Abstract
Grape products have been known to exert greater antioxidant and anti-obesity than anti-hyperglycemic effects in animals and humans. Omija is used as an ingredient in traditional medicine, and it is known to have an anti-hyperglycemic effect. We investigated whether the combined extracts of grape pomace and omija fruit (GE+OE) could reduce fat accumulation in adipose and hepatic tissues and provide beneficial effects against hyperglycemia and insulin resistance in type 2 diabetic mice. C57BL/KsJ-db/db mice were fed either a normal control diet or GE+OE (0.5% grape pomace extract and 0.05% omija fruit extract, w/w) for 7 weeks. GE+OE decreased plasma leptin and resistin levels while increasing adiponectin levels and reducing the total white adipose tissue weight. Furthermore, GE+OE lowered plasma free fatty acid (FFA), triglyceride, and total-cholesterol levels as well as hepatic FFA and cholesterol levels. Hepatic fatty acid synthase and glucose 6-phosphate dehydrogenase activities were decreased in the GE+OE group, whereas hepatic β-oxidation activity was increased. Furthermore, GE+OE supplementation not only reduced hyperglycemia and pancreatic β-cell failure but also lowered blood glycosylated hemoglobin and plasma insulin levels. The homeostasis model assessment of insulin resistance levels was also decreased and the decrease seems to be mediated by the lowered activities of hepatic glucose-6-phosphatase and phosphoenolpyruvate carboxykinases. The present data suggest that GE+OE may have the potential to reduce hyperglycemia, insulin resistance, and obesity in patients with type 2 diabetes.
Keywords: grape pomace, omija fruit, hyperglycemia, obesity, type 2 diabetes
INTRODUCTION
Type 2 diabetes is a chronic metabolic disorder characterized by insulin resistance in peripheral tissues and/or a relative deficiency in insulin secreting ability. Hyperinsulinemia is often seen in animals and humans with early stage type 2 diabetes (1). Obesity is closely linked to hyperinsulinemia, and the adipose tissue is known to secrete various metabolites and adipocytokines that may play a role in hyperinsulinemia (2). For example, increase in nonesterified fatty acid (NEFA) release from adipose tissue causes hyperinsulinemia and insulin resistance by inhibiting glucose transport/phosphorylation and by reducing the rates of glucose oxidation and glycogen synthesis (3). Excessive fat accumulation also results in an increase or decrease in the secretion of adipocytokines, which may play a major role in the pathogenesis of insulin resistance (4). Along with adipose tissue, the liver is a critical organ in metabolic regulation, and hepatic insulin signaling is important for the regulation of glucose and lipid homeostasis (5). Hepatic glucose production is elevated in the presence of hyperinsulinemia and insulin resistance, and it is a cause of increased fasting blood glucose (6). Hepatic insulin resistance is also strongly related to hepatic steatosis, and hepatic steatosis is present in animals with type 2 diabetes, such as C57BL/KsJ-db/db (db/db) mice (7).
Pérez-Jiménez and Saura-Calixto (8) reported that grape products (grapes, wine, grape skin, grape seed, grape pomace, and grape polyphenol extracts) exert hypolipidemic, anti-atherosclerotic, and antioxidant effects in animals and humans. The consumption of red wine by obese women was discussed with regard to the potential beneficial effects on insulin sensitivity (9); however, Ceriello et al. (10) found that there was no change in plasma glucose levels despite a beneficial effect against postprandial oxidative stress in type 2 diabetic patients after the consumption of a meal supplemented with grape products. Therefore, the hypoglycemic effects of grape products remain unclear. On the other hand, the omija (Schisandra chinensis) fruit has been used in traditional medicine for the treatment of cough, wheezing, dry mouth, hepatitis, and cardiovascular disease in East Asia. A recent study reported that it reduces postprandial hyperglycemia in in vitro and in vivo animal models by inhibiting the activities of intestinal α-glucosidase and pancreatic α-amylase (11). In addition, omija supplementation lowered lipid and glucose accumulation in the liver of normal diet- and hypercholesterolemic diet-fed mice (12).
In our previous study, we demonstrated that, compared to grape pomace ethanol extract (GE, 0.5%) alone, GE plus omija ethanol extract (GE+OE, GE+0.05% OE) ameliorated adiposity and hepatic steatosis in high-fat diet (HFD)-induced obese mice (13). In this study, we investigated whether GE+OE at the same dose as in our previous study (13) could reduce hyperglycemia and insulin resistance as well as fat accumulation in adipose and hepatic tissues in type 2 diabetic db/db mice, which are an animal model for diabetes, obesity, diabetic dyslipidemia, and hyperleptinemia caused by homozygous genetic leptin receptor deficiency (14).
