Abstract
Background
Subjective memory complaints reflect patient-identified deficits in memory and have been linked to increased risk of future dementia in nondemented (including cognitively intact) older adults.
Objectives
To assess the risk of incident dementia during follow-up for participants in the Prevention of Alzheimer’s Disease with Vitamin E and Selenium (PREADVISE) study who reported memory complaints at baseline.
Design
Double-blind, placebo controlled 2×2 randomized controlled trial that transformed into an observational cohort following discontinuation of supplementation in the SELECT parent trial.
Setting
PREADVISE participants were assessed at 130 local clinical study sites in the United States, Canada, and Puerto Rico during the controlled trial phase and were later followed by telephone from a centralized location during the observational phase.
Participants
PREADVISE enrolled a total of 7,547 nondemented men over the age of 60; 4,271 consented to participation in the observational study.
Measurements
Participants were interviewed at baseline for memory complaints. The Memory Impairment Screen (MIS) was administered to each participant at the annual memory screening. Participants who failed the MIS also received a more detailed neurocognitive assessment: an expanded Consortium to Establish a Registry in Alzheimer’s Disease (CERADe) neuropsychological battery was used during the RCT, and the modified Telephone Interview for Cognitive Status (TICS-m) was used during the observational study. Participants who failed the second screen were asked to have a memory work-up with a local physician and to share their medical records with PREADVISE. Subgroups of men who did not fail the MIS were also asked to complete the CERADe battery and TICS-m for validation purposes. Additional measures collected include self-reported medical history, medication use, and the AD8 Dementia Screening Test.
Results
After controlling for important risk factors for dementia, Cox proportional hazards regression revealed that men who reported memory changes at baseline had an 80% increase in the hazard of incident dementia compared to men who reported no SMC. Men who reported memory problems at baseline had almost a 6-fold increase in the hazard of incident dementia compared to men who reported no memory complaint.
Conclusions
Memory complaints in nondemented older men predicted future dementia. Men who reported that the changes in their memory were a problem were especially at risk, and the presence of common comorbidities like diabetes, sleep apnea, and history of head injury further exacerbated this risk.
Keywords: subjective memory complaints, dementia, longitudinal cohort
Introduction
Subjective memory complaints (SMC) reflect self-identified deficits in memory and may be observed spontaneously, as with the patient who presents to a physician’s office, or systematically, as with participants in a research study. Such complaints are common in older adults when solicited during the course of research, particularly when participants are asked about such complaints multiple times over the course of follow-up [1, 2]. Although SMC in nondemented older adults do not always correlate with deficits in objective cognitive testing, imaging studies have identified both structural and functional deficits associated with SMC [3–5], and clinicopathological correlation studies have identified increased Alzheimer’s type pathology in research participants who died cognitively intact but reported SMC [2].
Despite the methodological differences among studies that measure SMC, a growing body of evidence suggests that SMC made by apparently cognitively intact older adults predict future cognitive impairment, including dementia [1, 2, 6–8]. The current study draws on a large sample of longitudinally followed older men from the Prevention of Alzheimer’s Disease with Vitamin E and Selenium study (PREADVISE), which enrolled non-demented men who were followed for incident dementia for up to 12 years. We hypothesized that participants who reported SMC at baseline would be at increased risk for dementia even after controlling for important dementia risk factors including age, educational attainment, APOE, and comorbidities.
Methods
Participants
For details on recruitment and design of the National Institutes of Health (NIH) National Institute on Aging-sponsored PREADVISE trial, please see Kryscio et al. [9, 10] and Caban-Holt et al. [11]. Briefly, PREADVISE was designed as a double-blind, 2×2 factorial randomized controlled trial (RCT), the primary aim of which was to determine the effectiveness of the antioxidant supplements vitamin E and selenium in preventing incident AD (PREADVISE investigators remain blind to treatment status as of this writing and so treatment assignment is ignored). Beginning in 2002, PREADVISE recruited a subsample of participants age 62 and over (age 60 if of African-American descent) from the NIH National Cancer Institute-sponsored Selenium and Vitamin E Cancer Prevention Trial (SELECT) from 130 participating clinical sites in the US, Canada, and Puerto Rico. PREADVISE enrolled 7,547 non-demented men, and enrollment ceased in 2009. PREADVISE eligibility was determined by active SELECT enrollment at a participating study site, and absence of dementia and other active neurologic conditions that affect cognition, such as major psychiatric disorder (including depression), serious head injury (> 30-minute loss of consciousness within the last five years prior to enrollment), or substance abuse.
