SUMMARY
Background
Neuronal circuits in worms, flies and mammals are organized so as to minimize wiring length for a functional number of synaptic connections, a phenomenon called wiring optimization. However, the molecular mechanisms that establish optimal wiring during development are unknown. We addressed this question by studying the role of N-cadherin in the development of optimally wired neurite fascicles in the peripheral visual system of Drosophila.
Results
Photoreceptor axons surround the dendrites of two post-synaptic targets, lamina cells, within a concentric fascicle called a cartridge. N-cadherin is expressed at higher levels in lamina cells than in photoreceptors, and all genetic manipulations that invert these relative differences displace lamina cells to the periphery and relocate photoreceptor axon terminals into the center.
Conclusions
Differential expression of a single cadherin is both necessary and sufficient to determine cartridge structure by positioning the most adhesive elements that will make the most synapses at the core, surrounded by less adhesive elements that make fewer synapses. These results suggest a general model by which differential adhesion can be utilized to determine the relative positions of axons and dendrites to establish optimal wiring.
INTRODUCTION
All brains comprise complex networks of synaptic connections arranged within a compact structure. Ramon y Cajal proposed that these connections are arranged to minimize the total length of neurites, a phenomenon called wiring optimization that has been reported in vertebrates, flies and worms [1–4]. However, the developmental mechanisms that might specify “optimally wired” connections remain unknown. Here we describe how differences in adhesion direct assembly of an optimally wired structure.
The Differential Adhesion Hypothesis (DAH) postulates that cells minimize surface free tension, a property shaped by adhesion [5, 6]. As the DAH predicts, mixtures of two mutually adhesive cell types segregate so that the less adhesive population surrounds the more adhesive one [7–9]. Cortical tension counteracts cell adhesion, and surface tension results from integrating both forces [10–14]. During embryonic development, differential adhesion and cortical contraction mediate germ layer sorting [13], the establishment of tissue boundaries [15–17], and the formation of mosaic epithelia [18–20]. Cadherins, homophilic adhesion molecules, play prominent roles. In Drosophila, E-cadherin determines oocyte position in the egg chamber [21], while differential expression of N-cadherin (Ncad) regulates cone cell patterning in the eye [22]. In the chick spinal cord, differential MN-cadherin expression sorts neurons into distinct motor pools [23]. In the brain, cadherins regulate axon targeting, dendrite elaboration, and synapse formation [24, 25]. However, whether differential adhesion might determine the relative positions of synaptic partners is unknown.
To address this question we studied the development of cartridges, columnar fascicles that comprise the first neuropil of the Drosophila visual system, the lamina. The cartridge array represents a retinotopic map, with each cartridge receiving direct photoreceptor input from a single point in visual space. Cartridges contain the axon terminals of six photoreceptors (R cells, designated R1–R6), five lamina cells (L cells, designated L1–L5), as well as other neuron and glial types [26, 27]. Cartridges have an invariant cylindrical structure, with R cell axon terminals and L cell dendrites arranged coaxially, with L1 and L2 at the core, surrounded by R1–R6 and L3–L5 cells. This concentric arrangement, combined with volume exclusion, represents the optimally wired configuration, allowing synapse formation between all synaptic partners while minimizing dendrite and axon lengths [4]. However, the mechanisms that determine the positions of these cartridge elements remain unknown. Here we demonstrate that the relative positions of L cell neurites and R cell axon terminals within the cartridge are substantially determined by differential expression of Ncad, and thus by differential adhesion.
RESULTS
L cell neurites change position in the developing cartridge
Cartridge organization has been reported at the electron microscopic level in both adults and pupae [4]. Early in pupal development, at approximately 24hr after puparium formation (apf), L cells form a single fascicle that must undergo rearrangement to achieve the adult pattern [4, 26, 28]. However, when axons and dendrites within the cartridge change their relative positions is unknown. We focused on the outer six R cells and the five L cells, because these represent the dominant afferent columnar elements in every cartridge and contribute the largest synaptic populations [4, 29]. We labeled all R cells with anti-Chaoptin (mAb24B10) and individual L cells with mCD8GFP using mosaic analysis with a repressible cell marker (MARCM) [30]. We examined L cell positions beginning shortly before R cells extended to their target cartridges (28% apf), until the adult cartridge organization became apparent (48% apf). L cells were identified by the positions of their cell bodies, their shapes, and by the location of their neurites within the cartridge [31] (Figure S1 and Experimental Procedures). L cells are monopolar neurons that extend a single vertical neurite with laterally directed dendrites that are largely postsynaptic in the lamina, and axon terminals that are largely pre-synaptic in the second optical neuropil, the medulla [32]. Insofar as pre- and postsynaptic sites are not absolutely segregated, we could not strictly designate the vertical neurite as either dendritic or axonal, and so refer to this main process in the lamina as the primary neurite, defining its lateral branches as dendrites.
During late larval and early pupal development, R cell axons and L cell neurites established two distinct, but adjoining fascicles. At 28% apf, R1–R6 cells formed a sheet of growth cones across the lamina plexus, while L cells formed a tight fascicle from which small, bushy dendritic processes radiated outward (Figure 1A, L1–L5 clone). L4 formed two lateral branches at this stage (Figure S1), and L5 had only few lateral processes. At approximately 32% apf, R1–R6 growth cones extended away from their fascicle of origin to invade neighboring L cell fascicles [33]. At 38% apf, R cell growth cones surrounded L cell processes and invaded the L cell bundle, physically separating the neurites of L1 and L2 from those of L3–L5 (Figure 1A, B). By 48% apf, L1 and L2 neurites were located at the core of the cartridge, surrounded by R cell processes, while the primary neurites of L3, L4 and L5 were displaced to the outside of each fascicle (Figure 1A, B). Concurrently, L1–L3 elaborated short dendrites, forming a bottle brushlike structure that interdigitated between R cell processes. L4’s main neurite formed three distinct dendritic branches in the proximal lamina (Figure S1, Figure 1A), while L5’s main neurite is almost completely devoid of dendrites (Figure 1A, Figure S1). In summary, together with previous data, our observations demonstrate that L cell neurites initially form a single fascicle and suggest that interactions between R cells and L cells separate this fascicle, to create three groups of processes with distinct relative positions (with L1 and L2 at the core, R cell terminals in the middle, and the main neurites of L3, L4 and L5 at the periphery). As synaptic structures are not detectable until approximately 55% apf [44], these developmental rearrangements clearly precede synaptogenesis.