MATERIALS AND METHODS
Preparation of extracts
In this study, grape pomace (skin and stem) and omija fruits (Fructus Schisandrae) were used. The grapes (Vitis vinifera, Muscat bailey A species) and omija (S. chinensis Baillon) were purchased from Gyeongsangbuk-do, Korea. Samples were prepared by adding 2 L of 80% and 50% ethanol to 100 g of dried grape pomace and omija fruit, respectively; the extraction was performed at 80°C for 2 h after which the solution was cooled. It was then filtered (Whatman paper No. 2), concentrated using a rotary vacuum evaporator, and stored at −70°C. The final weight of the grape pomace ethanol extract was 19.9 g (recovery rate: 19.9%) and that of the omija fruit ethanol extract was 39.7 g (recovery rate: 39.7%). A 1 g sample of grape pomace ethanol extract contains 0.2 mg of resveratrol, 52 mg of total flavonoid, and 95 mg of total polyphenol. The same amount of omija fruit ethanol extract contains 8 mg of schizandrin, 7 mg of total flavonoid, and 32 mg of total polyphenol. The grape pomace and omija fruit ethanol extracts were formally identified by CJ Food R&D center, CJ Cheiljedang Corp., Seoul, Korea.
Animals and diets
Male C57BL/KsJ-db/db mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) at 4 weeks of age. The animals were individually housed at a constant temperature (24°C) and with a 12-h light/dark cycle, and fed a pelletized commercial non-purified diet for 1 week after arrival. The mice were then randomly divided into 2 groups (n=10) and fed the respective experimental diets for 7 weeks: a normal control diet (CON) and the GE+OE diet (0.5% grape pomace extract and 0.05% omija fruit extract with CON diet, w/w). The dose of the GE+OE used in the present study is the same as that used in our previous study using a different animal model (13). The composition of each diet is presented in Table 1. The mice had ad libitum access to food and distilled water during the experimental period. Their food intake and body weight were measured daily and weekly, respectively.
Table 1.
Composition of experimental diets (unit: % of diet)
| Ingredients | CON | GE+OE |
|---|---|---|
| Casein | 20 | 20 |
| D, L-Methionine | 0.3 | 0.3 |
| Sucrose | 49.999 | 49.449 |
| Cellulose | 5 | 5 |
| AIN-mineral | 3.5 | 3.5 |
| AIN-vitamin | 1 | 1 |
| Choline bitartrate | 0.2 | 0.2 |
| Corn Starch | 15 | 15 |
| Corn oil | 5 | 5 |
| tert-Butylhydroquinone | 0.001 | 0.001 |
| Grape pomace extract | 0.5 | |
| Omija extract | 0.05 | |
| Total | 100 | 100 |
CON, normal diet control; GE+OE, CON plus grape pomace extract (0.5%, w/w) combined with omija fruit extract (0.05%, w/w).
On the 7th week, mice were anaesthetized with diethyl ether and sacrificed after 12 h of fasting. Blood was taken from the inferior vena cava and then centrifuged at 1,000 g for 15 min at 4°C, and the plasma was separated to analyze plasma biomarkers. After blood collection, the liver and adipose tissues were promptly removed, rinsed, weighed, frozen in liquid nitrogen, and stored at −70°C. The pancreas was also removed, rinsed, and fixed in 1% hydrogen peroxide. This animal study protocol was approved by the Ethics Committee for Animal Studies at Kyungpook National University, Korea (approval No. KNU 2011-28).
Plasma and blood biomarkers
The levels of plasma insulin, leptin, resistin, and adiponectin were determined using a multiplex detection kit from Bio-Rad (Hercules, CA, USA). All samples were assayed in duplicate and analyzed with a Luminex 200 Labmap system (Luminex, Austin, TX, USA). Data analyses were performed using the Bio-Plex Manager software version 4.1.1 (Bio-Rad). The blood glucose concentration was measured using a glucose analyzer, GlucDr supersensor (Allmedicus, Anyang, Korea), with whole blood obtained from the tail veins after withholding food for 12 h. The blood glycosylated hemoglobin (HbA1c) concentration was measured using an analyzer (Micromat™ I Hemoglobin A1c Test, Bio-Rad), and the plasma glucose level was analyzed using a commercially available kit (Asan Pharm. Co., Ltd., Seoul, Korea). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as previously described: HOMA-IR=[fasting insulin concentration (mU/L)]×[fasting glucose concentration (mg/dL)×0.05551]/22.5.
Plasma and hepatic lipids
Plasma lipid concentrations were determined using commercially available kits; total-cholesterol, triglyceride, and high-density lipoprotein (HDL)-cholesterol (Asan Pharm. Co., Ltd.), free fatty acids (FFA; Wako Chemicals, Richmond, VA, USA), apolipoprotein (apo) A-I, and apoB (Eiken, Tokyo, Japan) levels were analyzed. The hepatic lipids were extracted using the method of Folch et al. (15), and hepatic lipid levels were analyzed using the same enzymatic kits used in the plasma analyses.
