1. Preface
1.1. Structure and Membership of the Writing Committee
Members of the Writing Committee included experienced clinicians and specialists in cardiology, cardiac rehabilitation, quality improvement, outcomes research, epidemiology, and performance measures (PMs) methodology, as well as patient advocates. The Writing Committee also included representatives from the American Association for Cardiovascular and Pulmonary Rehabilitation (AACVPR), the American Academy of Family Physicians (AAFP), the American Medical Association–Physician Consortium for Performance Improvement (AMA-PCPI), the American Nurses Association (ANA), the American Society of Health-System Pharmacists (ASHP), the National Committee for Quality Assurance (NCQA), and Mended Hearts, Inc.
1.2. Disclosure of Relationships With Industry
The American College of Cardiology (ACC)/American Heart Association (AHA) Task Force on Performance Measures makes every effort to avoid actual, potential, or perceived conflicts of interest that may arise as a result of relationships with industry or other entities. The work of the Writing Committee was supported exclusively by the ACC and the AHA, without commercial support. The Writing Committee members volunteered their time. All members of the Writing Committee, as well as those selected to serve as peer reviewers of this document, were required to disclose all current relationships and those existing within the 12 months before the initiation of the project. It was also required that the Writing Committee co-chairs and at least 50% of the Writing Committee have no relevant relationships with industry or other entities. Because the Writing Committee is defining general principles, rather than making specific PM recommendations, members’ relationships with pharmaceutical and device companies were not considered relevant to the topic.
Author and peer reviewer relationships with industry and other entities relevant to the document are included in Appendices 1 and 2. Additionally, to ensure complete transparency, the writing committee members’ comprehensive disclosure information, including RWI not relevant to the present document, is available as an online supplement. Disclosure information for the Task force is available as a separate online supplement.
2. The Need for Shared Accountability
PMs have been useful for measuring the quality of care, promoting accountability for care, and improving the care and outcomes for patients with acute and chronic medical conditions.1,2 To date, the conceptualization of PMs has generally been “clinician focused,” developed to help define the quality of care delivered by clinicians (both individually and collectively); however, the ultimate goal of performance measurement and assessment is to improve patient outcomes, including health status (quality of life, symptom burden, and functional status), morbidity, and mortality. Patient participation and engagement are integral to the success of any treatment plan. Disease treatment and health promotion activities typically require action from multiple parties, including clinicians, the broader healthcare team, and the system in which health care is delivered, as well as patients, family members, caregivers, and community-based support services. It is clear that patients who are actively engaged in self-care, defined as the ability to perform the activities necessary to achieve, maintain, or promote optimal health, are more likely to successfully achieve their treatment goals.3
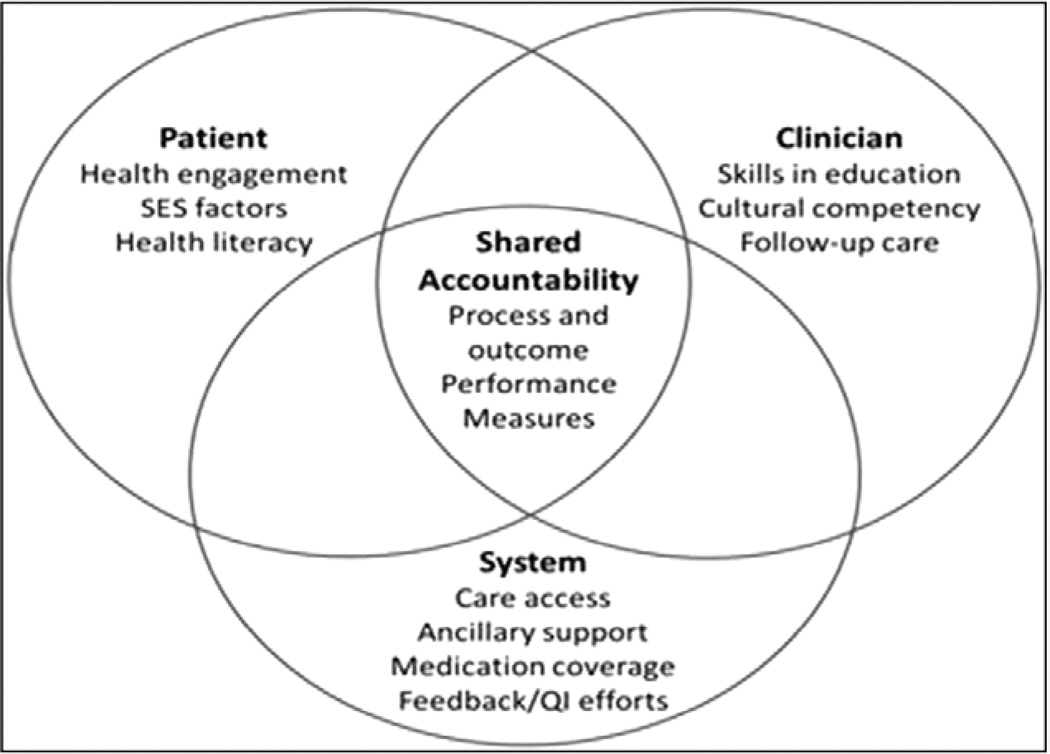
The Institute of Medicine has advocated for “shared accountability,” in which all stakeholders within the healthcare system and all members of the healthcare team(s), including the patient, are responsible for and contribute to the success of any measure.4 Underpinning this concept is recognition that the actions of clinicians and the patient are not independent but rather inextricably linked. Although the locus of control for treatment adherence and self-care is typically attributed to the patient, this can be influenced by patient factors (eg, health literacy, sociocultural factors, economic limitations), clinician factors (eg, skills in patient education, effective therapeutic communication, cultural competency, follow-up reinforcement), and healthcare system factors (eg, access to needed care, communication among caregivers, medication coverage, costs). These intricate interdependencies help illustrate the rationale for adopting the concept of shared accountability when PMs are under consideration (Figure 1). Note, here and throughout the document, the term “clinician” is meant to include not only physicians, but also the entire healthcare team (eg, nurses, pharmacists, physical therapists, social workers) and the systems of care in which the clinician works (eg, clinics, hospitals, health systems).
