CONSPECTUS
The potential immunotoxicity of nanoparticles that are currently being approved or in different phases of clinical trials or under rigorous in vitro and in vivo characterizations in several laboratories has recently raised special attention. Products with no apparent in vitro or in vivo toxicity may still trigger the various components of the immune system, unintentionally, and lead to serious adverse reactions. Cytokines are one of the useful biomarkers to predict the effect of biotherapeutics on modulating the immune system and for screening the immunotoxicity of nanoparticles, both in vitro and in vivo, and were found recently to partially predict the in vivo pharmacokinetics and biodistribution of nanomaterials. Control of polymer chemistry and supramolecular assembly provides a great opportunity for construction of biocompatible nanoparticles for biomedical clinical applications. However, the sources of data collected regarding immunotoxicities of nanomaterials are diverse and experiments are usually conducted using different assays and under specific conditions, making direct comparisons nearly impossible and, thus, tailoring properties of nanomaterials based on the available data is challenging. In this account, the effects of chemical structure, crosslinking, degradability, morphology, concentration and surface chemistry on the immunotoxicity of an expansive array of polymeric nanomaterials will be highlighted, with focus being given on assays conducted using the same in vitro and in vivo models and experimental conditions. Furthermore, numerical descriptive values have been utilized, uniquely, to stand for induction of cytokines by nanoparticles. This treatment of available data provides a simple and easy way to compare the immunotoxicity of various nanomaterials, and the values were found to correlate-well with published data. Based on the investigated polymeric systems in this study, valuable information has been collected that aids in the future design of nanomaterials for biomedical applications, which include: a) Immunotoxicity of nanomaterials is concentration- and dose-dependent; b) Synthesis of degradable nanoparticles is essential to decrease toxicity; c) Crosslinking minimizes the release of free polymeric chains and maintains high stability of nanoparticles, thereby, lowering their immunotoxicity; d) Lowering amine density for cationic polymers that are being utilized for nucleic acids delivery lowers the toxicity of nanoparticles; e) Among neutral, zwitterionic, anionic and cationic nanomaterials, neutral and cationic nanoparticles usually have the lowest and highest immunotoxicity, respectively; f) Morphology, dimension and surface chemistry have a great influence on the ability of nanomaterials to interact with the various components of the biological system and to modulate the immune system.
1. Immunotoxicity of Nanomaterials
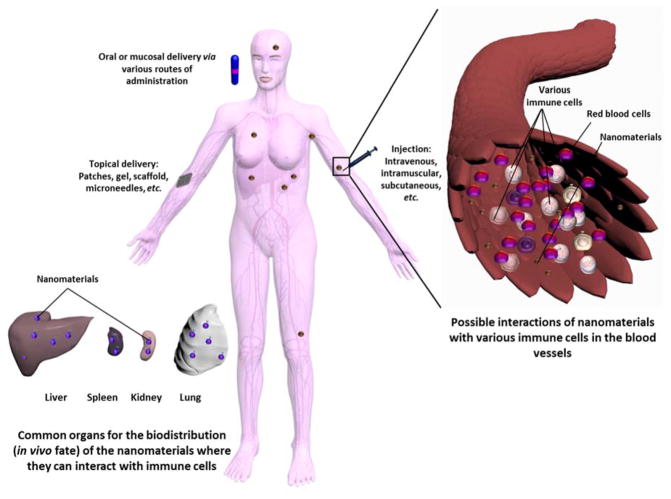
The potential immunotoxicity of nanoparticles has recently raised special attention due to their ability, like other exogenous materials, to unintentionally interact with various components of the immune system, which is usually harmful (Figure 1).1–3 Nanomaterials with no apparent in vitro or in vivo toxicity may still trigger the various components of the immune system via several mechanisms and mediators. For instance, development of poly(ethylene glycol) (PEG)-specific antibodies upon administration of PEG-coated liposomes in vivo has recently been observed, which raised questions towards the approved PEGylated therapeutics that are already in the market for use in humans.4 Immunogenicity of nanomaterials not only leads to serious adverse reactions that can terminate the therapy, but also reduces the therapeutic efficiency.5 The extent of interaction between nanoparticles and the immune system depends on their physicochemical properties (size, morphology, degradability, charge, hydrophobicity, presence of surface-decorating moieties, etc.), their drug payload, and the route of administration.3
Figure 1.
The possible interactions of nanoparticles with the components of the immune system after entering the body via various routes of administration (oral, mucosal, systemic or topical). Reproduced with permission from ref. 3. Copyright (2013) The Royal Society of Chemistry.
Although there are several markers (e.g. antibodies, complement proteins, etc.) for monitoring the modulation of the immune system upon treatment with nanoparticles, proinflammatory cytokines were utilized in this study as biomarkers to compare the immunotoxic effects of nanoparticles of various types. Cytokines are proteins that perform pleiotropic functions and play a pivotal role in regulating the immune system and in mediating adverse reactions (e.g. fever, hypotension, nausea), and sometimes deregulation of their expression can be life-threatening.6,7 Proinflammatory cytokines serve as mediators of inflammatory and immunologic reactions and activate functions of several inflammatory cells during acute inflammatory responses. Hence, it is advisable to monitor the levels of various cytokines to control undesirable responses to nanomaterials. Recently, our group has indicated that the induction of cytokines upon treatment with nanoparticles in vitro can be used as a tool to partially predict the in vivo pharmacokinetics of these materials,8 and others also have found that in vitro results work as a predictor for the in vivo biocompatibility of nanoparticles.9
Interactions between nanoparticles of different types with the immune system and plasma proteins have been studied elsewhere.10–12 However, comparing data from various laboratories and selecting safe and appropriate nanomaterials for a particular clinical application is challenging, due to several reasons. One of the main reasons is the variations in the experimental setup and conditions, and, hence, the level of expression of a particular marker in the control group itself will have different values in the same type of experiment in different laboratories. This variation becomes evident in immunotoxicity assays, where the expression of up to 100 different biomarkers is measured for nanostructures of various compositions.