Hepatic enzyme activities
Enzyme sources were prepared according to the method developed by Hulcher and Oleson (16) with slight modifications. The glucokinase (GK) activity was determined from liver samples homogenized in 9 volumes of a buffer containing 50 mmol/L Tris-HCl, pH 7.4, 100 mmol/L KCl, 10 mmol/L mercaptoethanol, and 1 mmol/L ethyl-enediaminetetraacetic acid (EDTA). Homogenates were centrifuged at 100,000 g for 1 h; the cytosol was used for the spectrophotometric assay as described by Davidson and Arion (17), in which the formation of glucose-6-phosphate from glucose at 37°C was coupled to its oxidation by glucose-6-phosphate dehydrogenase (G6PD) and nicotinamide adenine dinucleotide (NAD). The glucose-6-phosphatase (G6Pase) activity in the microsome was determined using a spectrophotometric assay according to the method by Alegre et al. (18). The reaction mixture contained the following: 100 mmol/L sodium Hepes (pH 6.5), 26.5 mmol/L glucose-6-phosphatase and 1.8 mmol/L EDTA, both previously adjusted to pH 6.5, 2 mmol/L NADP+, 0.6 IU/L mutarotase, and 6 IU/L glucose dehydrogenase. The phosphoenolpyruvate carboxykinase (PEPCK) activity was determined according to the method described by Bentle and Lardy (19). The reaction mixture contained the following in a 1 mL final volume: 77 mmol/L sodium Hepes, 1 mmol/L inosine 5′-diphosphate (IDP), 1 mmol/L MnCl2, 1 mmol/L di-thiothreitol, 0.25 mmol/L NADH, 2 mmol/L phosphoenolpyruvate, 50 mmol/L NaHCO3, and 7.2 U of malic dehydrogenase. The amount of protein in the enzyme sources was determined using the Bradford (20) method with bovine serum albumin as the standard. Fatty acid β-oxidation activity was measured spectrophotometrically by monitoring the reduction of NAD to NADH in the presence of palmitoyl-CoA, as described by Lazarow (21). The results were expressed as nmol/min per mg of protein. Fatty acid synthase (FAS) activity was determined according to the method described by Nepokroeff et al. (22) through monitoring the malonyl-CoA-dependent oxidation of NADPH at 340 nm, in which the activity represents the oxidized NADPH nmol/min per mg of protein. The G6PD activity was assayed using spectrophotometric methods according to the procedures described by Pitkänen et al. (23), in which the activity was expressed as the reduced NADPH nmol/min per mg of protein. The malic enzyme (ME) activity was determined as previously described by Ochoa (24). The cytosolic enzyme was mixed with 0.2 mM triethanolamine buffer (pH 7.4), 1.5 mM L-malate, 12 mM MnCl2, and 680 M NADP+, and the solution was then measured for 1 min at 340 nm (26°C) using a spectrophotometer. The phosphatidate phosphohydrolase (PAP) activity was determined using the method of Walton and Possmayer (25).
Histopathological analysis
Liver and epididymal fat were removed and fixed in a buffer solution of 10% formalin. Fixed tissues were processed routinely for paraffin embedding, and 4-μm sections were prepared and dyed with hematoxylin-eosin. Stained areas were viewed using an optical microscope with a magnifying power of ×200. For the immunohistochemical analysis of pancreatic β-cells, the islet was sectioned, fixed in 1% hydrogen peroxide, and washed in 0.01 M citrate buffer (pH 6.0). These sections were treated with blocking reagent [Ultra Tech horse-radish peroxidase (HRP)] to prevent nonspecific binding and incubated with monoclonal antibodies against insulin (Santa Cruz Biotech, Inc., Santa Cruz, CA, USA). Antibody reactivity was detected using HRP-conjugated biotin-streptavidin complexes and developed with diaminobenzidine tetrahydrochloride as the substrate. Stained areas were viewed using an optical microscope with a magnifying power of ×200.
Statistical analysis
The statistical analyses were performed with the statistical package for social science software program (SPSS, Inc., Chicago, IL, USA). Significant differences between the means were determined using the Student’s t-test. Differences were considered to be statistically significant when P<0.05. All data are expressed as the mean and standard error of the mean.
RESULTS
Body weight and plasma lipids levels
There was no significant difference in food intake between the CON and GE+OE groups (data not shown). The initial and final body weights also did not significantly differ between the groups (Table 2). However, GE+OE resulted in significantly lower plasma FFA, triglyceride, and total-cholesterol levels compared to that in the mice in the CON group (Table 2). The plasma apoB level was also decreased to a greater extent in the GE+OE group than in the CON group; however, there were no significant differences in plasma HDL-cholesterol and apoA-I levels between the groups (Table 2).
Table 2.