Figure 1.
The interdependencies of shared accountability in performance measurement. QI indicates quality improvement; and SES, socioeconomic status.
In the context of a shared PM, the concept of shared accountability must be defined, particularly as it relates to measure attribution. For many years, the nation’s quality and performance organizations have tracked clinician performance. Clinicians and hospitals have been held accountable for instituting evidence-based processes of care. Increasingly, PMs are being extended to evaluate whether patients follow prescribed care plans, as well as whether patients’ longitudinal health outcomes are improved. PMs are also increasingly being tied to important consequences, such as clinician ranking, reputation, and differential reimbursement.5 Nevertheless, the actions of clinicians can influence patients’ behavior and vice versa, and clinicians and patients together affect the outcome of the PM. These interdependencies are rarely incorporated into current PMs. The consequences of failing to meet the metrics of a PM are attributed to the clinician alone, even though patient behavior, system characteristics, and other healthcare team members contribute to success or failure.
This document will address the following issues related to shared-accountability PMs: 1) the definition, rationale, and scope; 2) examples of existing measures; 3) methodological challenges; 4) factors affecting feasibility; and 5) potential beneficial and adverse consequences.
3. Shared Accountability and PMs
3.1. General Overview
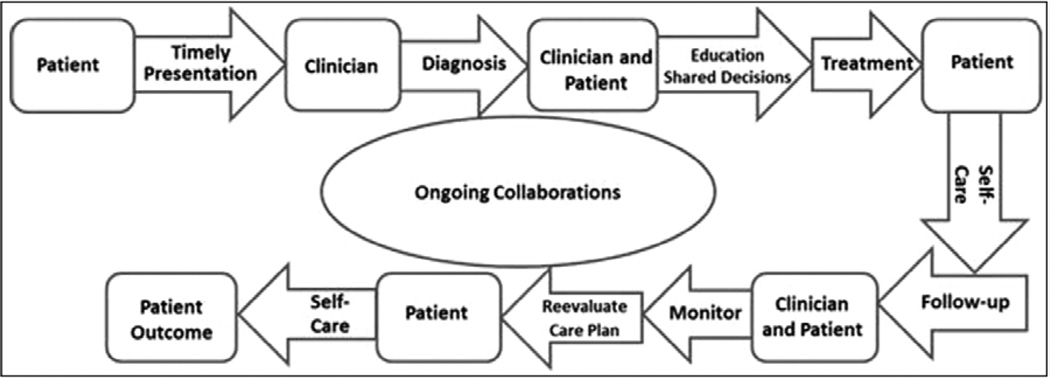
Figure 2 depicts the continuum of healthcare delivery.6 Clinicians must make the correct diagnosis, educate the patient on the diagnosis, engage the patient in jointly developing an appropriate treatment plan, monitor progress, and ensure the patient has appropriate support for self-care. Clinician follow-up should include assessing the patient’s response to treatment, making adjustments in the treatment plan as appropriate, and ensuring continuation of appropriate monitoring and support for self-care.
Figure 2.
A multistep framework of continuity of health care.6 Arrows represent the necessary steps in the continuity of care, ultimately leading to the patient’s outcome. Rounded boxes preceding the arrows indicate the person(s) responsible for each step. Adapted from Spertus et al.6
The general framework of shared accountability is predicated on partnerships between patients and clinicians, in which patients play an active role in setting goals, making treatment decisions, and assessing outcomes. Ideally, patients would be aware of what to watch for, contact their clinicians when symptoms arise, learn about their condition and what they can do to improve their health, implement agreed-on treatment plans and lifestyle changes, and follow up with their clinicians to assess outcomes and adjust the treatment plan. In this iterative process, the clinician, healthcare system, patient, and family members work together, with the end goal of improving patient-centered outcomes (symptoms, functional status, and quality of life), morbidity, and mortality. Clinicians and the healthcare system should facilitate this process by ensuring that patients have sufficient support and knowledge to actively participate in their health care. Key conceptual issues for shared accountability include 1) shared goal setting; 2) shared decision making; 3) shared care planning and monitoring, including patient feedback and self-care; and 4) assessment of patients’ longitudinal outcomes.
