the α-glucosidase and the α-amylase (26). Apolinário (27) reported that inulin shares many physiological traits with soluble dietary fiber, so they improve blood glucose levels. Dietary fiber has remnants of edible plant cell polysaccharides and associated substances resistant to hydrolysis by human digestive enzymes (28).
Edwards et al. (29) reported that soluble dietary fiber delays the access of glucose to the small intestine’s epithelium and reduces glucose absorption by resisting the convective effects of intestinal contractions, thereby blunting postprandial glucose peaks.
Effect of jicama extract on blood glucose level in vivo
The effect of jicama extract on blood glucose levels after a meal was studied in STZ-induced diabetic and normal mice. Postprandial blood glucose levels after consumption of jicama extract was significantly lower than levels of the control in diabetic mice (Fig. 3). Blood glucose levels of the control group increased to 383.7±11.5 mg/dL at 30 min, 402.5±15.3 mg/dL at 60 min after meal and decreased to 349.6±11.6 mg/dL at 120 min after meal. However, postprandial blood glucose levels were significantly decreased, when diabetic mice were fed jicama extract (342.0±15.7 mg/dL, 367.0±13.0 mg/dL, and 329.6±12.4 mg/dL at 30, 60, and 120 min, respectively). The postprandial blood glucose level was also significantly lower when the normal mice were fed with jicama extracts (Fig. 4). The AUC for glucose response of jicama extract fed group (658.1±18.0 mg·h/dL) was significantly lower (P<0.05) than that of the control group (742.5±24.7 mg·h/dL) in the diabetic mice (Table 3).
Fig. 3.
Blood glucose levels after the administration of jicama extract in streptozotocin-induced diabetic mice. Control (distilled water), jicama (200 mg/kg), and acarbose (100 mg/kg) were fed with orally with starch (2 g/kg). Each value is expressed as mean±SD of seven mice (n=42). A significant difference was identified at P<0.05 as analyzed by Duncan’s multiple range test.
Fig. 4.
Blood glucose levels after the administration of jicama extract in normal mice. Control (distilled water), jicama (200 mg/kg), and acarbose (100 mg/kg) were fed with orally with starch (2 g/kg). Each value is expressed as mean±SD of seven mice (n=42). A significant difference was identified P<0.05 as analyzed by Duncan’s multiple range test.
Table 3.
Area under the curve (AUC) of postprandial glucose responses of normal and streptozotocin-induced diabetic mice (unit: mg·h/dL)
| Group1) | AUC | |
|---|---|---|
|
| ||
| Normal mice | Diabetic mice | |
| Jicama | 259.32±34.07b | 685.18±26.00b |
| Acarbose | 204.58±25.98b | 653.57±21.38b |
| Control | 319.56±29.52a | 742.58±24.75a |
Jicama extract (200 mg/kg), acarbose (100 mg/kg), and control (distilled water) were co-administered orally with starch (2 g/kg). Each value is expressed as mean±SD of seven mice (n=42).
Values with different letters are significantly different at P<0.05 as analyzed by Duncan’s multiple range test.
The treatment goal for diabetic patients is to maintain near-normal levels of blood glucose level in both fasting and the postprandial states (30). Postprandial hyperglycemia is the first metabolic abnormality to occur in diabetes mellitus (3). Postprandial hyperglycemia performs the important function in the development of type 2 diabetes and complications associated with cardiovascular diseases.
As shown in Fig. 3 and Fig. 4, it seems that jicama extract has anti-hyperglycemic effect in STZ-induced diabetic and normal mice. The increase in postprandial blood glucose levels was suppressed significantly in both STZ-induced diabetic and normal mice which were fed with jicama extract. This result shows that jicama extract may delay the absorption of dietary carbohydrates, suppressing the increase of postprandial blood glucose levels. Moreover, jicama extract reduces the peak blood glucose level and the AUC. The hypoglycemic effect might be explained by oligofructose and inulin contained in jicama extracts playing a beneficial role in lowering the blood glucose levels. There are several mechanisms of soluble dietary fiber such as inulin and oligofructose to explain the decreased postprandial glucose curve. First, by delaying gastric emptying, after glucose more slowly enters into the blood stream, thus decreasing the postprandial increase in serum glucose (31). Second, the sequestration of carbohydrates ingested with the meal, retarding carbohydrates access to digestive enzymes (32). Third, soluble dietary fiber such as inulin increases the viscosity of small intestine fluid and hinders diffusion of glucose. So they help to delay the absorption and digestion of carbohydrates (33). All of these mechanisms might lower the rate of glucose absorption, and as a result, decrease the postprandial hyperglycemia. Bonsu (34) reported that inulin plays an important role in controlling postprandial glycemic levels and may have potential benefits in alleviating postprandial blood glucose levels.