Effects of the GE+OE extract on body weight and plasma lipids levels
| CON | GE+OE | |
|---|---|---|
| Initial body weight (g) | 31.65±0.52 | 31.63±0.60 |
| Final body weight (g) | 46.32±1.01 | 45.72±0.61 |
| Free fatty acid (mmol/L) | 1.32±0.06 | 1.19±0.04* |
| Triglyceride (mmol/L) | 3.09±0.18 | 2.67±0.12* |
| Total cholesterol (mmol/L) | 6.11±0.19 | 5.02±0.21* |
| HDL-cholesterol (mmol/L) | 1.08±0.04 | 1.08±0.03 |
| ApoB (mg/dL) | 7.24±1.00 | 3.51±0.44** |
| ApoA-I (mg/dL) | 49.22±0.62 | 47.44±1.14 |
Data are the means±standard errors (n=10).
P<0.05,
P<0.01 vs. CON.
Apo, apolipoprotein; CON, normal diet control; GE+OE, CON plus grape pomace extract (0.5%, w/w) combined with omija fruit extract (0.05%, w/w); HDL, high-density lipoprotein.
Fat weight, adipocyte size, and plasma adipokines levels
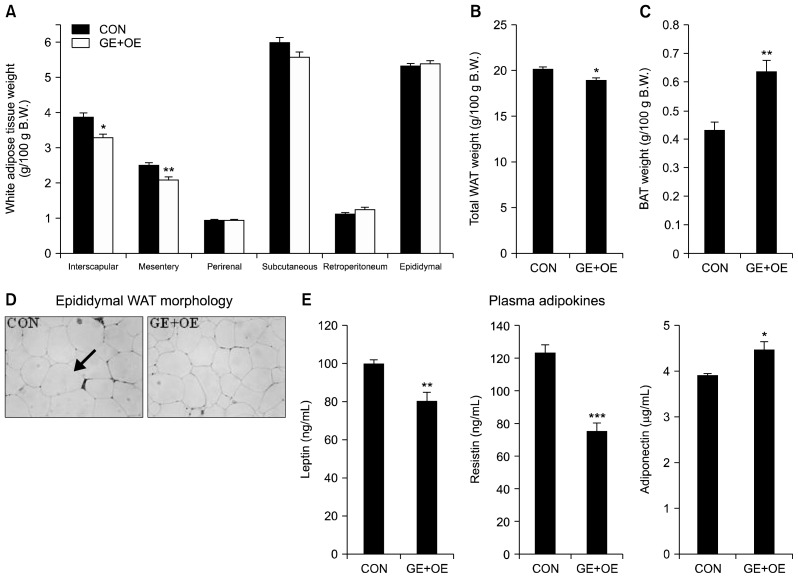
Compared to the CON group, supplementation with GE+OE resulted in significantly lower interscapular, mesentery, and total white adipose tissue (WAT) weights, while the brown adipose tissue weight was significantly higher (Fig. 1A–C). Furthermore, the GE+OE-supplemented db/db mice showed a smaller size of epididymal adipocytes than the mice in the CON group (Fig. 1D). Consistent with the WAT weight, the plasma leptin and resistin levels were significantly lower in the GE+OE group than in the CON group (Fig. 1E). On the other hand, the GE+OE group showed significantly higher levels of plasma adiponectin than the CON group (Fig. 1E).
Fig. 1.
Effects of the GE+OE extract on adipose tissue and plasma adipokines levels. (A) white adipose tissue (WAT) weight, (B) total WAT weight, (C) brown adipose tissue (BAT) weight, (D) epididymal WAT morphology, and (E) plasma adipokine levels. A~C and E: Data are the means±standard errors (n=10). *P<0.05, **P<0.01, ***P<0.001 vs. the normal control diet (CON). D: A representative photomicrograph of the epididymal WAT is shown at 200× magnification (n=10). Arrow, adipocytes; GE+OE, CON plus grape pomace extract (0.5%, w/w) combined with omija fruit extract (0.05%, w/w).