In September 2008, the SELECT Data Safety and Monitoring Committee recommended that study supplements be discontinued due to lack of efficacy on the primary endpoint (i.e., reduction of prostate cancer incidence) [12]. SELECT study sites closed over the next two years, and both PREADVISE and SELECT transitioned into observational cohort studies. Men who participated in the RCT were asked to continue in the cohort study, and 4,271 of the original 7,547 PREADVISE participants consented to continue annual dementia screenings, which were then conducted by telephone. The Memory Impairment Screen (MIS) [13] was used as the primary screening instrument in both the RCT and observational portions of the trial. Participants who obtained scores below cutoffs for intact cognition received a secondary screening instrument. The modified Telephone Interview for Cognitive Status (TICS-m) [14] was used during observational follow-up, replacing the expanded Consortium to Establish a Registry in Alzheimer’s Disease (CERADe) neuropsychological battery [15] used during the RCT. In addition, subgroups of participants who did not fail the MIS were also asked to complete the CERADe battery [16] and TICS-m. Annual screenings were completed in May 2014. All PREADVISE participants are included in the current study whether they participated in both the RCT and observational studies or just the RCT.
All research activities during both the RCT and observational phases of the study were approved by the University of Kentucky Institutional Review Board (IRB) as well as the IRBs at each SELECT study site. Each participant provided written informed consent.
Memory Complaint
All PREADVISE participants were asked during their baseline interview about changes in their memory. Men could report no change, a change, and if there was a change, whether they felt they had more problems with their memory than most other people their age (i.e., whether they considered the changes to be consistent with normal aging). A three-level ordinal variable was constructed accordingly.
APOE Genotype
APOE genotyping based on DNA samples was unavailable for 368 participants (4.9%). Genotypes were converted to an indicator variable for APOE-ε4, where thepresence of any 4 allele was considered positive. Multiple imputation (PROC MI; SAS 9.3®), based on logistic regression with family history of dementia as the predictor variable, was used to impute missing values for the indicator variable. Four imputed data sets were generated; participants with two or more imputations positive for APOE-ε4 were coded as APOE-ε4 positive. The proportion of APOE-ε4 positives was 26.9% in both the imputed and non-imputed data.
Case Ascertainment
Incident cases of dementia were identified using two methods. First, all men who scored 5 or less (out of 8) on either the immediate or delayed recall portions of the annually administered primary screening instrument, the Memory Impairment Screen were given a secondary screen. Failure on the secondary screen (T Score ≤ 35 on the CERAD battery, total score ≤ 35 on the TICS-m) triggered a request for the participant to obtain a memory work-up from a local clinician and forward the medical records to PREADVISE, where they were reviewed by a team of 2–3 expert neurologists and 2–3 expert neuropsychologists. Second, because some participants were reluctant to visit their doctors, additional longitudinal measures collected during the study were reviewed by the study investigators: the AD8 Dementia Screening Interview [17], self-reported medical history, self-reported medication use, and cognitive scores including the MIS, CERAD T Score, NYU Paragraph Delayed Recall [18], and TICS-m. Observing an AD8 total of ≥ 1 (at any time during follow-up) plus a self-reported diagnosis of dementia, use of memory enhancing prescription drug (i.e., donepezil, rivastigmine, galantamine, or memantine), or cognitive score below cutoffs for intact cognition (i.e., 1.5 SDs below expected performance) yielded a diagnosis of dementia, where the date of diagnosis was assigned to the earliest event.