Figure 1. Development of the lamina cartridge and Ncad expression in the lamina.
(A) Single cartridge cross-sections at 28, 38 and 48% apf, R cells labeled by mAb24B10 (magenta) and single L cells with mCD8GFP (green). All cartridges imaged are from the left dorsal hemisphere, except for L1–5, L1/2 and L5 at 28% apf. L4 cells also form lateral dendrite branches deeper in the lamina (see Figure S1). (B) Schematic organization of the R cell axons and L cell neurites in the lamina cartridge (R cells: magenta, L cells: green). (C, E) Plan view of the lamina at 28% apf (C) and 48% apf (E); R cells are labeled with mAb24B10 (red in merge) and Ncad (blue in merge). L cells are labeled by myr-EGFP (L cells, green in merge) driven by gcm-Gal4 (C) or GH146-Gal4 (E). (D) Intensity profile plot along the white scan line in (C) shows that Ncad (blue) is expressed at higher levels in L cells (green) than in R cells (magenta). Green and magenta bars denote L cell and R cell territory, respectively.
Ncad is expressed at higher levels in L cells than in R cells
The homophilic adhesion molecule Ncad is expressed by both R cells and L cells and mediates adhesive interactions between them, which are essential for R cells to extend to their target cartridges [34, 35]. We therefore considered the possibility that the same Ncad- mediated interaction could also subsequently determine the positioning of R cell terminals and L cell neurites within the target cartridge. By co-labeling R cells (mAb24B10), L cells (using gcm-Gal4 or GH146-Gal4 expressing myrEGFP, Figure S1) and Ncad, we observed that Ncad immunoreactivity was more intense in L cell processes than in R cell growth cones at 28% apf (Figure 1C). Line scans across single optical sections revealed that peaks in Ncad labeling coincided with peaks in the L cell label, while troughs in Ncad labeling coincided with peaks in R cell labeling (Figure 1D). While this analysis cannot distinguish whether L cell neurites contained higher surface density of Ncad, or simply had a larger surface area, these data suggest that L cell dendrites are more adhesive than R cell terminals. After R cells extended, R and L cell processes become intertwined, precluding this analysis at later stages. Yet, in line with the hypothesis that Ncad is expressed at higher levels in L cell processes, at 48% apf, Ncad protein was enriched in areas where R cell axons contacted L cell dendrites at the cartridge core (Figure 1E).
Relative differences in Ncad levels determine cartridge organization
According to the DAH, when cells of differential adhesiveness are mixed, segregation occurs such that the less adhesive cells surround the more adhesive ones [5]. Consistent with this notion, the pattern of Ncad immunolabeling suggested that L1 and L2 might be at the core of the cartridge, surrounded by R cells, because L1 and L2 are more adhesive. To test this possibility, we manipulated Ncad levels either in R cells, L cells, or both and examined cartridge organization (Figure 2). First, we used cell-type specific Gal4 drivers to knock down Ncad using RNA interference (RNAi) either in R or L cells (Figure S2). We used strong, early pan-R cell or pan-L cell drivers, ey3.5-Flp actin FRT stop FRT Gal4, or gcm-Gal4, respectively, and labeled the adult lamina using the presynaptic marker Csp2A. In the wild-type, Csp2A labeling revealed cartridge cross sections with six R cell profiles arranged in a circle around an unlabeled core, which contained the primary neurites of L1 and L2 and the dendrites of L1–L4 (Figure 2A). As demonstrated previously, reducing Ncad activity in either R cells or L cells caused R cell axon targeting defects, resulting in cartridges that contained variable numbers of R cell profiles (Figure 2A–C) [34, 35]. However, we observed a critical difference between these two manipulations. When Ncad was knocked down in R cells, Csp2a- labeled R cells surrounded an unlabeled center, even in cartridges that contained abnormal numbers of R cell terminals. By contrast, Ncad RNAi in L cells produced cartridges in which R cell processes were no longer excluded from the core, but instead filled the “hole” in Csp2a labeling (Figure 2A–C, K). We confirmed these results using null mutations in Ncad. While it is straightforward to generate R cell- specific mutants using ey3.5-Flp- mediated homologous recombination, no such tool is available for L cells. We therefore used a technique called FLICK (Figure S2) [36], to reproducibly knock out Ncad in >95% of L cells. In both experiments, we observed defects similar to those seen in the corresponding RNAi knock down condition (Figure 2E, F). Again, L cell loss of Ncad using FLICK resulted in cartridges with filled-in centers (Figure 2E, K). We observed a similar phenotype when Ncad was removed from all R cells (Figure 2K). However, these brains have severe defects in cartridge organization because Ncad plays many roles in early stages of R cell targeting, frequently resulting in cartridges that contain R cells, but lack L cells [29, 34].
Figure 2. Changes in Ncad expression levels affects cartridge organization.
(A–H) Adult laminas were labeled with the R cell marker Csp2a. Insets show magnified views of a single, representative cartridge. (A) Control: mCD8GFP. (B) L cell- specific Ncad RNAi. (C) R cell- specific Ncad RNAi. (D) Ncad RNAi in both cell types. (E) L cell - specific Ncad loss of function. (F) R cell- specific Ncad loss of function. (G) R cell- specific overexpression (OE) of Ncad-13b. (H) R cell- specific OE of Ncad-13a. (I) Schemata of observed phenotypes. (J) Quantification of Ncad expression in R cells vs. L cells (see Supplemental Experimental Procedures). If Ncad levels were relatively higher in R cells than L cells (dark gray), values were made negative. Light gray denotes genotypes where Ncad is relatively enriched in L cells. All groups are significantly different from control with at least p<0.05 (ANOVA, n=6 brains per group). (K) Quantification of cartridge defects, n= 151–288 cartridges per genotype. All groups, except Ncad overexpression (OE) in L cells are significantly different from control with p<0.001 (Fisher’s exact test).