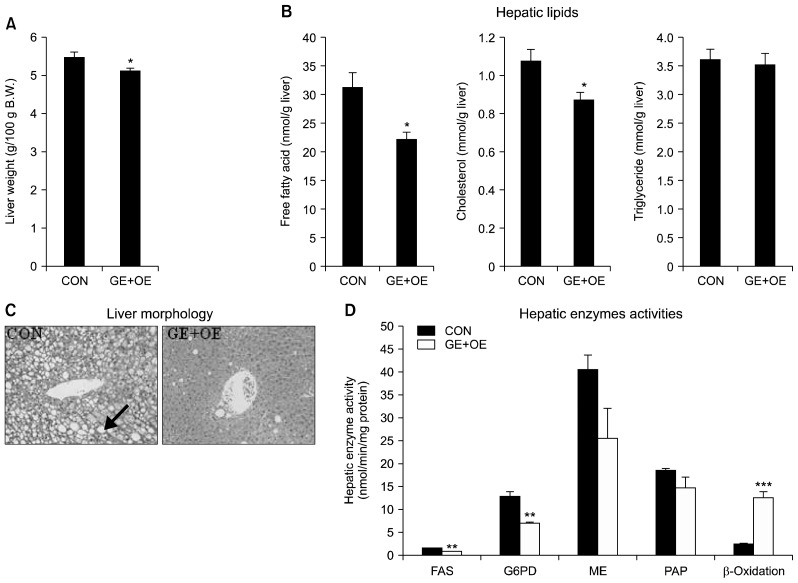
Liver weight, hepatic lipids levels, hepatic morphology, and lipid-regulating enzymes activities
Compared to the CON group, GE+OE supplementation resulted in significantly lower liver weight (Fig. 2A). Hepatic FFA and cholesterol levels were also significantly lower in the GE+OE group than in the CON group (Fig. 2B). Moreover, GE+OE markedly decreased the number and size of liver fat droplets, although hepatic triglyceride levels were not significantly altered by supplementation with GE+OE (Fig. 2B, 2C). GE+OE supplementation not only decreased the activities of hepatic FAS and G6PD, the enzymes for de novo fatty acid synthesis, but also increased hepatic β-oxidation activity (Fig. 2D). Compared to the CON group, mice in the GE+OE group showed lower hepatic ME and PAP activities; the activities were decreased by 37% and 20%, respectively, in the GE+OE group, although the differences were not statistically significant (Fig. 2D).
Fig. 2.
Effects of the GE+OE extract on hepatic lipids and hepatic lipid-regulating enzymes. (A) liver weight, (B) hepatic lipid levels, (C) liver morphology, and (D) hepatic lipid-regulating enzyme activities. A, B, and D: Data are the means±standard errors (n=10).*P<0.05, **P<0.01, ***P<0.001 vs. the normal control diet (CON). C: A representative photomicrograph of the liver is shown at 200× magnification (n=10). Arrow, lipid droplets; GE+OE, CON plus grape pomace extract (0.5%, w/w) combined with omija fruit extract (0.05%, w/w); FAS, fatty acid synthase; G6PD, glucose-6-phosphate dehydrogenase; ME, malic enzyme; PAP, phosphatidate phosphohydrolase.
Blood markers of glycemia and insulin resistance, hepatic glucose-regulating enzymes activities, and pancreatic insulin expression
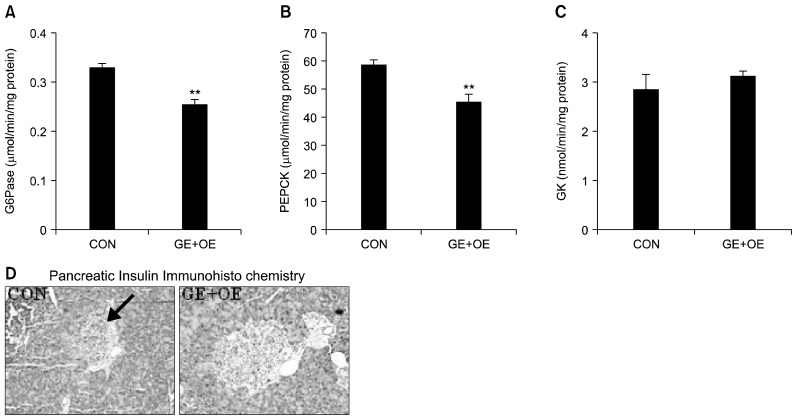
The initial fasting blood glucose level did not differ between the CON and GE+OE groups (Table 3). However, the final fasting blood glucose level was significantly lower in the GE+OE group than in the CON group (Table 3). GE+OE supplementation also significantly lowered plasma glucose and blood HbA1c levels in db/db mice (Table 3). Levels of plasma insulin as well as the HOMA-IR, a surrogate marker for insulin resistance, were significantly lowered in GE+OE group (Table 3). The beneficial effects of GE+OE on hyperglycemia and insulin resistance were supported by decreased hepatic gluconeogenic G6Pase and PEPCK activities (Fig. 3A, 3B). There was no significant difference in hepatic GK activity between the groups (Fig. 3C). Immunohistochemical staining of the pancreatic tissues showed more insulin-stained islets in GE+OE-supplemented mice than in those from the CON group (Fig. 3D).
Table 3.
Effects of the GE+OE extract on blood markers of glycemia
| CON | GE+OE | |
|---|---|---|
| Initial blood glucose (mg/dL) | 130.54±15.11 | 130.11±11.09 |
| Final blood glucose (mg/dL) | 515.50±29.31 | 389.84±2.63* |
| Plasma glucose (mg/dL) | 750.92±6.77 | 688.24±22.79* |
| HbA1c (%) | 12.55±0.26 | 10.53±0.38*** |
| Insulin (ng/mL) | 6.56±0.82 | 3.94±0.58* |
| HOMA-IR | 214.04±12.05 | 123.92±12.03** |
Data are the means±standard errors (n=10).