Attenuating postprandial blood glucose levels is especially important for patients with type 2 diabetes. Postprandial hyperglycemia is related to diverse metabolic disorders and diseases (35,36). Many synthetic compounds have been used in the treatment for diabetes. However, they have been generally associated with undesirable side effects (37). Jicama extract may be a good candidate for the natural anti-diabetic materials. In our study, the extract showed the anti-hyperglycemia effect at the test dose (200 mg/kg, mouse). Based on the findings of our animal study, jicama extract (0.02 g/kg, human) might be safe and effective to lowering postprandial hyperglycemia. In conclusion, jicama extract has high inhibitory activity against α-glucosidase and α-amlyase activities. Furthermore, jicama extract may lower postprandial blood glucose levels.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Kastorini CM, Panagiotakos DB. Dietary patterns and prevention of type 2 diabetes: from research to clinical practice; a systematic review. Curr Diabetes Rev. 2009;5:221–227. doi: 10.2174/157339909789804341. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Baron AD. Postprandial hyperglycemia and α-glucosidase inhibitors. Diabetes Res Clin Pract. 1998;40:S51–S55. doi: 10.1016/S0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 4.Ceriello A, Taboga C, Tonutti L, Quagliaro L, Piconi L, Bais B, Da Ros R, Motz E. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short- and long-term simvastatin treatment. Circulation. 2002;106:1211–1218. doi: 10.1161/01.CIR.0000027569.76671.A8. [DOI] [PubMed] [Google Scholar]
- 5.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Shamhart PE, Luther DJ, Adapala RK, Bryant JE, Petersen KA, Meszaros JG, Thodeti CK. Hyperglycemia enhances function and differentiation of adult rat cardiac fibroblasts. Can J Physiol Pharmacol. 2014;92:598–604. doi: 10.1139/cjpp-2013-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang W, Huang J, Liu Q, Lin F, He Z, Zeng Z, He L. Neutrophil-lymphocyte ratio is a reliable predictive marker for early-stage diabetic nephropathy. Clin Endocrinol (Oxf) 2015;82:229–233. doi: 10.1111/cen.12576. [DOI] [PubMed] [Google Scholar]
- 8.Bril V. Neuromuscular complications of diabetes mellitus. Continuum (Minneap Minn) 2014;20:531–544. doi: 10.1212/01.CON.0000450964.30710.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stitt AW, Lois N, Medina RJ, Adamson P, Curtis TM. Advances in our understanding of diabetic retinopathy. Clin Sci (Lond) 2013;125:1–17. doi: 10.1042/CS20120588. [DOI] [PubMed] [Google Scholar]
- 10.Tan MC, Wong TW, Ng OC, Joseph A, Hejar AR. Metabolic syndrome components and prevalence of cardiovascular disease among type 2 diabetic patients in Malaysia. Southeast Asian J Trop Med Public Health. 2014;45:226–235. [PubMed] [Google Scholar]
- 11.He K, Shi JC, Mao XM. Safety and efficacy of acarbose in the treatment of diabetes in Chinese patients. Ther Clin Risk Manag. 2014;10:505–511. doi: 10.2147/TCRM.S50362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joshi SR, Ramachandran A, Chadha M, Chatterjee S, Rathod R, Kalra S. Acarbose plus metformin fixed-dose combination in the management of type 2 diabetes. Expert Opin Pharmacother. 2014;15:1611–1620. doi: 10.1517/14656566.2014.932771. [DOI] [PubMed] [Google Scholar]
- 13.van de Laar FA, Lucassen PL, Akkermans RP, van de Lisdonk EH, Rutten GE, van Weel C. α-Glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28:154–163. doi: 10.2337/diacare.28.1.154. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe J, Kawabata J, Kurihara H, Niki R. Isolation and identification of α-glucosidase inhibitors from tochucha (Eucommia ulmoides) Biosci Biotechnol Biochem. 1997;61:177–180. doi: 10.1271/bbb.61.177. [DOI] [PubMed] [Google Scholar]
- 15.Salimifar M, Fatehi-Hassanabad Z, Fatehi M. A review on natural products for controlling type 2 diabetes with an emphasis on their mechanisms of actions. Curr Diabetes Rev. 2013;9:402–411. doi: 10.2174/15733998113099990076. [DOI] [PubMed] [Google Scholar]
- 16.Kim SK, Choi HJ, Won JF, Park JH, Lee IJ, Park SY. Introduction of yam bean (Pachyrhizus spp.) in Korea. Korean J Plant Res. 2009;22:546–551. [Google Scholar]