These results suggested that the “filled-in” cartridge phenotype associated with reducing Ncad in L cells was caused by the displacement of L1 and L2 neurites from the core of the cartridge to the periphery. This extrusion might be caused by increased Ncad adhesion between R cells, relative to the adhesion between L cells. We therefore predicted that reducing Ncad simultaneously in R cells and L cells should rescue the phenotype of filled-in cartridges. We expressed Ncad RNAi using a combination of both R and L cell Gal4 drivers and, as predicted, reverted cartridges to their normal morphology (while R cell targeting remained severely disrupted; Figure 2D, K). Thus, loss of the central cartridge “hole” in L cell-specific Ncad RNAi was caused by a relative increase in adhesion between R cells with respect to adhesion between L cells, rather than by independent reduction of Ncad levels in either cell type alone.
We next tested whether fascicle structure could be re-programmed by inverting the relative levels of Ncad in R cells compared with L cells using cell-type specific overexpression. If Ncad acts by differential adhesion, overexpressing Ncad in R cells should push L cell dendrites to the cartridge periphery, causing a filled-in cartridge. We tested four different Ncad isoforms, two of which caused increased Ncad levels in R cells relative to L cells (Figure 2J, Figure S2). Consistent with our prediction, when the Ncad isoform Ncad-13b was overexpressed in R cells, filling in of cartridges was observed (Figure 2G). Overexpressing the Ncad-13a isoform in R cells produced an intermediate “open-cartridge” arrangement in which R cell axons incompletely surrounded the central hole in Csp2a staining (Figure 2I), despite Ncad levels being clearly higher in R cells than in L cells in these animals (Figure 2J). As both Ncad isoforms are of similar adhesivity [37], the difference in phenotype observed likely reflected differences in the levels of overexpression (Figure 2K, Figure S2). We also sought to test the effect of Ncad overexpression in L cells using gcm-Gal4. As predicted, since this manipulation does not invert the relative Ncad levels, we observed no defects in cartridge arrangement. However, we also did not observe elevation of Ncad protein levels in L cell dendrites compared to control, possibly because gcm-Gal4 expression declines early in pupal development. In summary, these results demonstrate that all manipulations that inverted the relative level of Ncad activity between L cells and R cells repositioned the primary L1 and L2 neurites from the core of the cartridge to the periphery (Figure 2J, K).
These experiments did not allow us to directly visualize L cell neurite positions. Given that we have no Gal4-independent label for all L cells, we generated GFP-labeled mitotic clones either in L cells or R cells via MARCM. Using heat-shock mediated Flipase (Flp) expression, we generated large, contiguous Ncad mutant R cell or L cell clones in an otherwise Ncad heterozygous background. However, large clones were rare (being present in fewer than 1 in 20 animals for R cells and fewer than 1 in 100 for L cells), allowing for analysis only in mid-pupal development, when we can easily score the presence of clones by GFP expression. As we observed previously, while loss of Ncad in R cells affected axon target choice, this manipulation did not cause defects in cartridge organization (Figure 3A). In contrast, loss of Ncad in L cells significantly altered cartridge organization, with R cells and L cells forming adjacent, but separated fascicles (Figure 3B). This phenotype was observed in all cartridges in which most L cells were mutant. Furthermore, we observed that Ncad mutant L cells lacked the bottle-brush dendrites seen in wild-type clones. Instead, they formed only few dendritic processes at this stage (Figure 3B, insets).
Figure 3. Ncad loss in L cells, but not in R cells separates R cell and L cell fascicles.
Large Ncad mutant MARCM clones either in R cells (A) or L cells (B), positively labeled with mCD8GFP at 48% apf (green). All R cells are labeled with mAb24B10 (magenta). Insets show single, representative cartridges.
In summary, these data demonstrate that knockdown or loss of Ncad in L cells, but not in R cells, resulted in the separation of R cell and L cell fascicles. When Ncad levels are higher in L cells than in R cells, cartridge organization is normal. Conversely, when Ncad levels are higher in R cells than in L cells, their fascicles separate. Removal of Ncad from either cell population strongly reduces Ncad-mediated homophilic adhesion between them. Thus, the change of L cell position in the cartridge cannot merely have resulted from a failure of adhesion between R cells and L cells. Rather, cartridge organization must reflect a change in the levels of Ncad adhesion within each of these cell populations relative to one another.
Ncad mutant L1/L2 neurites are mispositioned
To determine the positions and morphologies of cartridge elements in Ncad mutants at higher resolution, and to test whether Ncad loss affected synapse formation, we used electron microscopy (EM) to analyze cartridges in L cell- specific Ncad mutants generated using FLICK. In cartridge cross sections we recognized R cells by their dark cytoplasm and the presence of glial invaginations (capitate projections), and distinguished L cell profiles by their pale cytoplasm (see Experimental Procedures) [26]. We reconstructed L cell profiles in four control and five Ncad mutant cartridges (Figure 4A–D). As we had observed using light microscopy, when Ncad was removed specifically from L cells, the primary neurites of both L1 and L2 lay at the periphery of the majority of cartridges, while the positions of L3 and L4 were unchanged, resulting in R cells occupying the cartridge core (Figure 4A, B). We also examined R cell clones. As complete loss of Ncad throughout larval and pupal development in R cells causes severe targeting and structural defects, we used a hypomorphic mutation, Ncad936, which removes half of the splice forms of Ncad [38]. Consistent with the light microscopic data, EM revealed no change in cartridge organization, with L1/ L2 neurites always located at the cartridge core (Figure 4E, F).
Figure 4. EM analysis of Ncad mutant cartridges.