P<0.05,
P<0.01,
P<0.001 vs. CON.
CON, normal diet control; GE+OE, CON plus grape pomace extract (0.5%, w/w) combined with omija fruit extract (0.05%, w/w); HbA1c, blood glycosylated hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance.
Fig. 3.
Effects of the GE+OE extract on hepatic glucose-regulating enzymes and staining for pancreatic insulin. (A) hepatic glucose-6-phosphatase (G6Pase), (B) phosphoenolpyruvate carboxykinase (PEPCK), and (C) glucokinase (GK) activities, and (D) immunohistochemical staining for pancreatic insulin. A~C: Data are the means±standard errors (n=10). **P<0.01 vs. the normal control diet (CON). D: Representative photomicrographs of the pancreas are shown at 200× magnification (n=10). Arrow, β-cell; GE+OE, CON plus grape pomace extract (0.5%, w/w) combined with omija fruit extract (0.05%, w/w).
DISCUSSION
The db/db mouse, a good animal model for type 2 diabetes, exhibits a severe obesity phenotype due to its inability to produce the leptin receptor long form (26). The db/db mouse also displays diabetic dyslipidemias, such as hypertriglyceridemia and hypercholesterolemia as well as hepatic steatosis (27,28). In this study, GE+OE supplementation reduced adiposity, and plasma and hepatic lipids levels in type 2 diabetic db/db mice. These findings are in accordance with our previous study, where GE+OE significantly decreased total WAT weight and plasma FFA levels and ameliorated hepatic steatosis in HFD-induced obese mice (13).
Hyperlipidemia in db/db mice contributes to accelerated hepatic lipid accumulation, and increased hepatic lipogenesis is associated with fat accumulation in the adipose tissue of subjects with obesity (29,30). Kelley et al. (31) also observed that hepatic steatosis in type 2 diabetes was related to dyslipidemia and greater amounts of visceral adipose tissue. Meanwhile, it was shown that hepatic lipid-regulating enzyme activities could be changed by diet (32). GE+OE supplementation lowered the activity of hepatic FAS, which is a key lipogenic enzyme catalyzing the terminal steps in the de novo biogenesis of fatty acids, and G6PD, which provides reductive potential in the form of NADPH for fatty acids biogenesis. In addition, GE+OE increased hepatic β-oxidation, which may contribute to lower plasma and hepatic lipids levels, and suppress adiposity.
In the present study, we observed hypoglycemic effects as well as anti-obesity effects from GE+OE supplementation in type 2 diabetic db/db mice. GE+OE feeding to db/db mice for 7 weeks decreased fasting blood glucose levels and those of HbA1c, which reflects the mean glucose concentration over the previous two to three months and is a risk parameter for monitoring the potential development of late diabetic complications (33). Moreover, mice supplemented with GE+OE had decreased plasma glucose and insulin levels, and a decreased HOMA-IR, which assesses insulin resistance, with recovering pancreatic β-cell mass and failure. The db/db mice initially show increased insulin secretion from the pancreatic β-cell to compensate for the insulin resistance associated with obesity (34). Subsequently, these mice exhibit β-cell failure as a result of either inadequate compensatory increase in β-cell mass or the loss of the ability of the existing β-cell mass to respond to glucose, leading to insufficient insulin release by the pancreatic β-cells (35). Diabetes is aggravated by altered glucose metabolism activity of the hepatic enzymes in type 2 diabetic db/db mice (36). Increased activities of G6Pase and PEPCK contribute to an increase in hepatic glucose production and fasting hyperglycemia (37). Among the many genes involved in glucose homeostasis, PEPCK gene expression is up-regulated in most forms of diabetes and contributes to an increased hepatic glucose output (38). The present study demonstrated that GE+OE supplementation reduced hyperglycemia by inhibiting G6Pase and PEPCK activities in the livers of db/db mice.
Obesity may be an inflammatory condition increasing the production of proinflammatory adipokines and leading to chronic activation of the innate immune system, which ultimately causes impairment in glucose tolerance and type 2 diabetes (39). Many adipokines are involved in the pathogenesis of the chronic inflammation and insulin resistance associated with obesity and type 2 diabetes (40). Leptin is one of the adipokines abundantly released by the adipose tissue, and its circulating level predicts increased visceral adiposity (41). Most obese mouse models, such as db/db mice, show high levels of circulating leptin, and hyperleptinemia is associated with obesity-associated insulin resistance (42). Obese mice are resistant to the effects of leptin administration, and leptin resistance can develop in the face of a high circulating level of leptin (4). Leptin is also reported to play important roles in a complex metabolic response to glucose as well as in lipid metabolism (43). Resistin is another adipose tissue-specific hormone whose levels are increased during adipogenesis (44). Plasma resistin levels are also elevated in animal models of insulin resistance, such as ob/ob mice and db/db mice (44). On the other hand, low plasma adiponectin levels are associated with obesity and type 2 diabetes (45). We observed that GE+OE supplementation significantly lowered plasma leptin and resistin levels and significantly increased plasma adiponectin levels in type 2 diabetic mice.