- 17.Fernandez MV, Warid WA, Loaiza JM, Montiel A. Developmental patterns of jicama (Pachyrhizus erosus (L.) Urban) plant and the chemical constituents of roots grown in Sonora, Mexico. Plant Foods Hum Nutr. 1997;50:279–286. doi: 10.1007/BF02436074. [DOI] [PubMed] [Google Scholar]
- 18.Mussury RM, Scalon SP, Silva MA, Silva TF, Gomes H, Gassi R. Postharvest conservation of the tuberous roots of Pachyrhizus ahipa (Wedd) Parodi. An Acad Bras Cienc. 2013;85:761–768. doi: 10.1590/S0001-37652013005000035. [DOI] [PubMed] [Google Scholar]
- 19.Kumalasari ID, Nishi K, Harmayani E, Raharjo S, Sugahara T. Immunomodulatory activity of Bengkoang (Pachyrhizus erosus) fiber extract in vitro and in vivo. Cytotechnology. 2014;66:75–85. doi: 10.1007/s10616-013-9539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prashanth D, Padmaja R, Samiulla DS. Effect of certain plant extracts on α-amylase activity. Fitoterapia. 2001;72:179–181. doi: 10.1016/S0367-326X(00)00281-1. [DOI] [PubMed] [Google Scholar]
- 21.Kalra S. Alpha glucosidase inhibitors. J Pak Med Assoc. 2014;64:474–476. [PubMed] [Google Scholar]
- 22.Moorthy NS, Ramos MJ, Fernandes PA. Studies on α-glucosidase inhibitors development: magic molecules for the treatment of carbohydrate mediated diseases. Mini Rev Med Chem. 2012;12:713–720. doi: 10.2174/138955712801264837. [DOI] [PubMed] [Google Scholar]
- 23.Nieto C. Agronomical and bromatological studies in jicama (Polymnia sonchifolia Poep et Endl.) Arch Latinoam Nutr. 1991;41:213–221. [PubMed] [Google Scholar]
- 24.Meyer D, Bayarri S, Tárrega A, Costell E. Inulin as texture modifier in dairy products. Food Hydrocolloids. 2011;25:1881–1890. doi: 10.1016/j.foodhyd.2011.04.012. [DOI] [Google Scholar]
- 25.Yun JW. Fructooligosaccharides-occurrence, preparation, and application. Enzyme Microb Technol. 1996;19:107–117. doi: 10.1016/0141-0229(95)00188-3. [DOI] [Google Scholar]
- 26.Oku T, Tokunaga T, Hosoya N. Nondigestiblility of a new sweetener, “Neosugar”, in the rat. J Nutr. 1984;114:1574–1578. doi: 10.1093/jn/114.9.1574. [DOI] [PubMed] [Google Scholar]
- 27.Apolinário AC, de Lima Damasceno BP, de Macêdo Beltrão NE, Pessoa A, Converti A, da Silva JA. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr Polym. 2014;101:368–378. doi: 10.1016/j.carbpol.2013.09.081. [DOI] [PubMed] [Google Scholar]
- 28.Cherbut C. Inulin and oligofructose in the dietary fibre concept. Br J Nutr. 2002;87:S159–S162. doi: 10.1079/BJN2002532. [DOI] [PubMed] [Google Scholar]
- 29.Edwards CA, Johnson IT, Read NW. Do viscous polysaccharides slow absorption by inhibiting diffusion or convection? Eur J Clin Nutr. 1988;42:307–312. [PubMed] [Google Scholar]
- 30.Lebovitz HE. Postprandial hyperglycaemic state: importance and consequences. Diabetes Res Clin Pract. 1998;40:S27–S28. [PubMed] [Google Scholar]
- 31.Blackburn NA, Redfern JS, Jarjis H, Holgate AM, Hanning I, Scarpello JH, Johnson IT, Read NW. The mechanism of action of guar gum in improving glucose tolerance in man. Clin Sci (Lond) 1984;66:329–336. doi: 10.1042/cs0660329. [DOI] [PubMed] [Google Scholar]
- 32.Pastors JG, Blaisdell PW, Balm TK, Asplin CM, Pohl SL. Psyllium fiber reduces rise in postprandial glucose and insulin concentrations in patients with non-insulin-dependent diabetes. Am J Clin Nutr. 1991;53:1431–1435. doi: 10.1093/ajcn/53.6.1431. [DOI] [PubMed] [Google Scholar]
- 33.Ou S, Kwok K, Li Y, Fu L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J Agric Food Chem. 2001;49:1026–1029. doi: 10.1021/jf000574n. [DOI] [PubMed] [Google Scholar]
- 34.Bonsu NK, Johnson CS, McLeod KM. Can dietary fructans lower serum glucose? J Diabetes. 2011;3:58–66. doi: 10.1111/j.1753-0407.2010.00099.x. [DOI] [PubMed] [Google Scholar]
- 35.Dennis JW, Laferté S, Waghorne C, Breitman ML, Kerbel RS. Beta 1–6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987;236:582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- 36.Gruters RA, Neefjes JJ, Tersmette M, de Goede RE, Tulp A, Huisman HG, Miedema F, Ploegh HL. Interference with HIV-induced syncytium formation and viral infectivity by inhibitors of trimming glucosidase. Nature. 1987;330:74–77. doi: 10.1038/330074a0. [DOI] [PubMed] [Google Scholar]
- 37.Yale JF. Oral antihyperglycemic agents and renal disease: new agents, new concepts. J Am Soc Nephrol. 2005;16:S7–S10. doi: 10.1681/ASN.2004110974. [DOI] [PubMed] [Google Scholar]