(A–F) Individual cartridge cross sections, (A, C, E) or their corresponding traced and color-coded profiles (B, D, F). Primary neurites of L1 and L2 are indicated by asterisks. (A, B) Cartridges with Ncad FLICK mutant L cells, including supernumerary L2 cells. (Bi) Same reconstruction as in B, but only showing R cell terminals and the primary neurites of L1 and L2. (C, D) Cartridges innervated by Ncad936 mutant eye containing one of each identified L-cell type (L1–L4). (E, F) Ncad FLICK control cartridges. (G–P) 3D reconstructions of R cell axon terminals and L cell dendrites in the Ncad L cell FLICK mutant (G–K), or the R cell mutant Ncad936 (L–P). (Q, R) Synapses in cartridges with Ncad FLICK mutant L-cells, L-cell elements color-coded as in A–F. (Q) Normal tetrad synapse with T-bar ribbon (arrow). (R) Abnormal synapse formed between two Ncad FLICK mutant L2 cells with T-bar ribbon. Scale bars: (C) 2 μm; (H) 200 nm.
Interestingly, L1 and L2 cells that were both mutant for Ncad and located at the cartridge periphery formed asymmetric lateral dendrites that invaded the cartridge interior, like those of L3 (Figure 4G–J, compare to Figure S3). By contrast, when Ncad was removed from R cells, the dendrites of all L cells were normal (Figure 4L–P). Furthermore, L cells mutant for Ncad had relatively complex dendrites in adult animals, but displayed greatly diminished complexity when observed in mid pupal development (Figure 3). We infer that the loss of Ncad delays dendrite development. In addition, we observed no changes in the ultrastructure or composition of R cell to L cell synapses in either Ncad R cell or L cell mutants, compared to controls (Figure 4Q) [26], suggesting that Ncad loss did not change cell fate or synaptic connectivity. We did, however, observe a striking reduction in synapse numbers in both mutants, with R cells forming four or five times fewer tetrads than in control (Table 1), a phenotype that falls at the extreme of the synapse numbers previously reported in a wide range of photoreceptor mutants [39]. Finally, we note that when we did observe abnormally large cartridges containing increased numbers of R cells and L cells, we also observed abnormal synaptic contacts between these supernumerary L cells, similar to those previously reported when neighboring cartridges fuse [40]. For example, we observed abnormal dyadic synapses between two L2 cells within the same cartridge (Figure 4R). Nonetheless, the vast majority of synapses we observed in these abnormal cartridges occurred between the same synaptic partners as in wild-type animals. In summary, our EM data confirmed our findings with light microscopy and showed that mis-positioning L1 or L2 neurites did not affect their synaptic partnerships with R cells.
Ncad determines the positions of individual L cells in the lamina cartridge
To test whether Ncad acts cell autonomously to determine L cell position within the cartridge, we made single L cells mutant for Ncad using MARCM. As shown previously, we observed no R cell targeting defects in these single L cell clones, allowing us to dissociate Ncad’s role in axon targeting from its role in cartridge structure (data not shown) [35]. We observed that the neurites of all single Ncad mutant L3, L4 or L5 cells remained at the periphery of the cartridge as in wild-type (Figure 5A–C). In contrast, approximately 60% of Ncad mutant L2 cells were displaced from the cartridge core to the periphery, forming asymmetric dendrites that projected into the cartridge, like those of L3 (Figure 5B, C, E). L1 cells mutant for Ncad displayed a qualitatively similar phenotype, with 20% of L1 cells located at the periphery (Figure 5C, E). This difference in expressivity between L1 and L2 likely reflects an asymmetry in cartridge structure early in development. After R cell growth cones have extended laterally, L1 is located at the core of the newly formed cartridge, while L2 sits at the anterior edge and must be actively surrounded by R cell processes to reach the cartridge core, perhaps making it more susceptible to the loss of Ncad (Figure 1A, at 38% apf). We note that the observed defects in L1 and L2 positioning were not caused by a change in cell fate, because both L1 and L2 mutant axons reached their correct stratum in the medulla, as observed previously (Figure S4) [31].
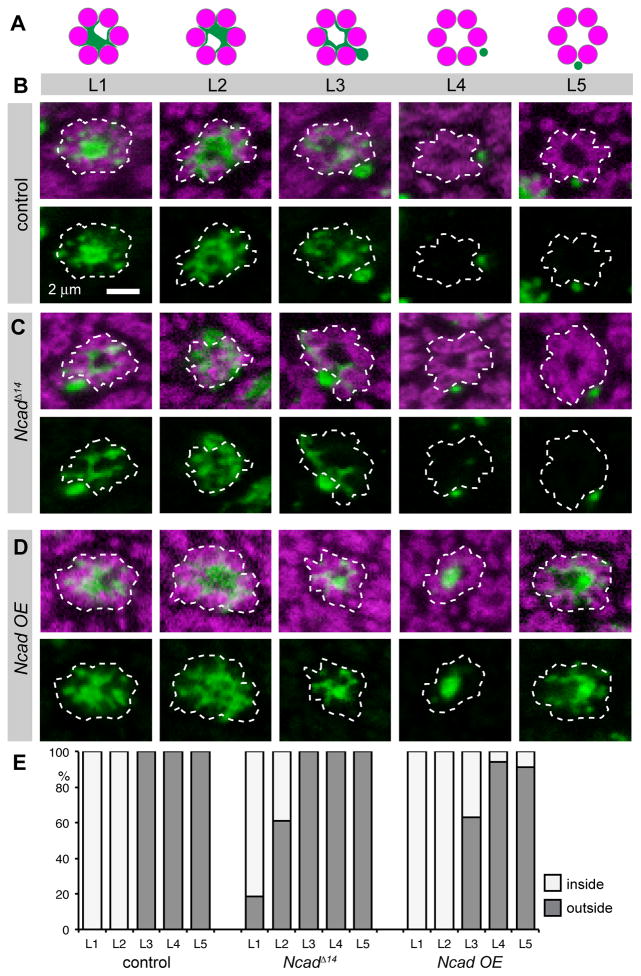
Figure 5. Ncad cell autonomously regulates L cell neurite position.