The present data suggest that GE+OE not only improves hyperglycemia and insulin resistance but also reduces adiposity and lipid accumulation in the plasma and liver, which seems to be mediated through the regulation of hepatic enzymes involved in glucose and lipid metabolism, and to be related to the levels of plasma adipokines and protection of the pancreas. GE+OE may have potential to improve hyperglycemia, insulin resistance, dyslipidemia, hepatic steatosis, and obesity in patients with type 2 diabetes.
ACKNOWLEDGEMENTS
This work was supported by the High Value-added Food Technology Development Program (No. 110129-3), the Ministry for Food, Agriculture, Forestry and Fisheries, the National Research Foundation of Korea grant (2015R1A5A6001906, NRF-2012M3A9C4048818), and the Center for Women In Science, Engineering and Technology (WISET) Grant funded by the Ministry of Science, ICT & Future Planning of Korea (MSIP) under the Program for Returners into R&D, Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res. 2005;36:197–209. doi: 10.1016/j.arcmed.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines · adipocyte-derived bioactive substances. Ann NY Acad Sci. 1999;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 3.Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajala MW, Scherer PE. Minireview: The adipocyte–at the crossroads of energy homeostasis, inflammation, and atherosclersis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 5.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell. 2000;6:87–97. doi: 10.1016/S1097-2765(05)00015-8. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. doi: 10.7326/0003-4819-131-4-199908170-00008. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2300–2308. doi: 10.3748/wjg.v18.i19.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pérez-Jiménez J, Saura-Calixto F. Grape products and cardiovascular disease risk factors. Nutr Res Rev. 2008;21:158–173. doi: 10.1017/S0954422408125124. [DOI] [PubMed] [Google Scholar]
- 9.Cordain L, Melby CL, Hamamoto AE, O’Neill DS, Cornier MA, Barakat HA, Israel RG, Hill JO. Influence of moderate chronic wine consumption on insulin sensitivity and other correlates of syndrome X in moderately obese women. Metabolism. 2000;49:1473–1478. doi: 10.1053/meta.2000.17672. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Bortolotti N, Motz E, Lizzio S, Catone B, Assaloni R, Tonutti L, Taboga C. Red wine protects diabetic patients from meal-induced oxidative stress and thrombosis activation: a pleasant approach to the prevention of cardiovascular disease in diabetes. Eur J Clin Invest. 2001;31:322–328. doi: 10.1046/j.1365-2362.2001.00818.x. [DOI] [PubMed] [Google Scholar]
- 11.Jo SH, Ha KS, Moon KS, Lee OH, Jang HD, Kwon YI. In vitro and in vivo anti-hyperglycemic effects of omija (Schizandra chinensis) fruit. Int J Mol Sci. 2011;12:1359–1370. doi: 10.3390/ijms12021359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang XY, Yu ZL, Pan SY, Zhang Y, Sun N, Zhu PL, Jia ZH, Zhou SF, Ko KM. Supplementation with the extract of Schisandrae Fructus pulp, seed, or their combination influences the metabolism of lipids and glucose in mice fed with normal and hypercholesterolemic diet. Evid Based Complement Alternat Med. 2014;2014:472638. doi: 10.1155/2014/472638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho SJ, Jung UJ, Park HJ, Kim HJ, Park YB, Kim SR, Choi MS. Combined ethanol extract of grape pomace and omija fruit ameliorates adipogenesis, hepatic steatosis, and inflammation in diet-induced obese mice. Evid Based Complement Alternat Med. 2013;2013:212139. doi: 10.1155/2013/212139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of western diet feeding. Metabolism. 2000;49:22–31. doi: 10.1016/S0026-0495(00)90588-2. [DOI] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 16.Hulcher FH, Oleson WH. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J Lipid Res. 1973;14:625–631. [PubMed] [Google Scholar]
- 17.Davidson AL, Arion WJ. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: physiological implications of higher cellular activity. Arch Biochem Biophys. 1987;253:156–167. doi: 10.1016/0003-9861(87)90648-5. [DOI] [PubMed] [Google Scholar]
- 18.Alegre M, Ciudad CJ, Fillat C, Guinovart JJ. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal Biochem. 1988;173:185–189. doi: 10.1016/0003-2697(88)90176-5. [DOI] [PubMed] [Google Scholar]