Statistical Analysis
Group differences in baseline characteristics were assessed with ANOVA and chi-square statistics. Survival time was calculated as the time in years between the diagnosis date and the baseline date. Men without evidence of dementia were censored at their last annual follow-up. Censoring was assumed to be non-informative. Kaplan-Meier product-limit estimation was used to obtain unadjusted survival estimates and log-rank statistics. Cox proportional hazards regression was used to obtain unadjusted and adjusted estimates for the hazard ratio. Adjusted models included main effects for baseline age, years of education, race (black vs. not black), APOE genotype (at least one ε4 vs. no ε4s), and baseline self-reported comorbidities including history of head injury with less than 30-minute loss of consciousness, diabetes, hypertension, and sleep apnea (all coded as present/absent). Interaction terms between memory complaint and each independent variable were also tested. The proportional hazards assumption was tested by creating time interaction variables for each independent variable in the model. Lack of statistical significance (p < 0.05) for the time interaction was taken as support for the proportional hazards assumption. Sensitivity analyses comparing the results observed from complete cases only with the fully imputed dataset were conducted to assess the impact of the imputed APOE data. All analyses were conducted using SAS 9.3® (SAS Institute, Inc., Cary, NC).
Results
Memory complaints were common at baseline: 22.0% reported memory changes, and 1.6% reported memory problems (Table 1). Participation in the observational study was less common for participants who reported memory problems at baseline (44.7%) than men who reported changes (53.9%) or did not complain (57.6%) (p<0.001). Mean baseline age of all participants was 67.5±5.3 years, and while absolute age differences across memory complaint groups were not large (Table 1), men who did not complain were significantly younger than both men who reported changes (p<0.001) and men who reported problems (p=0.035). There were no differences in educational attainment or APOE-ε4 positivity, but men who complained of memory changes or problems were less likely to be black (p<0.001). Less frequent reporting of memory complaints among black participants may be associated with their younger mean age at enrollment (by about 1.5±5.3 years, p<0.001). History of head injury (p<0.001) was more common in men who made either type of complaint, and sleep apnea was more common in men who complained of memory problems (p=0.002). No differences were observed for the remaining medical comorbidity risk factors. Baseline MIS scores were significantly lower in men who complained of memory problems than men who did not complain at all (p=0.004) and men who complained of memory changes (p=0.004). As with age, absolute differences in mean MIS scores were small.
Table 1.
PREADVISE baseline participant characteristics (N=7,547)
| Characteristic* | All Subjects (N=7,547) | No Memory Complaint (n=5,762) | Memory Change (n=1,662) | Memory Problem (n=123) |
|---|---|---|---|---|
| Age, y† | 67.5±5.3 | 67.2±5.2 | 68.5±5.6 | 67.5±5.3 |
| Education, y | 15.0±2.7 | 15.0±2.7 | 15.0±2.7 | 14.6±2.9 |
| Black race‡ | 756 (10.0) | 627 (10.9) | 121 (7.3) | 8 (6.5) |
| Baseline MIS§ | 7.6±0.7 | 7.6±0.7 | 7.6±0.7 | 7.4±0.8 |
| APOE-ε4 | 2,029 (26.9) | 1,543 (26.8) | 446 (26.8) | 40 (32.5) |
| Hypertension | 2,998 (39.7) | 2,290 (39.7) | 660 (39.7) | 48 (39.0) |
| Diabetes | 858 (11.4) | 657 (11.4) | 185 (11.1) | 16 (13.0) |
| Sleep apnea|| | 552 (7.3) | 407 (7.1) | 126 (7.6) | 19 (15.5) |
| Head injury{ | 997 (13.2) | 650 (11.3) | 321 (19.3) | 26 (21.4) |
Results presented are mean±SD or frequencies with proportions.
Mean baseline age for men who reported No Memory Complaint is less than Memory Change (p<0.001) and Memory Problem (p=0.035).
Proportion of black participants is significantly higher in the No Memory Complaint group than either Memory Change or Problem (p<0.001).