Previously, like other groups, we compared either the number of the induced cytokines or the levels of induction of cytokines among the different types of nanoparticles and the control untreated group. However, comparing the immunotoxic effects of nanoparticles based on comparing the expressions of tens of cytokines was tedious, in particular, when one set of nanoparticles induced expression of higher amounts of specific cytokines, and lower levels of other cytokines, as compared to another set of nanoparticles. Hence, in this account, we have used calculated numerical values that represent the secretion of cytokines using a single value for each set of nanoparticles. These values were found to corroborate the previously published data across types of polymeric systems. Several examples on various types of polymers and nanoparticles are highlighted herein. Only data reported using nanoparticles that did not contain detectable amounts of endotoxins are included. Overexpression of cytokines is calculated only for cytokines with significant differences from control (p < 0.05), and are normalized to the value of the control in each experiment. The controls are either untreated cells for in vitro assays, or animals injected with saline for in vivo assays. Then, the values exceeding 1 (i.e. higher than the control) are summed and used as numerical theoretical values for evaluating the immunotoxicity of the nanoparticles (i.e. the higher the immunotoxicity index value, the higher the predicted immunotoxicity). Worth mentioning is that the concentration of nanoparticles at which the immunotoxicity index is calculated must be provided because the index value depends on the concentration of the tested materials.
The expression of each cytokine or the detailed discussion and explanation of the data are beyond the scope of this review, but rather this account highlights the effects of chemical structure, crosslinking, degradability, morphology, concentration and surface chemistry on the immunotoxicity of an expansive array of degradable and non-degradable polymeric nanomaterials, with focus being given on assays carried out using the same in vitro or in vivo models and experimental conditions. It is expected that the data reported in this account can be utilized as a valuable guide towards the rational design of clinically-viable and biocompatible nanopharmaceuticals via controlling the synthetic polymer chemistry and supramolecular assembly of various polymeric precursors.
2. Effect of Polymeric Coating
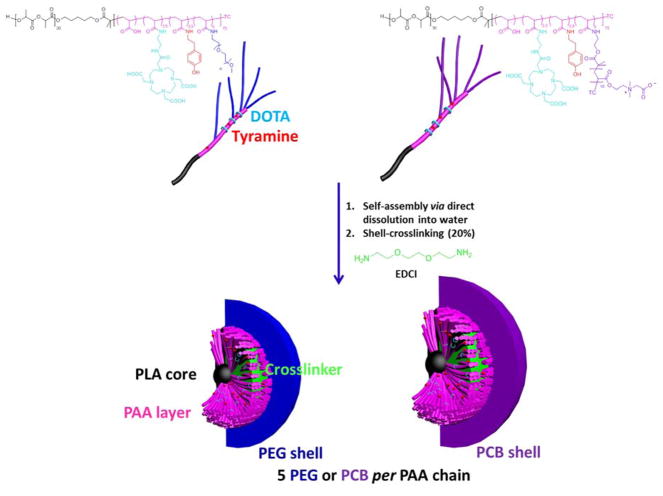
We have previously synthesized poly(acrylic acid)-block-polylactide (PAA-b-PLA) copolymers, functionalized with 1,4,7,10-tetraazacyclododecanetetraacetic acid (DOTA) and tyramine (for potential radiolabeling), and the PAA shell was grafted with PEG (PEG5k or PEG2k, where 5k and 2k represent the polymer molecular weights in g/mol) and poly(carboxybetaine) (PCB5k or PCB2k, where 5k and 2k represent the polymer molecular weights in g/mol) of various molecular weights, to examine the effect of polymeric coating and type of polymer (PEG vs. PCB) on the immunotoxicity, pharmacokinetics and biodistribution of the formed shell crosslinked knedel-like nanoparticles (SCKs) (Figure 2).8,13 Polymers or nanoparticles were incubated with RAW 264.7 mouse macrophages at a non-cytotoxic concentration (500 μg/mL) for 24 h, followed by measurement of the levels of 23 cytokines (interleukin (IL)-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (P40), IL-12 (P70), IL-13, IL-17, eotaxin, granulocyte-colony-stimulating factor (G-CSF), granulocyte macrophage-colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), keratinocyte-derived chemokine (KC), monocyte chemotactic protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, regulated upon activation normal T-cell expressed and presumably secreted (RANTES) and tumor necrosis factor-α (TNF-α)) utilizing a multiplex assay.8
Figure 2.
Chemical structures of DOTA- and tyramine-functionalized PEG- and PCB-g-PAA-b-PLA copolymers, their self-assembly in water and crosslinking to form SCKs, with PLA degradable cores, PAA crosslinked shells, DOTA and tyramine available functionalities, and a hydrophilic shell of either PEG or PCB. TC = SC(=S)SC12H25, trithiocarbonate chain end from the RAFT polymerization chemistry. Reproduced with permission from ref. 8. Copyright (2013) Elsevier.
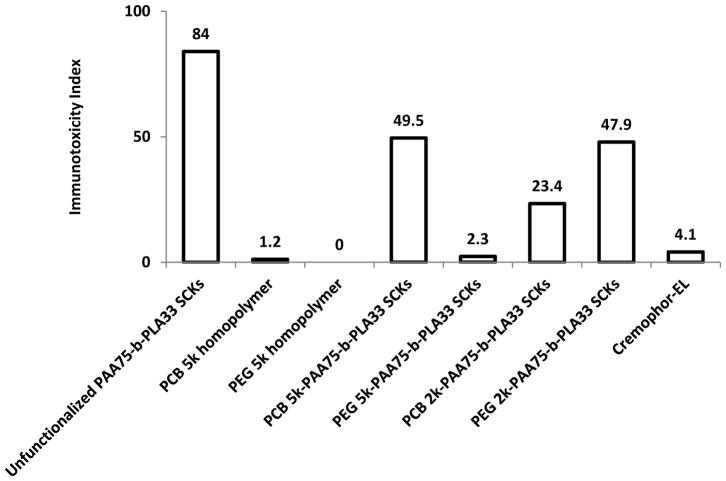
The uncoated nanoparticles resulted in high cellular release of most of the measured cytokines. Functionalization with DOTA and tyramine was also observed to enhance the release of the tested cytokines, as compared to the unfunctionalized SCKs. PCB5k-SCKs significantly induced the production of several cytokines, as compared to PEG5k-SCKs and the control-untreated cells, probably due to the better shielding efficiency provided by PEG vs. PCB polymers to the PAA-b-PLA SCKs. Low immunotoxicity was observed for Cremophor-EL, a well-known low molecular weight surfactant that is used in several commercial preparations, although it is known for inducing hypersensitivity reactions and peripheral neuropathy in vivo.14 No cytokine secretions were induced by the free polymers (PEG or PCB) at various tested concentrations. As an extension from these results, it is clear that each nanoparticle should be evaluated per se, as controls of homopolymers may not be comparable in free and assembled states. It is also evident that steric stabilization is critical in minimizing the toxicity of nanoparticles.