(A) Schemata showing L1–L5 neurites in wild-type cartridges. (B–D) Single mCD8GFP- labeled (green) L cells either wild-type (B), Ncad mutant (C) or overexpressing Ncad using elavC155-Gal4 (D) using MARCM in an Ncad heterozygous background, counterstained with mAb24B10 (all R cells, magenta). Single cartridge profiles are outlined by dashed white line. All cartridges, except L4 in control and L5 in Ncad OE are from the dorsal hemisphere, the equator is to the right in all panels. (E) Percentage of L cells localized at the core (inside) or periphery (outside) of the cartridge, control n=19–44, Ncad loss n=28–38, Ncad overexpression n=18–87. Ncad mutant L1 and L2, as well as Ncad overexpressing L3 differ significantly different from control with p<0.05 (Fisher’s exact test).
Consistent with our observations of large L cell clones, loss of Ncad in single L cells affected dendrite formation, reducing dendritic area in L1–L3 (Figure S4), and frequently causing L4 to lose at least one of its collateral branches (data not shown). These data suggest that Ncad- mediated adhesion of R cells to L cells promotes the formation or stabilization of dendrites. We also examined whether the location of the primary L1 or L2 neurites in the cartridge could be a secondary outcome of their different dendrite sizes, an effect that would be revealed by a correlation between dendrite size and neurite position. However, no such correlation was observed (Figure S4).
We next examined whether increasing Ncad expression in single L3, L4 and L5 cells would be sufficient to move their primary neurites into the cartridge core from their normal positions at the periphery. Indeed, Ncad overexpression in single L3 cells frequently caused their primary neurites to move into the core (37%, Figure 5D, E). These shifted L3 neurites also formed symmetrically radiating dendrites, thus resembling L1 and L2. Notably, overexpressing Ncad in L3 did not change the shape or the location of the L3 cell body, nor was the size of the L3 dendritic tree affected (Figure S4, data not shown). Similarly, Ncad overexpression in single L4 or L5 cells also moved the neurites of these cells to the cartridge core, albeit only rarely (6% and 9%, respectively). Interestingly, when neurites of L4 or L5 were displaced to the interior of the cartridge, they were either enlarged, or displayed ectopic dendrites (Figure 4C). This suggests that the relatively weak expressivity seen in these manipulations of L4 and L5 might reflect the fact that these cells have relatively simple neurites and hence a small surface area, precluding significant increases in adhesion despite increased levels of Ncad. Finally, we note that this change in position was not caused by a fate transformation, because when L4 and L5 cells overexpressed Ncad, they still expressed their characteristic fate marker, the Brain Specific Homeobox protein, and L4 still formed its characteristic dendrite collaterals (data not shown). In summary, our data demonstrate that changing Ncad expression in single L cells can change the positions that their primary neurites occupy within the cartridge.
DISCUSSION
We have demonstrated that Ncad- mediated differential adhesion instructs the organization of the lamina cartridge, ensuring that the primary neurites of the L cells L1 and L2 are located at the core, surrounded by the axon terminals of R1–R6 photoreceptors and the neurites of L3–L5. Ncad is normally expressed at higher levels in L cells than in R cells. Furthermore, all genetic manipulations that resulted in higher levels of Ncad expression in R cells relative to L cells displace L1 and L2 from the core to the periphery. Conversely, Ncad overexpression in L3, L4 or L5 can move the primary neurites of these cells to the core. Thus, relative differences in Ncad levels between R cells and L cells are both necessary and can be sufficient to determine the relative placement of axons and primary neurites. These data support a simple developmental mechanism that specifies a critical feature of an efficient wiring diagram.
Differential adhesion patterns lamina cartridges
The DAH compares adhesive strength, also called adhesive work (W), amongst cells of type a (Wa), amongst cells of type b (Wb) and between both populations (Wab) [5]. The relative differences in adhesive work determine the spatial arrangement of the two cell types in the equilibrium state of a mixed aggregate. Initially formulated to explain the behavior of individual embryonic cells, our data demonstrate that differential adhesion can also account for much of the organization of primary neurites in a synaptic fascicle. We can define the adhesive work of R cells as Wr, the adhesive work of L1 and L2 as Wl, and the adhesion between R cells and L cells as Wrl (Figure 6). In wild-type, L1 and L2 neurites express higher levels of Ncad than R cells and are surrounded by R cell axon terminals, observations that are consistent with the relative magnitude of adhesive work being Wl > Wrl > Wr. When Ncad is lost in R cells or overexpressed in L cells, the absolute levels of Ncad expression change, but the relative levels do not. As a result the relative order of adhesive work does not change, and cartridge architecture is unaffected. In contrast, when Ncad is lost in L cells, R cell and L cell fascicles separate, suggesting that they adhere much less to each other than to themselves. These data suggest that the relative order of adhesive strength has become Wr > Wl > Wrl. However, as R cells and L cells still adhere to themselves and to each other after losing Ncad expression, there must be additional molecules that contribute to the overall adhesive balance. Recent studies demonstrate that the opposing actions of cell adhesion and cortical tension drive cell sorting [10–15, 19, 41, 42]. We found that expression levels of Ncad were sufficient to instruct the sorting of primary neurites in a fascicle, suggesting that differential adhesion might be the main driving force. However, Ncad may also change cytoskeletal tension, a possibility consistent with the fact that cadherin signaling can reduce cortical actin levels [10, 43].
Figure 6. Differential adhesion between R cells and L cells guides cartridge assembly.

The experimentally observed neurite configurations of the lamina cartridge can be predicted by differences in adhesive work (W) [6].