Mean baseline MIS scores were significantly lower in men who reported a Memory Problem than either men who did not complain (p=0.004) or men who reported a Memory Change (p=0.004).
Sleep apnea was more common among men who reported a Memory Problem (p=0.002) than either men who reported a Memory Change or did not complain.
Head injury was more common in men who reported a Memory Change or Memory Problem than men who did not complain (p<0.001).
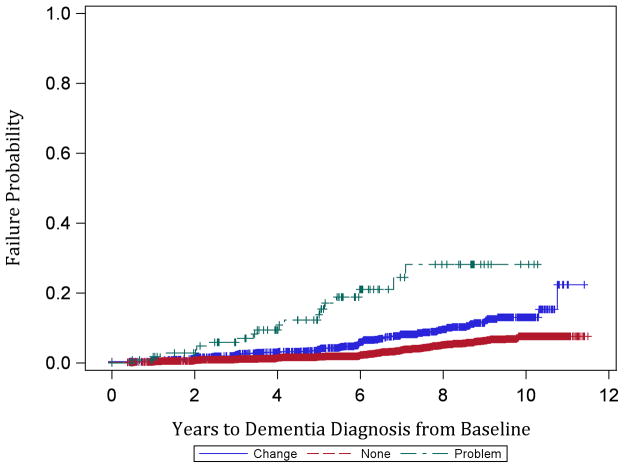
Participants were followed for an average of 5.7±2.8 years and accrued a total of 42,930.2 person-years of observation. The proportion of dementia cases observed by complaint status was as follows: no baseline memory complaint = 3.1% (181/5,762), memory change = 6.7% (111/1,662), and memory problem = 14.6% (18/123) (p<0.001, chi-square test). Case ascertainment was made by medical records review for 174/310 (56.1%) and by review of AD8 and medical history, medications, and cognitive testing for 136/310 (43.9%). The distribution of case ascertainment methods did not vary by complaint status (p=0.69). Kaplan-Meier estimates of 11-year cumulative incidence were 7.6% in the no memory complaint group, 21.8% in the memory change group, and 28.2% in the memory problem group (Figure 1). Incidence rates per 10,000 person-years were 55.1 for participants who made no complaint, 116.6 for participants who reported memory change, and 332.3 for participants who reported memory problems. Not only were participants who complained of memory changes and problems more likely to develop dementia during follow-up than those who did not complain, they also developed dementia more quickly (Figure 1; p<0.001, log-rank test).
Figure 1.
Kaplan-Meier product-limit estimates of failure probability by baseline memory complaint status. Red (bottom) = No Memory Complaint, Blue (middle) = Memory Change, Green (top) = Memory Problem. Censored observations are indicated with a ‘+’ symbol.
The unadjusted hazard ratio (HR) for a reported memory change vs. no memory complaint was 2.11 (95% CI: 1.66–2.67), and the HR for memory problem vs. no complaint was 6.67 (95% CI: 4.46–11.76). Both HRs were attenuated slightly following adjustments for demographics and comorbid conditions: 1.84 (95% CI: 1.44–2.36) for memory changes vs. no complaint, and 6.46 (95% CI: 4.11–10.87) for memory problems vs. no complaint. In addition to memory complaints, black race, APOE-ε4 carrier status, history of head injury, and baseline age were significantly associated with incident dementia (Table 2). There was a significant race by memory complaint interaction such that black men who reported memory problems at baseline had a dramatically increased hazard for dementia compared to black men who reported no complaint: HR = 34.5 (12.82, 100.0). The latter confidence interval is wide because the number of black participants reporting memory problems at baseline was small (n=8), and 3/8 developed dementia. Hence, these results should be interpreted with appropriate caution. HR estimates for the other strata in this interaction were comparable to the estimates from the main effects model (Table 2). Results for all models were unaffected by the inclusion or exclusion of the imputed APOE data (data not shown).
Table 2.