Nanoparticles that had lower in vitro immunotoxicity were correlated with longer blood circulation time and lower clearance in the immune organs (for instance PEG5k-SCKs vs. PCB5k-SCKs, the detailed information is available in reference 13). The PCB2k-SCKs had a similar or slightly better biodistribution profile than the PEG2k analog, which was also correlated with the higher immunotoxicity of PEG2k-SCKs, as compared to PCB2k analogs. Higher adsorption of proteins on PCB polymers and nanoparticles was also observed, as compared to PEGylated nanoparticles. Comparing the type of grafted polymer coating for the PEG5k- and PCB5k-SCKs is reasonable, due to the similar physicochemical characteristics (size and zeta-potential values). However, SCKs coated with the 2 kDa PCB had three times the size of PEG2k-coated nanoparticles. Hence, the differences in immunotoxicity and characteristics observed for this particular type of nanoparticle (i.e. coated with the 2 kDa PCB chains) might be attributed to the size differences of the nanoparticles rather than the type of polymeric coating. It was also observed that SCKs coated with the 2 kDa PEG resulted in massive release of most of the tested cytokines, as compared to PEG5k-SCKs, which corroborate the better pharmacokinetic profiles of the nanoparticles coated with PEG of higher molecular weights (the detailed information is available in reference 13), and with the better shielding provided by those polymers as indicated by the almost neutral zeta-potential values, as compared to the negatively-charged surface of nanoparticles coated with the shorter PEG polymeric chains, and the uncoated nanoparticles.
In vivo, the PCB-coated nanoparticles resulted in secretions of higher amounts of cytokines as compared to the PEG-coated nanoparticles, whereas, Cremophor-EL surfactant had slightly higher immunotoxicity, as compared to PCB-based nanoparticles, after injection in mice at a dose of 4 mg/kg (3 h post-injection). Hence, cytokines might be useful as biomarkers for nanoparticle immunotoxicity as well as for partial prediction of in vivo pharmacokinetics and biodistribution profiles.
As a summary to the analyses of the effects of nanoparticle surface chemistry, the high immunotoxicity of charged nanoparticles could be reduced but not eliminated by grafting with hydrophilic neutral or zwitterionic polymers that, as homopolymers, have negligible immunotoxicity. Modification with other functionalities, such as DOTA and tyramine, without polymer grafts further aggravated the immune response to the particles. For this particular type of nanoparticle, PEG-coated nanoparticles appeared to be promising as a drug delivery vehicle with low immunotoxicity both in vitro and in vivo and with better pharmacokinetic profile, in comparison to PCB-coated analogs. In vitro release of cytokines worked as a partial predictor of the in vivo pharmacokinetics of nanoparticles, and depended on the types of shell coating, shielding efficiency provided to the core, and size of the nanoparticles. In general, the immunotoxicity index is usually lower for in vivo data, as compared to data obtained from in vitro cell-based assays, which might be explained by the different environments, and the dilution of the nanoparticles upon intravenous injection. Worth mentioning is that Figures 3 and 4 summarize ca. 60 figures and ca. 190 values (when the data are presented in individual figures for each cytokine) that could be replaced with 11 descriptive values in the two figures (when data are presented in terms of immunotoxicity indices). In addition, these values are in agreement with the conclusions from the corresponding published data,8 without any need for repetition of the experiments, but rather through applying simple formula for calculating the immunotoxicity indices of the various tested materials.
Figure 3.
Induction of mouse cytokines, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (P40), IL-12 (P70), IL-13, IL-17, eotaxin, G-CSF, GM-CSF, IFN-γ, KC, MCP-1, MIP-1α, MIP-1β, RANTES and TNF-α following the treatment of RAW 264.7 cells with various polymeric materials. The cytokine induction is expressed by the immunotoxicity index for the polymeric materials tested at a concentration of 500 μg/mL after 24 h incubation with the cells.
Figure 4.
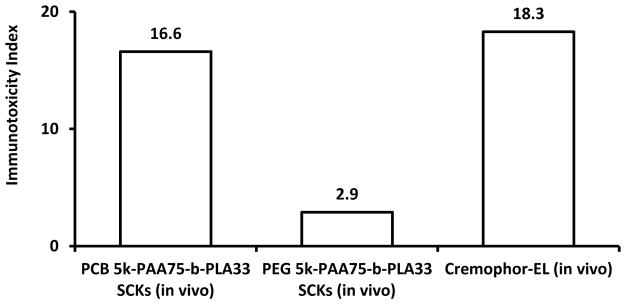
Expression of 23-mouse cytokines in serum of mice treated with PEG5k- or PCB5k-based SCKs and Cremophor-EL at 4 mg/kg as expressed by the immunotoxicity index (3 h post-injection).
3. Effect of Surface charge, Crosslinking and Biodegradability
Although the surface chemistry, degree of crosslinking and rate of degradation of nanomaterials are able to be varied according to the chemical compositions and structures, and there are attempts to tune those characteristics to meet the needs of cargoes to be delivered and therapeutic applications, it remains an unmet challenge to accurately study the nanomaterial properties, as a function of time, especially in complex biological media.15–17 Nonetheless, it is important to study the effects of these structural modifications, at least taking into consideration the initial starting point (which can be accurately characterized) on the toxicity of nanomaterials. According to recent literature data, and as discussed in this section, it is clear that nanoparticles having the collective properties of cationic surface, non-crosslinked unimers and non-degradable polymers/crosslinkers potentiate higher interactions with the biological components and induce higher cyto-/immuno-toxicities.
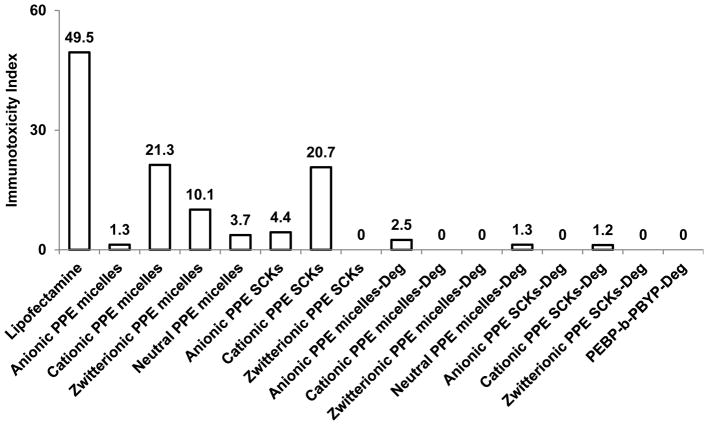
In one attempt to synthesize biodegradable nanoparticles with control over their surface chemistry (non-ionic, anionic, cationic, and zwitterionic) and with a possibility of crosslinking, polyphosphoester (PPE)-based micelles and crosslinked nanoparticles were prepared using a quick and efficient synthetic strategy.18 The immunotoxicities of the PPE micelles, SCKs and their degradation products were studied at a concentration of 5 μg/mL, using the same method described in the previous section.17 The PPE nanoparticles of various surface charges, crosslinking extents and their degradation products have demonstrated high compatibility with RAW 264.7 macrophages as indicated by low levels of the secreted proinflammatory cytokines, as compared to Lipofectamine, a commercially available transfection agent that is commonly utilized for delivery of nucleic acids.