Ncad is required for dendrite and synapse formation
Ncad mutant L cells displayed a transient delay in dendrite elaboration that is apparent in mid pupal development (Figure 3), but not detectable in adult animals (Figure 4). While loss of Ncad in single L1, L2 or L3 cells had only a modest effect on dendrite size, removal of Ncad from all L cells resulted in a dramatic reduction of dendrites at mid-pupal stages. Similarly, loss of Ncad in mushroom body neurons or in vertebrate neuronal cultures reduces dendrite field sizes and spine numbers [44–46], suggesting that Ncad- mediated adhesion promotes dendrite formation or stabilization in a wide range of neuron types. As this phenotype is transient in L cells, we infer that other dendritogenic molecules can compensate for the loss of Ncad. Consistent with this developmental delay in dendrite elaboration, we observed a striking reduction in the number of synapses between R cells and L cells. As this phenotype was similar when Ncad was lost in R cells or in L cells, we attribute this deficit to reduced homophilic adhesion between future synaptic partners, independent of their position within the cartridge. As only the removal of Ncad from L cells causes defects in cartridge structure, while removing it from R cells does not, we infer that these defects in synaptogenesis reflect an additional role for Ncad.
Differential adhesion positions neurites to generate an efficient wiring network
This study describes how differential expression of a single cadherin mediates the assembly of a concentric synaptic structure, the lamina cartridge, with the most adhesive elements at its core and the least adhesive ones in the periphery. This arrangement of elements is optimally wired [4] such that the position of a neurite (either dendritic or axonal) within the cartridge correlates with the number of synapses it forms. L1 and L2 each receive the largest number of synaptic contacts (more than 250 each) and sit at the cartridge core, surrounded by R cells, each of which forms about 150 synapses, while L3 has about 80, L4 about 20, and L5 less than 15 synapses [4, 29]. We note that neurites of another cartridge element, amacrine cells, also form large numbers of synapses, but are located at the cartridge periphery. However, amacrine cells are not columnar elements and innervate a number of cartridges [4]. Thus different rules may guide the wiring of such wide-field elements.
R cell terminals surround L cells in many insect species, including all Diptera, as well as ancestral insects, such as dragonflies [47]. This concentric arrangement has persisted across these species even though the pattern by which R cells select their target cartridge has changed dramatically [48, 49]. Thus, this concentric cartridge arrangement is uncoupled from earlier targeting events and likely of functional importance. The sorting of neuronal processes based on differential adhesion provides a simple means to achieve wiring efficiency, representing a general strategy that may be widely applied to achieve optimally wired configurations.
EXPERIMENTAL PROCEDURES
Fly stocks
The following fly strains were used: NcadΔ14 FRT40A [35], Ncad936 FRT40A [38], gcm-Gal4 [50], GH146-Gal4 [51], UAS-mCD8GFP, FRT40A gmr-Hid, UAS-Flp, QUAS-mCD8GFP (Bloomington Stock Center), gmr-Flp [52], ey3.5-Flp [53], actin-FRT-yellow+-FRT-Gal4/ actin>stop>Gal4 (gift from G. Struhl), UAS-Ncad-7b–13b–18a and UAS-Ncad-7b–13a–18a [54], Ncad-IR 1092 [55], UAS-myrEGFP [56], 27G05-Flp and tara-Gal4 (gift from L. Zipursky), P(XP)d10879 and pBac(WH)f00896 insertions (Exelixis collection at Harvard University). For MARCM analysis the following strains were used: elavC155-Gal4 hs-Flp UAS-mCD8GFP, tub-Gal80 FRT40A, and FRT40A, tub-Gal4 UASmCD8GFP [30]. For a detailed list of all parental stocks and crosses used in the experiments, please see Supplemental Tables 1 and 2.
Clonal analysis
Single-cell MARCM clones in L cells were generated using 27G05-Flp orhs-Flp. For the latter, third instar larvae were heat shocked for 22 minutes at37°C, 24–32 h prior to 0% apf. To generate large R cell and L cell clones using hs-Flp, first instar larvae were heat shocked for 50 minutes at 37°C about 96 hprior to 0% apf.
Immunohistochemistry & Imaging
Brains of pupae and adult flies were dissected in 2% paraformaldehyde (0.1 M L-lysine containing 0.05M phosphate buffer). Pupal brains were fixed for 55 min and adult brains for 60 min at 20–22°C, and washed in PBS with 0.5% Triton X-100. The following antibodies were used: anti-GFP (chicken 1:1000, Abcam), anti-Bsh (guinea pig 1:500) from C.H. Lee, anti-mAb24B10 (mouse 1:10), anti-Csp2a (6D6, mouse 1:10), anti-Ncad (rat DN-Ex8, 1:100) and anti-Elav (rat 7E8A10, 1:50) from Developmental Studies Hybridoma Bank. The following secondary antibodies were used: anti-chicken Alexa-488, anti-mouse Cy3, anti-mouse 546 IgG1, anti-rat Cy5, anti-rat Alexa-633, anti-rabbit Cy3, anti-mouse Alexa-488, anti-guinea pig Alexa 594, and anti-guinea pig Alexa 633 (all goat, all at 1:200, from Life Technologies).
Images were acquired using a Leica TCS SP2 AOBS confocal microscope, using a 100x/ N.A. 1.4 or a 40x/ N.A. 1.25 lens and were rendered and analyzed using Bitplane Imaris and Fiji/Image J. Figures were prepared using Adobe Photoshop and Illustrator. Statistics were calculated using Graph Pad Prism. For details on image analysis and quantification please see Supplemental Experimental Procedures.
Electron microscopy
Please see Supplemental Experimental Procedures for details.
Supplementary Material
HIGHLIGHTS.