Cox proportional hazards regression model results. Bolded results are significant at p < 0.05.
| Comparison | Main Effects Model Adjusted HR (95% CI) |
Interaction Model Adjusted HR (95% CI) |
|---|---|---|
| Memory Change vs. No Complaint | 1.87 (1.47–2.38) | |
| Memory Problem vs. No Complaint | 6.01 (3.68–9.74) | |
| Memory Change vs. No Complaint (Black = Y) | 2.46 (1.15–5.27) | |
| Memory Problem vs. No Complaint (Black = Y) | 35.7 (12.99–100.0) | |
| Memory Change vs. No Complaint (Black = N) | 1.81 (1.41–2.33) | |
| Memory Problem vs. No Complaint (Black = N) | 4.50 (2.55–7.94) | |
| Baseline age, 1 year | 1.11 (1.09–1.13) | 1.11 (1.09–1.13) |
| Black vs. not black race | 1.86 (1.28–2.70) | |
| Education, 1 year | 0.97 (0.93–1.01) | 0.97 (0.93–1.01) |
| APOE-ε4 carrier vs. not | 1.91 (1.52–2.41) | 1.88 (1.49–2.38) |
| Diabetes vs. none | 1.07 (0.75–1.51) | 1.08 (0.76–1.53) |
| Hypertension vs. none | 0.91 (0.72–1.16) | 0.91 (0.72–1.15) |
| Sleep apnea vs. none | 1.34 (0.91–1.99) | 1.34 (0.90–1.99) |
| Head injury vs. none | 1.39 (1.03–1.88) | 1.36 (1.01–1.85) |
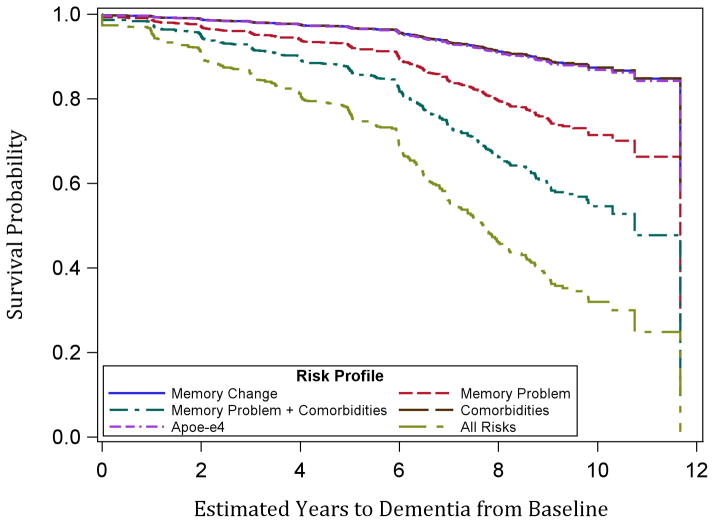
Time to dementia was roughly the same given the presence of any one of the following risks at baseline: memory change, APOE-ε4 positive, and all comorbidities (hypertension, diabetes, history of head injury, and sleep apnea) (Figure 2). Time to dementia was substantially decreased given a baseline memory problem, and presence of all considered risk factors (including memory problem but excluding memory change) further accelerated onset. This suggests that prevention and management of comorbid conditions may be especially important in delaying the onset of dementia for non-demented men who report problems with their memory.
Figure 2.
Estimated years to dementia diagnosis based on Cox regression results for hypothetical participants with different risk profiles. All hypothetical participants are white, age 70 at baseline, and have 12 years of education. ‘Comorbidities’ indicates presence of hypertension, diabetes, sleep apnea, and history of head injury.
Discussion
A simple measure of SMC (i.e., “Have you noticed any changes in your memory?”) provided important information about the potential for transition to dementia within an average of 6 years of follow-up in this large cohort of older men. An additional probe for men who reported changes in their memory (“Do you feel that you have more problems with your memory than most people?”) revealed significant additional risk. Both measures were associated not only with increased risk of dementia but also shorter time to diagnosis from baseline. This association does not appear to be the result of differences in age, educational attainment, APOE-ε4, or common comorbidities including hypertension, diabetes, sleep apnea, and head injury. Black men who complained of memory problems were especially at risk for dementia compared to black men who did not complain, but definitive conclusions are difficult to draw given the small number who reported memory problems at baseline.