When evaluated by the number of induced cytokines, degradation products of the micelles, SCKs and the poly(2-ethylbutyl phospholane)-block-poly(butynyl phospholane) (PEBP-b-PBYP) precursor induced minimal immunotoxicities. For the intact micelles, the immunotoxicity was ranked as cationic micelles (13 cytokines) > zwitterionic (7 cytokines) > neutral (3 cytokines) > anionic micelles (1 cytokine), while for the SCKs, the immunotoxicity was ranked as cationic SCKs (12 cytokines) > anionic SCKs (3 cytokines) > zwitterionic SCKs (no cytokine induction) (due to the synthetic chemistry approach employed, there was no neutral SCK). Comparing the immunotoxicity of nanoparticles based on the immunotoxicity index rather than the number of induced cytokines demonstrated the same pattern of ranking with a single numerical value for each of the tested nanoparticles (Figure 5). Furthermore, it could provide predictable values for comparing the immunotoxicity. For instance, the immunotoxicity index of cationic micelles (highest immunotoxicity among the tested formulations) was less than half of the value of Lipofectamine (21.3 vs. 49.5).
Figure 5.
Induction of the 23 mouse cytokines following the treatment of RAW 264.7 cells with PPE-micelles and SCKs of various surface charges and their degradation products. The cytokine induction is expressed by the immunotoxicity index for the polymeric materials tested at a concentration of 5 μg/mL after 24 h incubation with the cells.
Cationic nanoparticles, even when crosslinked and made up of degradable precursors, induced higher release of cytokines, as compared to nanoparticles of other surface charges, which is well-reported in the literature, due to their greater capacity to interact with the membranes of cells and with other biological (macro)molecules.19–21 Crosslinking zwitterionic micelles to afford the zwitterionic SCKs significantly reduced their immunotoxicities. In vitro degradation data have demonstrated a superior stability of the anionic SCKs as compared to the anionic micelles. The increased immunotoxicity of anionic micelles upon crosslinking might be due to the higher stability of the crosslinked micelles towards producing the degradation products that induce minimal toxicity. It is important that the intact nanoparticles and their degradation products be studied independently, as illustrated in the data summarized in Figure 5, and in other reports. For instance, a degradation product from the cationic degradable PPE-based nanoparticles imparted an anti-inflammatory character serendipitously, by efficiently inhibiting the transcription of inducible nitric oxide synthase and preventing the overproduction of nitric oxide, although the nanoparticles were not loaded with any specific therapeutic drug.22
Non-degradable poly(acrylic acid)-b-polystyrene (PAA-b-PS) micelles were prepared and subsequently transformed into SCKs by crosslinking the shell domains of the micelles via amidation chemistry with nominal crosslinking of 10% and 30%, for delivery of cisplatin.23 The effect of crosslinking on the immunotoxicity of nanoparticles was tested using the same method described previously. Nanoparticles either free or loaded with cisplatin were not immunotoxic at low concentration (ca. 0.08 μg/mL), whereas high secretion of cytokines was observed for both micelles and SCKs at a concentration of 32 μg/mL (Figure 6). According to the method of analysis used in the study at that time, there was an increase in immunotoxicity upon increasing the degree of crosslinking. After reanalyzing the data and calculating the immunotoxicity index, it was found that the immunotoxicity of the micelles was reduced upon increasing the degree of crosslinking.
Figure 6.
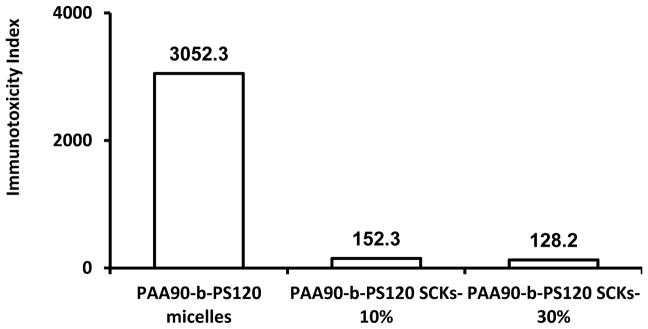
Induction of the 23 mouse cytokines following the treatment of RAW 264.7 cells with PAA-b-PS micelles and SCKs of varying crosslinking degrees. The cytokine induction is expressed by the immunotoxicity index for the polymeric materials tested at a concentration of 32 μg/mL after 24 h incubation with the cells.