Neurites in synaptic fascicles change position during development
N-cadherin is differentially expressed in pre- and postsynaptic neurites
Relative, not absolute, N-cadherin levels determine the placement of neurites
N-cadherin loss affects dendrite and synapse development
Acknowledgments
We thank Liqun Luo, Chi-Hon Lee, Iris Salecker, Gary Struhl, Larry Zipursky, the Bloomington and Harvard University Stock Centers, as well as the Developmental Studies Hybridoma Bank and the Vienna Drosophila RNAi Center for fly strains and antibodies. We are grateful to Zhiyuan Lu for cutting ultrathin sections, Rita Kostyleva for early analyses of Ncad936, Patrick Macdonald for EM reconstructions, and finally to Erin Barnhart, Jenn Esch, Andrew Huberman and Jacob Bendor for helpful comments on the manuscript. This work was supported by a postdoctoral fellowship from Walter V. and Idun Berry (T.S.), and by funding from the National Eye Institute, R01 EY-015231 (T.R.C.) and R01 EY-03592 (I.A.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cajal R. Textura del Sistema Nervioso del Hombre y de los Vertebrados. Madrid: Nicolas Moya; 1899. [Google Scholar]
- 2.Chen BL, Hall DH, Chklovskii DB. Wiring optimization can relate neuronal structure and function. Proc Natl Acad Sci U S A. 2006;103:4723–4728. doi: 10.1073/pnas.0506806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klyachko VA, Stevens CF. Connectivity optimization and the positioning of cortical areas. Proc Natl Acad Sci U S A. 2003;100:7937–7941. doi: 10.1073/pnas.0932745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera-Alba M, Vitaladevuni SN, Mischenko Y, Lu Z, Takemura SY, Scheffer L, Meinertzhagen IA, Chklovskii DB, de Polavieja GG. Wiring economy and volume exclusion determine neuronal placement in the Drosophila brain. Curr Biol. 2011;21:2000–2005. doi: 10.1016/j.cub.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinberg MS. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963;141:401–408. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg MS. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool. 1970;173:395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- 7.Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol. 2003;253:309–323. doi: 10.1016/s0012-1606(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 8.Foty RA, Pfleger CM, Forgacs G, Steinberg MS. Surface tensions of embryonic tissues predict their mutual envelopment behavior. Development. 1996;122:1611–1620. doi: 10.1242/dev.122.5.1611. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci U S A. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amack JD, Manning ML. Knowing the boundaries: extending the differential adhesion hypothesis in embryonic cell sorting. Science. 2012;338:212–215. doi: 10.1126/science.1223953. [DOI] [PubMed] [Google Scholar]
- 11.Kafer J, Hayashi T, Maree AF, Carthew RW, Graner F. Cell adhesion and cortex contractility determine cell patterning in the Drosophila retina. Proc Natl Acad Sci U S A. 2007;104:18549–18554. doi: 10.1073/pnas.0704235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, Heisenberg CP. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 13.Maitre JL, Berthoumieux H, Krens SF, Salbreux G, Julicher F, Paluch E, Heisenberg CP. Adhesion functions in cell sorting by mechanically coupling the cortices of adhering cells. Science. 2012;338:253–256. doi: 10.1126/science.1225399. [DOI] [PubMed] [Google Scholar]
- 14.Schotz EM, Burdine RD, Julicher F, Steinberg MS, Heisenberg CP, Foty RA. Quantitative differences in tissue surface tension influence zebrafish germ layer positioning. Hfsp J. 2008;2:42–56. doi: 10.2976/1.2834817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang LH, Chen P, Lien MT, Ho YH, Lin CM, Pan YT, Wei SY, Hsu JC. Differential adhesion and actomyosin cable collaborate to drive Echinoid-mediated cell sorting. Development. 2011;138:3803–3812. doi: 10.1242/dev.062257. [DOI] [PubMed] [Google Scholar]
- 16.Laplante C, Nilson LA. Differential expression of the adhesion molecule Echinoid drives epithelial morphogenesis in Drosophila. Development. 2006;133:3255–3264. doi: 10.1242/dev.02492. [DOI] [PubMed] [Google Scholar]
- 17.Milan M, Weihe U, Perez L, Cohen SM. The LRR proteins capricious and Tartan mediate cell interactions during DV boundary formation in the Drosophila wing. Cell. 2001;106:785–794. doi: 10.1016/s0092-8674(01)00489-5. [DOI] [PubMed] [Google Scholar]
- 18.Bao S, Cagan R. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Dev Cell. 2005;8:925–935. doi: 10.1016/j.devcel.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Bao S, Fischbach KF, Corbin V, Cagan RL. Preferential adhesion maintains separation of ommatidia in the Drosophila eye. Dev Biol. 2010;344:948–956. doi: 10.1016/j.ydbio.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Togashi H, Kominami K, Waseda M, Komura H, Miyoshi J, Takeichi M, Takai Y. Nectins establish a checkerboard-like cellular pattern in the auditory epithelium. Science. 2011;333:1144–1147. doi: 10.1126/science.1208467. [DOI] [PubMed] [Google Scholar]
- 21.Godt D, Tepass U. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature. 1998;395:387–391. doi: 10.1038/26493. [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Carthew RW. Surface mechanics mediate pattern formation in the developing retina. Nature. 2004;431:647–652. doi: 10.1038/nature02952. [DOI] [PubMed] [Google Scholar]
- 23.Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- 24.Hadjieconomou D, Timofeev K, Salecker I. A step-by-step guide to visual circuit assembly in Drosophila. Curr Opin Neurobiol. 2011;21:76–84. doi: 10.1016/j.conb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Hirano S, Takeichi M. Cadherins in brain morphogenesis and wiring. Physiol Rev. 2012;92:597–634. doi: 10.1152/physrev.00014.2011. [DOI] [PubMed] [Google Scholar]
- 26.Meinertzhagen IA, O’Neil SD. Synaptic organization of columnar elements in the lamina of the wild-type in Drosophila melanogaster. J Comp Neurol. 1991;305:232–263. doi: 10.1002/cne.903050206. [DOI] [PubMed] [Google Scholar]