- 19.Bentle LA, Lardy HA. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J Biol Chem. 1976;251:2916–2921. [PubMed] [Google Scholar]
- 20.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 21.Lazarow PB. Assay of peroxisomal β-oxidation of fatty acids. Methods Enzymol. 1981;72:315–319. doi: 10.1016/S0076-6879(81)72021-4. [DOI] [PubMed] [Google Scholar]
- 22.Nepokroeff CM, Lakshmanan MR, Porter JW. Fatty acid synthase from rat liver. Mothods Enzymol. 1975;35:37–44. doi: 10.1016/0076-6879(75)35136-7. [DOI] [PubMed] [Google Scholar]
- 23.Pitkänen E, Pitkänen O, Uotila L. Enzymatic determination of unbound D-mannose in serum. Eur J Clin Chem Clin Biochem. 1997;35:761–766. doi: 10.1515/cclm.1997.35.10.761. [DOI] [PubMed] [Google Scholar]
- 24.Ochoa S. “Malic” enzyme. A. “Malic” enzyme from pigeon liver and wheat germ. Methods Enzymol. 1955;1:739–753. doi: 10.1016/0076-6879(55)01129-4. [DOI] [Google Scholar]
- 25.Walton PA, Possmayer F. Mg2-dependent phosphatidate phosphohydrolase of rat lung: development of an assay employing a defined chemical substrate which reflects the phosphohydrolase activity measured using membrane-bound substrate. Anal Biochem. 1985;151:479–486. doi: 10.1016/0003-2697(85)90208-8. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84:491–495. doi: 10.1016/S0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 27.Nishina PM, Lowe S, Wang J, Paigen B. Characterization of plasma lipids in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism. 1994;43:549–553. doi: 10.1016/0026-0495(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 28.Lombardo YB, Hron WT, Sobocinski KA, Menahan LA. A metabolic profile of fed and fasting genetically obese mice at 4–5 months of age. Horm Metab Res. 1984;16:37–42. doi: 10.1055/s-2007-1014894. [DOI] [PubMed] [Google Scholar]
- 29.Schäffler A, Schölmerich J, Büchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue–emerging role in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:273–280. doi: 10.1038/ncpgasthep0186. [DOI] [PubMed] [Google Scholar]
- 30.Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2002;282:E46–E51. doi: 10.1152/ajpendo.2002.282.1.E46. [DOI] [PubMed] [Google Scholar]
- 31.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–E916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 32.Likimani TA, Wilson RP. Effects of diet on lipogenic enzyme activities in channel catfish hepatic and adipose tissue. J Nutr. 1982;112:112–117. doi: 10.1093/jn/112.1.112. [DOI] [PubMed] [Google Scholar]
- 33.Miedema K. Towards worldwide standardisation of HbA1c determination. Diabetologia. 2004;47:1143–1148. doi: 10.1007/s00125-004-1453-0. [DOI] [PubMed] [Google Scholar]
- 34.Kasuga M. Insulin resistance and pancreatic β cell failure. J Clin Invest. 2006;116:1756–1760. doi: 10.1172/JCI29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Jr, Kempf J, Zinman B, Haffner SM. Elevations in markers of liver injury and risk of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes. 2004;53:2623–2632. doi: 10.2337/diabetes.53.10.2623. [DOI] [PubMed] [Google Scholar]
- 37.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 38.Davies GF, Khandelwal RL, Wu L, Juurlink BH, Roesler WJ. Inhibition of phosphoenolpyruvate carboxykinase (PEPCK) gene expression by troglitazone: a peroxisome proliferator-activated receptor-γ (PPARγ)-independent, antioxidant-related mechanism. Biochem Pharmacol. 2001;62:1071–1079. doi: 10.1016/S0006-2952(01)00764-X. [DOI] [PubMed] [Google Scholar]
- 39.Tataranni PA, Ortega E. A burning question: does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes. 2005;54:917–927. doi: 10.2337/diabetes.54.4.917. [DOI] [PubMed] [Google Scholar]
- 40.Paquot N, Tappy L. Adipocytokines: link between obesity, type 2 diabetes and atherosclerosis. Rev Med Liege. 2005;60:369–373. [PubMed] [Google Scholar]
- 41.Tong J, Fujimoto WY, Kahn SE, Weigle DS, McNeely MJ, Leonetti DL, Shofer JB, Boyko EJ. Insulin, C-peptide, and leptin concentrations predict increased visceral adiposity at 5- and 10-year follow-ups in nondiabetic Japanese Americans. Diabetes. 2005;54:985–990. doi: 10.2337/diabetes.54.4.985. [DOI] [PubMed] [Google Scholar]
- 42.Steppan CM, Lazar MA. Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab. 2002;13:18–23. doi: 10.1016/S1043-2760(01)00522-7. [DOI] [PubMed] [Google Scholar]
- 43.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 44.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 45.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]