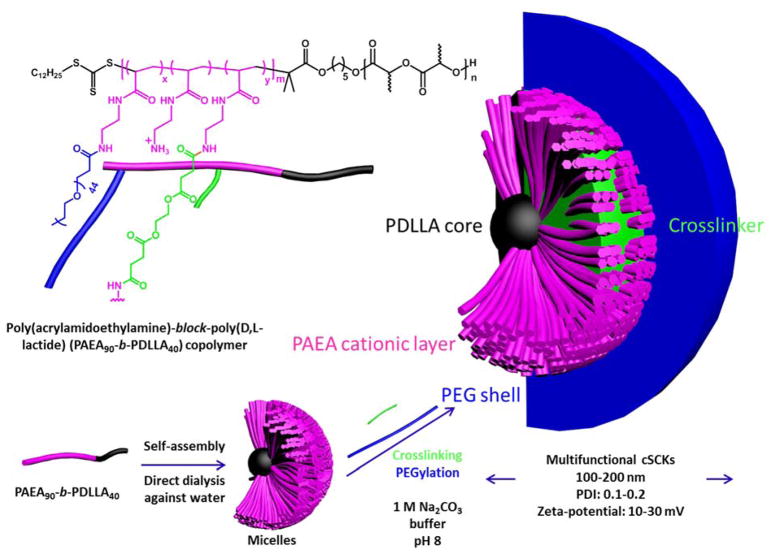
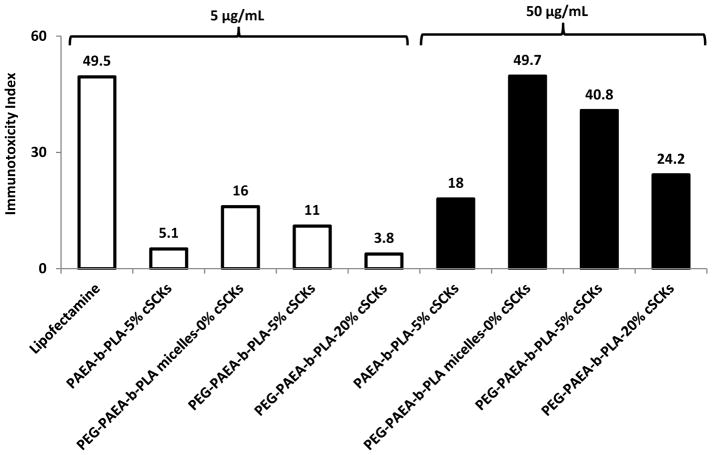
In another study, comparisons of the effects of PEGylation side-by-side to crosslinking degrees were investigated. Poly(acrylamidoethylamine)-block-poly(DL-lactide) (PAEA90-b-PDLLA40) copolymers were synthesized and self-assembled in water to yield micelles (Figure 7).24 The effects of grafting PEG on the micelle surface and the degree of crosslinking of the PAEA layer on the immunotoxicity were studied. Lipofectamine induced significantly higher expression of almost all the tested cytokines as compared to the cationic SCKs (cSCKs), PEGylated or not (Figure 8). The general conclusion after calculating the immunotoxicity index is that both PEGylation and crosslinking are important to reduce the immunotoxicity of nanoparticles. Although there could be several contributing factors, the reduced immunotoxicity is expected to be mainly due to the limitations of the flexibility of the shell with crosslinking, and the hydrophilic layer that surrounds the cationic nanoparticles, which both hinder the nanoparticles from rapid dissociation and interaction with the surrounding biomolecules. Increasing the concentration of nanoparticles, regardless of the degree of crosslinking, was generally associated with enhanced levels of the secreted cytokines, which is in agreement with dose-dependent immunotoxicity of nanomaterials.25,26
Figure 7.
Schematic illustration for the preparation of the micelles and cSCKs via the self-assembly of the amphiphilic diblock copolymer PAEA90-b-PDLLA40, followed by crosslinking and/or PEGylation, including the overall chemical structure showing the polymer backbone with the possibilities for conjugation with PEG and the crosslinker, where m = 90, n = 40, and x and y are determined by the particular sample: 5%-cSCK x = 0, y = 0.05; PEG-micelles x = 0.05, y = 0; PEG-5%-cSCKs x = 0.05, y = 0.05; and PEG-20%-cSCKs x = 0.05, y = 0.2. Reproduced with permission from ref. 24. Copyright (2013) The Royal Society of Chemistry.
Figure 8.
Induction of the 23 mouse cytokines following the treatment of RAW 264.7 cells with Lipofectamine, 5% crosslinked non-PEGylated cSCKs, non-crosslinked PEG-micelles, 5%- and 20%-crosslinked PEG-SCKs at 5 μg/mL (white bars) and 50 μg/mL (black bars). The cytokine induction is expressed by the immunotoxicity index for the polymeric materials tested at concentrations of 5 and 50 μg/mL after 24 h incubation with the cells.
4. Effect of Structural Modifications Along the Polymer Backbone
For delivery of nucleic acids, cSCKs with polystyrene core and poly(acrylamidoethylamine) (PAEA) shell were prepared for efficient electrostatic complexation with negatively-charged nucleic acids.27 Incorporation of varying amounts of the low- and high-pKa histamine and primary amines into the shell was performed to test the effect on toxicity and transfection efficiency of the resulting nanoparticles. Histamine was incorporated aiming at increasing the buffering capacity of the nanoparticles and, thus, enhancing their transfection efficiency. The immunotoxicities of the 0%- and 15%-histamine-cSCKs were studied by measuring the levels of 23 cytokines upon treatment of RAW 264.7 mouse macrophages with the nanoparticles for 24 h. Generally, lower secretion of the cytokines was observed from cells treated with the histamine-modified cSCKs, as compared to the cSCKs with 100% primary amines. A similar trend was observed for most of the tested cytokines, which indicate the potential immunotoxicity of the nanoparticles with higher primary amine density (i.e. 0%-His-cSCKs). The incorporation of histamine into the polymer precursor significantly reduced the cytotoxicity of the nanoparticles, as indicated by the higher IC50 value of the 15%-His-cSCKs as compared to that of the 0%-His-cSCKs (20.7 vs. 16.3 μg/mL, p < 0.05). The reduced cyto- and immuno-toxicity of the histamine-modified cSCKs might be due to their limited ability to interact with the cell membrane and stimulate the secretions of cytokines, which might result from the lower charge density along the cationic polymer backbone.
5. Effect of Morphology and Control of Polymeric Nanostructures via Supramolecular Assembly
Few studies have focused on the relationship between the nanoparticle shape and the cytokine release pattern either in vitro or in vivo, which is challenging as it depends on several variables, inncluding the type of cell line and animal model. For instance, in comparison of spherical and sheet-like zinc oxide nanoparticles of approximately similar specific surface area, the spherical nanoparticles induced higher and lower release of TNF-α in RAW 264.7 mouse macrophages and in mouse primary dendritic cells, as compared to the sheet-like nanoparticles, respectively.28
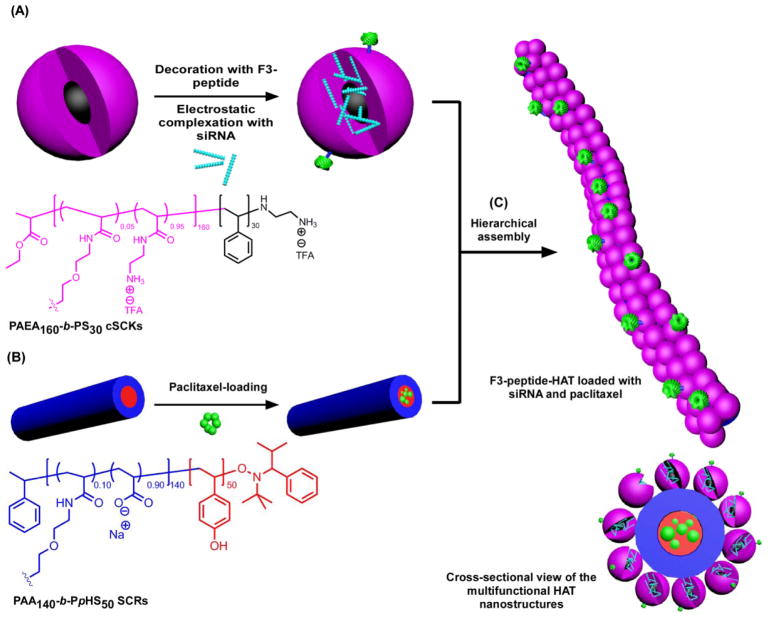
Multifunctional hierarchically assembled theranostic (HAT) nanostructures were previously constructed via templating cationic spherical nanoparticles (cSCKs) onto anionic shell cross-linked rod-like nanoparticles (SCRs) (Figure 9).29,30 The functionalities of these HAT nanostructures include a cationic surface for complexation of nucleic acids (siRNA) and surface decoration with active targeting moieties (F3-tumor homing peptide), anionic cylinders for solubilization of hydrophobic anticancer drugs (paclitaxel) in their cores, together with sites for radiolabeling, and elongated morphologies for potential prolongation in the blood circulation time after in vivo administration. Although beyond the scope of this account, these sophisticated polymeric structures were found to have a unique intracellular trafficking mechanism “stick and trigger” via binding to cell surfaces with the cationic spheres followed by intracellular release of these spheres while the cylindrical parts remained near the cell surface, which allowed for exceptional transfection efficiency.