This study supports previous findings that link SMC with future cognitive impairment (including dementia) [1, 2, 6–8]. Given this growing body of evidence, and the results from imaging studies that have linked SMC to structural and functional changes in the brains of cognitively intact older adults, SMC should be taken seriously by clinicians. SMC plus deficits in objective cognitive testing have long indicated a diagnosis of at least Mild Cognitive Impairment [19, 20], while SMC without objective impairments may be dismissed as indicative of depression [21, 22] or “worried well” [1]. In other words, SMC in cognitively intact older adults should be considered prognostic rather than diagnostic.
This study has some limitations. Although we lack of a measure of depressive symptoms at baseline, we note that the exclusion criteria for PREADVISE precluded enrollment for any man who had been diagnosed with or was under treatment for depression or anxiety in the four months prior to the baseline visit. Dementia diagnoses generated in the absence of a medical records review may be less accurate. However, application of the case criteria (i.e., AD8 > 1 plus at least one other indicator) to participants where the diagnosis was known demonstrated good agreement (data not shown). Ascertainment of dementia was also limited by the shift from an RCT to an observational study, particularly for those participants who did not continue in the study, so cases were likely missed. However, the SMC group with the highest proportion of participants in the observational study was the group who did not complain at baseline, which implies that if dementia cases were missed due to nonparticipation in the observational study, the no complaint group was the least likely to be affected. This also suggests that baseline SMC may be related to probability of drop out in longitudinal studies and would have implications for AD prevention trials.
Other limitations include the assumption of uninformative censoring, which is less likely to be valid in cohorts of older adults [23]. A competing risk approach that takes death into account may be more appropriate for these data as indicated by the effect estimate for hypertension, which appears to be protective against dementia (albeit not significant). If hypertension increases the risk of death such that the participant dies before dementia can manifest, then hypertension would appear to “protect” against dementia. Alternatively, use of antihypertensive medications may decrease the risk of dementia [24], and high blood pressure may have different effects on dementia risk depending on age (e.g., mid-life vs. late-life).
Strengths of the current study include large sample and long participant follow-up. Although SMC was measured with a simple probe, we considered two levels of SMC. We also considered the effect of common medical comorbidities that have been linked to increased risk of dementia, as well as how demographic, genetic, and medical characteristics may interact with SMC.
In the absence of objective cognitive impairment, SMC in nondemented older adults are important indicators of increased risk for future cognitive impairment. As such, they may be useful in identifying persons at high risk for transition to dementia in clinical trials. However, because SMC are common and often do not progress to pathological states, future research is needed to develop methods for identifying which SMC are truly predictive of cognitive impairment and which are not.
Acknowledgments
Funding
PREADVISE (NCT00040378) is supported by NIA R01 AG019421. Additional support for the current study comes from NIA R01 AG038651 and NIA P30 AG028383. SELECT was supported by NCI CA37429. NCI was involved in the design of SELECT. Otherwise, the sponsors had no role in the design and conduct of the current study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
We gratefully acknowledge all PREADVISE volunteers for their many years of participation and Drs. Herman Buschke, John Crowley, Gregory Jicha, Gregory Cooper, Steven Estus, Donna Wilcock, and the late Dr. William Markesbery for their contributions to the study. We also thank the PREADVISE support staff and the Cancer Research and Biostatistics group for assistance with study procedures and data management.
Contributor Information
EL Abner, Email: erin.abner@uky.edu.
RJ Kryscio, Email: kryscio@email.uky.edu.
AM Caban-Holt, Email: amcaba2@uky.edu.
FA Schmitt, Email: fascom@email.uky.edu.