- 27.Tuthill JC, Nern A, Holtz SL, Rubin GM, Reiser MB. Contributions of the 12 neuron classes in the fly lamina to motion vision. Neuron. 2013;79:128–140. doi: 10.1016/j.neuron.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meinertzhagen IA, Piper ST, Sun XJ, Frohlich A. Neurite morphogenesis of identified visual interneurons and its relationship to photoreceptor synaptogenesis in the flies, Musca domestica and Drosophila melanogaster. Eur J Neurosci. 2000;12:1342–1356. doi: 10.1046/j.1460-9568.2000.00033.x. [DOI] [PubMed] [Google Scholar]
- 29.Meinertzhagen IA, Sorra KE. Synaptic organization in the fly’s optic lamina: few cells, many synapses and divergent microcircuits. Prog Brain Res. 2001;131:53–69. doi: 10.1016/s0079-6123(01)31007-5. [DOI] [PubMed] [Google Scholar]
- 30.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 31.Nern A, Zhu Y, Zipursky SL. Local N-cadherin interactions mediate distinct steps in the targeting of lamina neurons. Neuron. 2008;58:34–41. doi: 10.1016/j.neuron.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takemura SY, Lu Z, Meinertzhagen IA. Synaptic circuits of the Drosophila optic lobe: the input terminals to the medulla. J Comp Neurol. 2008;509:493–513. doi: 10.1002/cne.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meinertzhagen IA, Hanson TE. In: The Development of the optic lobe. Bate M, Martinez-Arias A, editors. Vol. 2. Cold Spring Harbor Laboratory Press; 1993. pp. 1363–1491. [Google Scholar]
- 34.Lee CH, Herman T, Clandinin TR, Lee R, Zipursky SL. N-cadherin regulates target specificity in the Drosophila visual system. Neuron. 2001;30:437–450. doi: 10.1016/s0896-6273(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 35.Prakash S, Caldwell JC, Eberl DF, Clandinin TR. Drosophila N-cadherin mediates an attractive interaction between photoreceptor axons and their targets. Nat Neurosci. 2005;8:443–450. doi: 10.1038/nn1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hakeda-Suzuki S, Berger-Muller S, Tomasi T, Usui T, Horiuchi SY, Uemura T, Suzuki T. Golden Goal collaborates with Flamingo in conferring synaptic-layer specificity in the visual system. Nat Neurosci. 2011;14:314–323. doi: 10.1038/nn.2756. [DOI] [PubMed] [Google Scholar]
- 37.Yonekura S, Ting CY, Neves G, Hung K, Hsu SN, Chiba A, Chess A, Lee CH. The variable transmembrane domain of Drosophila N-cadherin regulates adhesive activity. Mol Cell Biol. 2006;26:6598–6608. doi: 10.1128/MCB.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nern A, Nguyen LV, Herman T, Prakash S, Clandinin TR, Zipursky SL. An isoform-specific allele of Drosophila N-cadherin disrupts a late step of R7 targeting. Proc Natl Acad Sci U S A. 2005;102:12944–12949. doi: 10.1073/pnas.0502888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, Schulze KL, Cao Y, Verstreken P, Clandinin TR, Fischbach KF, et al. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. 2006;16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frohlich A, Meinertzhagen IA. Cell recognition during synaptogenesis is revealed after temperature-shock-induced perturbations in the developing fly’s optic lamina. J Neurobiol. 1993;24:1642–1654. doi: 10.1002/neu.480241208. [DOI] [PubMed] [Google Scholar]
- 41.Brodland GW. The Differential Interfacial Tension Hypothesis (DITH): a comprehensive theory for the self-rearrangement of embryonic cells and tissues. J Biomech Eng. 2002;124:188–197. doi: 10.1115/1.1449491. [DOI] [PubMed] [Google Scholar]
- 42.Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, Said A, Julicher F, Dahmann C. Increased cell bond tension governs cell sorting at the Drosophila anteroposterior compartment boundary. Curr Biol. 2009;19:1950–1955. doi: 10.1016/j.cub.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bozdagi O, Wang XB, Nikitczuk JS, Anderson TR, Bloss EB, Radice GL, Zhou Q, Benson DL, Huntley GW. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J Neurosci. 2010;30:9984–9989. doi: 10.1523/JNEUROSCI.1223-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurusu M, Katsuki T, Zinn K, Suzuki E. Developmental changes in expression, subcellular distribution, and function of Drosophila N-cadherin, guided by a cell-intrinsic program during neuronal differentiation. Dev Biol. 2012;366:204–217. doi: 10.1016/j.ydbio.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- 47.Arnett-Kibel C, Meinertzhagen IA, Dowling JE. Cellular and synaptic organization in the lamina of the dragon-fly Sympetrum rubicundulum. Proc R Soc Lond B Biol Sci. 1977;196:385–413. [Google Scholar]
- 48.Braitenberg V. Patterns of projection in the visual system of the fly. I. Retina-lamina projections. Exp Brain Res. 1967;3:271–298. doi: 10.1007/BF00235589. [DOI] [PubMed] [Google Scholar]
- 49.Meinertzhagen IA. The organization of perpendicular fibre pathways in the insect optic lobe. Philos Trans R Soc Lond B Biol Sci. 1976;274:555–594. doi: 10.1098/rstb.1976.0064. [DOI] [PubMed] [Google Scholar]
- 50.Chotard C, Leung W, Salecker I. glial cells missing and gcm2 cell autonomously regulate both glial and neuronal development in the visual system of Drosophila. Neuron. 2005;48:237–251. doi: 10.1016/j.neuron.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 51.Stocker RF, Heimbeck G, Gendre N, de Belle JS. Neuroblast ablation in Drosophila P[Gal4] lines reveals origins of olfactory interneurons. J Neurobiol. 1997;32:443–456. doi: 10.1002/(sici)1097-4695(199705)32:5<443::aid-neu1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- 53.Bazigou E, Apitz H, Johansson J, Loren CE, Hirst EM, Chen PL, Palmer RH, Salecker I. Anterograde Jelly belly and Alk receptor tyrosine kinase signaling mediates retinal axon targeting in Drosophila. Cell. 2007;128:961–975. doi: 10.1016/j.cell.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 54.Ting CY, Yonekura S, Chung P, Hsu SN, Robertson HM, Chiba A, Lee CH. Drosophila N-cadherin functions in the first stage of the two-stage layer-selection process of R7 photoreceptor afferents. Development. 2005;132:953–963. doi: 10.1242/dev.01661. [DOI] [PubMed] [Google Scholar]
- 55.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 56.Schwabe T, Neuert H, Clandinin TR. A network of cadherin-mediated interactions polarizes growth cones to determine targeting specificity. Cell. 2013;154:351–364. doi: 10.1016/j.cell.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.