Figure 9.
Construction of HAT nanostructures as a template for the co-delivery of siRNA and paclitaxel: (A) Electrostatic complexation of PAEA160-b-PS30 cSCKs and nucleic acids (e.g. siRNA) and decoration of the surface of nanoparticles with targeting ligands (e.g. F3 peptides); (B) Loading of hydrophobic drugs (e.g. paclitaxel) into SCRs composed of PAA140-b-PpHS50. (C) Hierarchical-assembly of cSCKs and SCRs to form the HAT nanoassemblies and the cross-sectional view of the multifunctional HAT nanostructures. Reproduced with permission from ref. 29. Copyright (2013) American Chemical Society.
The immunotoxicities of cSCKs and HATs (each at 5 μg/mL), and anionic cylinders (0.5 μg/mL) were studied by the same method described previously. Generally, the highest secretion of cytokines was observed from cells treated with the cylinders, followed by cSCKs, whereas the HATs were found to be relatively the least immunotoxic (Figure 10). The strong induction of cytokine release from cells upon treatment with anionic cylinders (0.5 μg/mL) was surprising and could be explained by similarities to bacterial dimensions and surface properties.31 Increase in the release of cytokines from cells treated with the cylinders was also observed upon increasing the concentration of the nanoparticles. Spherical cationic nanoparticles usually induce the secretions of high amounts of cytokines due to their non-specific binding to cell membranes, and, also probably due to their similarities to viral dimensions.31 Binding of these cationic spheres onto the surface of the anionic cylinders (i.e. HAT nanostructures) reduced the release of the cytokines, perhaps due to two main reasons. First, lowering the charge density and limiting the free movement of the cationic nanoparticles afforded lower possibilities of interactions between nanoparticles and the cell membranes and biomolecules in the medium. Second, masking the bacterial-like properties of the negatively charged anionic cylinders reduced the immunostimulatory effects of the unbound anionic cylinders. The lower immunotoxicity of HATs was associated with higher efficiency in siRNA binding and transfection efficiency, as compared to the cSCKs,29,30 in addition to their potential ability to carry more than one cargo and extend the blood circulation time in vivo.32 The calculation of the immunotoxicity index was in accordance with the conclusions of this study.
Figure 10.
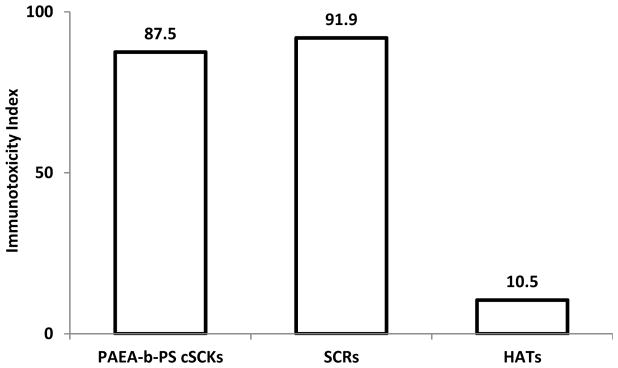
Induction of the 23 mouse cytokines following the treatment of RAW 264.7 cells with cSCKs (5 μg/mL), SCRs (0.5 μg/mL) or HATs (5 μg/mL) for 24 h. The cytokine induction is expressed by the immunotoxicity index for the SCRs (0.5 μg/mL) and cSCKs and HATs (5 μg/mL) after 24 h incubation with the cells.
6. Concluding Remarks
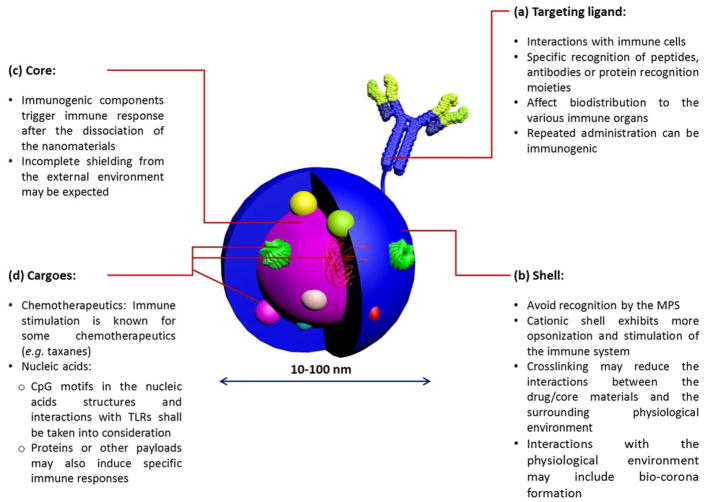
Immunotoxicity of nanomaterials must be evaluated on an individual basis, as it depends on the overall composition of the nanoparticle, in addition to the embedded cargoes and moieties utilized to decorate the surface of nanoparticles (Figures 11 and 12). However, it is advisable based on the collected data to construct nanoparticles from degradable precursors and to impart nanoparticles with greater kinetic stability via crosslinking some of their components at a degree that will need to be optimized for each type of nanoparticle. Shielding the core of nanomaterials with a neutral layer of PEG continues to be the most efficient way to prepare stealth nanoparticles with minimal immunotoxicity, although the safety of PEGylated biotherapeutics remains controversial. The length of the polymer grafts that comprise the hydrophilic shell and degree of crosslinking are among the most important parameters to be controlled precisely. Morphology and surface chemistry appear to also contribute significantly to the capacity of nanomaterials to interact with the surrounding biomolecules in the biological environment. Future studies should be designed to take into consideration effects of overall particle mechanical properties, which may also be a contributing factor to immunotoxicity and other biological responses. Such studies would require that the particle size, shape and surface chemistry be held constant, while only altering the particle rigidity vs. flexibility.