References
- 1.Gifford KA, Liu D, Lu Z, Tripodis Y, et al. The source of cognitive complaints predicts diagnostic conversion differentially among nondemented older adults. Alzheimer’s & Dement. 2014;10:319–27. doi: 10.1016/j.jalz.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology. 2014:83. doi: 10.1212/WNL.0000000000000856. to appear October 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumas JA, Kutz AM, McDonald BC, et al. Increased working memory-related brain activity in middle-aged women with cognitive complaints. Neurobiol Aging. 2013;34:1145–47. doi: 10.1016/j.neurobiolaging.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hafkemeijer A, Altmann-Schneider I, Oleksik AM, et al. Increased functional connectivity and brain atrophy in elderly with subjective memory complaints. Brain Connectivity. 2013;3:353–62. doi: 10.1089/brain.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–6. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am J Psych. 1999;156:531–7. doi: 10.1176/ajp.156.4.531. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. Journal of the American Geriatrics Society. 2004;52:2045–51. doi: 10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- 8.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s & Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kryscio RJ, Mendiondo MS, Schmitt FA, Markesbery WR. Designing a large prevention trial: statistical issues. Stat Med. 2004;23(2):285–296. doi: 10.1002/sim.1716. [DOI] [PubMed] [Google Scholar]
- 10.Kryscio RJ, Abner EL, Schmitt FA, et al. A randomized controlled Alzheimer’s disease prevention trial’s evolution into an exposure trial: The PREADVISE trial. J Nutr Health Aging. 2013;17:72–5. doi: 10.1007/s12603-012-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caban-Holt A, Abner E, Kryscio RJ, Crowley JJ, Schmitt FA. Age-expanded normative data for the Ruff 2&7 Selective Attention Test: evaluating cognition in older males. Clin Neuropsychol. 2012;26:751–68. doi: 10.1080/13854046.2012.690451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman PJ, Hartline JA, Tangen CM, et al. Moving a randomized clinical trial into an observational cohort. Clin Trials. 2013;10:131–42. doi: 10.1177/1740774512460345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the memory impairment screen. Neurology. 1999;52:231–8. doi: 10.1212/wnl.52.2.231. [DOI] [PubMed] [Google Scholar]
- 14.de Jager CA, Budge MM, Clarke R. Utility of TICS-M for the assessment of cognitive function in older adults. Int J Geriatr Psych. 2003;18:318–24. doi: 10.1002/gps.830. [DOI] [PubMed] [Google Scholar]
- 15.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 16.Mathews M, Abner E, Caban-Holt A, Kryscio R, Schmitt F. CERAD practice effects and attrition bias in a dementia prevention trial. Int Psychogeriatr. 2013;25:1115–23. doi: 10.1017/S1041610213000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galvin JE, Roe CM, Powlishta KK, et al. A brief informant interview to detect dementia. Neurology. 2005;65:559–64. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 18.Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psych Neur. 1999 Winter;12(4):168–79. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 20.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairement due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montejo P, Montenegro M, Fernandez MA, Maestu F. Subjective memory complaints in the elderly: prevalence and influence of temporal orientation, depression, and quality of life in a population-based study in the city of Madrid. Aging Ment Health. 2011;15:85–96. doi: 10.1080/13607863.2010.501062. [DOI] [PubMed] [Google Scholar]
- 22.Benito-León J, Mitchell AJ, Vega S, Bermejo-Pareja F. A population-based study of cognitive function in older people with subjective memory complaints. J Alzheimer’s Dis. 2010;22:159–70. doi: 10.3233/JAD-2010-100972. [DOI] [PubMed] [Google Scholar]
- 23.Murphy TE, Han L, Allore HG, Peduzzi PN, Gill TM, Lin H. Treatment of death in the analysis of longitudinal studies of gerontological outcomes. J Gerontol. 2011;66A:109–14. doi: 10.1093/gerona/glq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasar S, Xia J, Yao W, Furberg CD, et al. Antihypertensive drugs decrease risk of Alzheimer disase. Ginkgo Evaluation of Memory Study. Neurology. 2013;81:896–903. doi: 10.1212/WNL.0b013e3182a35228. [DOI] [PMC free article] [PubMed] [Google Scholar]