Figure 11.
The general composition of a multifunctional nanoparticle for biomedical delivery applications is illustrated with highlighting some important considerations for the design of nanoparticles of low immunogenicity. Reproduced with permission from ref. 3. Copyright (2013) The Royal Society of Chemistry.
Figure 12.
Summary of the important features that should be considered for the future design of clinical nanopharmaceutical products.
In some cases, in vivo administration of even low doses of nanoparticles may induce hypersensitivity reactions and biochemical changes through “complement activation-related pseudoallergy”.33 There are also several attempts to delay the detection of nanomaterials by the immune system. For instance, Discher and coworkers have designed nanobeads decorated with CD47 peptide as a “marker of self” to mimic “self” cells, and, thus avoid recognition by the immune system.34 Upon intravenous injection, the attached peptide delayed the clearance and prolonged the blood circulation time of the nanoparticles and allowed for higher accumulation in the tumor tissues of treated mice.
Finally, immunotoxicity index appears as a descriptive numerical value that can be utilized to partially predict and simply compare the immunotoxicity of various biomolecular products. The most attractive feature is that from one numerical value (immunotoxicity index), to a great extent, the levels of immunotoxicities of several nanomaterials could be expressed and compared (as long as the measurements were conducted at equivalent concentrations). At the time when these papers were published, immunotoxicity index was not available and the explanation of the data was much more complicated. We expect a much easier understanding and corroboration of experimental data in the future, and, thus the information summarized in this account will provide useful guidelines for the design of clinically viable nanopharmaceutical products of high efficiency and low toxicity and immunogenicity.
Acknowledgments
We greatly appreciate the dissertation efforts of Ritu Shrestha, Sandani Samarajeewa, Shiyi Zhang, Fuwu Zhang, and Ang Li, which provided nanoparticle materials having various structures, compositions, shapes and surface chemistries, and experiments by Stephanie F. Pollack and collaborative interactions with Professor Yongjian Liu, each of which contributed to an important database for understanding the behavior of nanomaterials and a guide for constructing biocompatible nanoparticles. We gratefully acknowledge financial support from the National Heart Lung and Blood Institute of the National Institutes of Health as a Program of Excellence in Nanotechnology (HHSN268201000046C), the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01-DK082546) and the National Science Foundation (DMR-1105304). The Welch Foundation is gratefully acknowledged for support through the W. T. Doherty-Welch Chair in Chemistry, Grant No. A-0001. Financial support from the Egyptian Ministry of Scientific Research – Science and Technology Development Fund (Demand-driven Project 5688 and Reintegration Grant 5362), and the Ministry of Higher Education (CEP2-007-ASSU) are gratefully acknowledged (M.E.).
Biographies
Mahmoud Elsabahy is Director of the Assiut International Center of Nanomedicine at Alrajhy Liver Hospital, and Assistant Professor at the Department of Pharmaceutics, Faculty of Pharmacy, Assiut University, Egypt, and Assistant Director of the Laboratory for Synthetic-Biologic Interactions at the Department of Chemistry, Texas A&M University, Texas, USA. He received a B.S. in Pharmaceutical Sciences from Assiut University, and then he finished his M.Sc. and Ph.D. degrees from the Faculty of Pharmacy, University of Montreal (Canada). Research interests include the rational design and evaluation of pharmaceutical nanocarriers for delivery of therapeutics and diagnostics via various routes of administrations, for which he has received several prestigious awards. He is one of the members of the Senior Professors Council at Misr University for Science and Technology, Egypt. He is also one of the first 15 official members of the national “Egyptian Young Academy of Science” (Academy of Scientific Research and Technology, Ministry of Scientific Research, Egypt).
Karen L. Wooley received a B.S. in Chemistry from Oregon State University in 1988 and a Ph.D. in polymer/organic chemistry from Cornell University in 1993. She began as an Assistant Professor at Washington University in St. Louis in 1993, was promoted in 1999 to Full Professor, and was installed as a James S. McDonnell Distinguished University Professor in Arts & Sciences in 2006. In 2009, Karen relocated to Texas A&M University, undertaking a position as the W. T. Doherty-Welch Chair in Chemistry, and being awarded the title of University Distinguished Professor in 2011. Research areas include the synthesis and characterization of degradable polymers, unique macromolecular architectures and complex polymer assemblies, and the design and development of well-defined nanostructured materials, for which she has received several awards, including the 2014 American Chemical Society Award in Polymer Chemistry, a 2014 Royal Society of Chemistry Centenary Prize, and the 2015 Oesper Award. Karen serves as an Associate Editor for the Journal of American Chemical Society.
References
- 1.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 2.Dobrovolskaia MA, Germolec DR, Weaver JL. Evaluation of nanoparticle immunotoxicity. Nat Nanotechnol. 2009;4:411–414. doi: 10.1038/nnano.2009.175. [DOI] [PubMed] [Google Scholar]
- 3.Elsabahy M, Wooley KL. Cytokines as biomarkers of nanoparticle immunotoxicity. Chem Soc Rev. 2013;42:5552–5576. doi: 10.1039/c3cs60064e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172:38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Tan Y, Li S, Pitt BR, Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum Gene Ther. 1999;10:2153–2161. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 6.Breslin S. Cytokine-release syndrome: overview and nursing implications. Clinical journal of oncology nursing. 2007;11:37–42. doi: 10.1188/07.CJON.S1.37-42. [DOI] [PubMed] [Google Scholar]
- 7.Schneider CK, Kalinke U, Lower J. TGN1412--a regulator’s perspective. Nat Biotechnol. 2006;24:493–496. doi: 10.1038/nbt0506-493. [DOI] [PubMed] [Google Scholar]
- 8.Elsabahy M, Li A, Zhang F, Sultan D, Liu Y, Wooley KL. Differential immunotoxicities of poly(ethylene glycol)- vspoly(carboxybetaine)-coated nanoparticles. J Control Release. 2013;172:641–652. doi: 10.1016/j.jconrel.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin W, Ma G, Ji F, Zhang J, Wang L, Suna H, Chen S. Biocompatible long-circulating star carboxybetaine polymers. J Mater Chem B. 2015;3:440–448. doi: 10.1039/c4tb01477d. [DOI] [PubMed] [Google Scholar]
- 10.Dwivedi PD, Tripathi A, Ansari KM, Shanker R, Das M. Impact of nanoparticles on the immune system. J Biomed Nanotechnol. 2011;7:193–194. doi: 10.1166/jbn.2011.1264. [DOI] [PubMed] [Google Scholar]
- 11.Hussain S, Vanoirbeek JA, Hoet PH. Interactions of nanomaterials with the immune system. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;2:169–183. doi: 10.1002/wnan.166. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li A, Luehmann HP, Sun G, Samarajeewa S, Zou J, Zhang S, Zhang F, Welch MJ, Liu Y, Wooley KL. Synthesis and in vivo pharmacokinetic evaluation of degradable shell cross-linked polymer nanoparticles with poly(carboxybetaine) versus poly(ethylene glycol) surface-grafted coatings. ACS Nano. 2012;6:8970–8982. doi: 10.1021/nn303030t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–685. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 15.Zou J, Zhang F, Zhang S, Pollack SF, Elsabahy M, Fan J, Wooley KL. Poly(ethylene oxide)-block-polyphosphoester-graft-paclitaxel conjugates with acid-labile linkages as a pH-sensitive and functional nanoscopic platform for paclitaxel delivery. Advanced healthcare materials. 2014;3:441–448. doi: 10.1002/adhm.201300235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samarajeewa S, Ibricevic A, Gunsten SP, Shrestha R, Elsabahy M, Brody SL, Wooley KL. Degradable cationic shell cross-linked knedel-like nanoparticles: Synthesis, degradation, nucleic acid binding, and in vitro evaluation. Biomacromolecules. 2013;14:1018–1027. doi: 10.1021/bm3018774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elsabahy M, Zhang S, Zhang F, Deng ZJ, Lim YH, Wang H, Parsamian P, Hammond PT, Wooley KL. Surface charges and shell crosslinks each play significant roles in mediating degradation, biofouling, cytotoxicity and immunotoxicity for polyphosphoester-based nanoparticles. Sci Rep. 2013;3:3313. doi: 10.1038/srep03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Zou J, Zhang F, Elsabahy M, Felder SE, Zhu J, Pochan DJ, Wooley KL. Rapid and versatile construction of diverse and functional nanostructures derived from a polyphosphoester-based biomimetic block copolymer system. J Am Chem Soc. 2012;134:18467–18474. doi: 10.1021/ja309037m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang TL, Aljuffali IA, Lin CF, Chang YT, Fang JY. Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: lipid nanoparticles versus polymeric nanoparticles. Int J Nanomedicine. 2015;10:371–385. doi: 10.2147/IJN.S73017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novo L, Takeda KM, Petteta T, Dakwar GR, van den Dikkenberg JB, Remaut K, Braeckmans K, van Nostrum CF, Mastrobattista E, Hennink WE. Targeted Decationized Polyplexes for siRNA Delivery. Mol Pharm. 2015;12:150–161. doi: 10.1021/mp500499x. [DOI] [PubMed] [Google Scholar]
- 21.Novo L, Rizzo LY, Golombek SK, Dakwar GR, Lou B, Remaut K, Mastrobattista E, van Nostrum CF, Jahnen-Dechent W, Kiessling F, Braeckmans K, Lammers T, Hennink WE. Decationized polyplexes as stable and safe carrier systems for improved biodistribution in systemic gene therapy. J Control Release. 2014;195:162–175. doi: 10.1016/j.jconrel.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen Y, Zhang S, Zhang F, Pavia-Sanders A, Fan J, Taylor JSA, Wooley KL. Polyphosphoester-based cationic nanoparticles serendipitously release integral biologically-active components to serve as novel degradable inducible nitric oxide synthase inhibitors. Adv Mater. 2013;25:5609–5614. doi: 10.1002/adma.201302842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Elsabahy M, Zhang S, Lin LY, Zou J, Wooley KL. Shell crosslinked knedel-like nanoparticles for delivery of cisplatin: effects of crosslinking. Nanoscale. 2013;5:3220–3225. doi: 10.1039/c3nr34320k. [DOI] [PubMed] [Google Scholar]
- 24.Elsabahy M, Samarajeewa S, Raymond JE, Clark C, Wooley KL. Shell-crosslinked knedel-like nanoparticles induce lower immunotoxicity than their non-crosslinked analogs. J Mater Chem B. 2013;1:5241–5255. doi: 10.1039/C3TB20668H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J Invest Dermatol. 2007;127:143–153. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142:416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shrestha R, Elsabahy M, Florez-Malaver S, Samarajeewa S, Wooley KL. Endosomal escape and siRNA delivery with cationic shell crosslinked knedel-like nanoparticles with tunable buffering capacities. Biomaterials. 2012;33:8557–8568. doi: 10.1016/j.biomaterials.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heng BC, Zhao X, Tan EC, Khamis N, Assodani A, Xiong S, Ruedl C, Ng KW, Loo JS. Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles. Arch Toxicol. 2011;85:1517–1528. doi: 10.1007/s00204-011-0722-1. [DOI] [PubMed] [Google Scholar]
- 29.Elsabahy M, Shrestha R, Clark C, Taylor S, Leonard J, Wooley KL. Multifunctional hierarchically assembled nanostructures as complex stage-wise dual-delivery systems for coincidental yet differential trafficking of siRNA and paclitaxel. Nano Lett. 2013;13:2172–2181. doi: 10.1021/nl4006645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha R, Elsabahy M, Luehmann H, Samarajeewa S, Florez-Malaver S, Lee NS, Welch MJ, Liu Y, Wooley KL. Hierarchically Assembled Theranostic Nanostructures for siRNA Delivery and Imaging Applications. J Am Chem Soc. 2012;134:17362–17365. doi: 10.1021/ja306616n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rettig L, Haen SP, Bittermann AG, von Boehmer L, Curioni A, Kramer SD, Knuth A, Pascolo S. Particle size and activation threshold: a new dimension of danger signaling. Blood. 2010;115:4533–4541. doi: 10.1182/blood-2009-11-247817. [DOI] [PubMed] [Google Scholar]
- 32.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szebeni J, Bedocs P, Csukas D, Rosivall L, Bunger R, Urbanics R. A porcine model of complement-mediated infusion reactions to drug carrier nanosystems and other medicines. Adv Drug Deliv Rev. 2012;64:1706–1716. doi: 10.1016/j.addr.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “Self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]