3.2. Examples of Shared-Accountability Measures
In this section, we provide a few examples of current PMs and then discuss how the concept of shared accountability can be incorporated or made more explicit.
3.2.1. Longitudinal Process Adherence
Traditionally, process PMs have focused on acute conditions and have been cross sectional, measuring care delivered at a point in time or over a relatively short period of time. However, it is increasingly recognized that a longitudinal timeline should be considered for PMs. The reasons for this are 2-fold. To be effective, most treatments need to be delivered consistently over time. Additionally, we have a growing body of evidence that longitudinal treatment adherence is quite poor. For example, although beta blockers are prescribed for 95% of patients at hospital discharge after an acute myocardial infarction, almost one half of patients will no longer be adherent to beta blockers by 6 months, even among employed populations with medication coverage.7
Achieving (or failing to achieve) longitudinal medication adherence is a shared responsibility. The discharging clinician should discuss with patients their preferences for treatment, prescribe the appropriate medications, and educate patients about the effects and side effects of the medication and the importance of medication adherence. The patient should fill the prescription, take the medication as prescribed, and call the clinician if adverse effects occur. In addition, at follow-up, the outpatient clinician must review these prescribed medications and the patient’s experience with taking the medications and make appropriate modifications that reflect up-to-date evidence and clinical practice guidelines, as well as the patient’s experience with the medication. Clinicians, family members, and caregivers can all play important roles in understanding and supporting medication adherence. These activities are not performed in isolation. Each of these parties can indirectly influence the others, which may increase or decrease longitudinal adherence. To increase the likelihood of adherence to medication prescribed at hospital discharge, a clinician can select an affordable medication or one specifically covered by the patient’s formulary. Additionally, both inpatient and outpatient clinicians should provide patients and their caregivers with sufficient information on why adherence is important and should query them about their concerns and any real or potential barriers to adherence at initial prescription and at each patient encounter.
3.2.2. Intermediate Patient Outcome Metrics — Reaching Target Goals (Blood Pressure, Hemoglobin A1c)
PMs have traditionally been limited in scope to the evaluation of specific processes of care. Many assess whether a clinician prescribed a medication to treat a specific cardiac risk factor, without measuring whether the impact of the drug or target goal was achieved (eg, angiotensin-converting enzyme inhibitors for patients with left ventricular dysfunction). Some PMs address only whether an intermediate outcome was achieved. For treatment of hypertension, current PMs assess whether blood pressure (BP) was measured and whether BP goals were achieved (eg, BP <140/90 mmHg). This might be acceptable in a setting in which the BP goal was reached; however, in settings in which the BP goal was not achieved, it would be informative to have other process measures, such as treatment intensification or alteration by clinician(s) in response to elevated BP levels.8
In this example, the success of achieving the intermediate outcome is a shared responsibility, dependent on multiple parties. The patient first seeks care from a clinician to obtain a diagnosis of hypertension; the clinician must recognize when treatment is required and prescribe the appropriate medication. Then the patient purchases the medication, takes it as prescribed, and follows up with the clinician to convey his or her experience with taking the medication and to report benefits and any adverse side effects. This may involve home monitoring of BP to assess whether the BP is controlled throughout the day. If the first medication choice or dose titration does not achieve the desired goal, several iterations of this process will be required. If the patient is followed by several clinicians, each of whom could reasonably be considered responsible for treating BP, the plan of care may become unclear, and the patient may be at risk for poor BP control or adverse events. Success is then dependent on coordinated care by all of these clinicians.
Achieving BP control is just one of several proposed intermediate outcome measures. Tighter glucose control, as measured by hemoglobin A1c levels, is another important intermediate outcome. Weight loss and smoking cessation are other potential examples of intermediate outcome measures that require lifestyle modification, although to some patients, these may be important outcomes in themselves. At first consideration, weight loss may seem to be a treatment that is solely under a patient’s locus of control, but this too may be partially shared with a healthcare clinician. The clinician, in this example, could work jointly with the patient in setting a target weight goal and exploring the patient-specific difficulties in achieving the goal. The clinician could then take additional actions to support the patient in achieving this goal, such as referring the patient to a dietician; recommending exercise programs or peer support groups; and even considering psychological, pharmacological, or surgical interventions, as needed, to achieve the desired lifestyle modification.
3.2.3. Example of Shared Accountability for Clinical Events and Patient Outcomes
The emphasis of current outcome-focused performance metrics has been on assessing for the occurrence of specific clinical events (eg, death, hospital readmissions). The aim of health care is to improve patient outcomes, yet many factors, including patient behavior, can affect outcomes. An example of this interplay can be found in the Centers for Medicare and Medicaid Services’ hospital performance metric for readmission after acute myocardial infarction or heart failure. Although use of evidence-based therapies and best-practice discharge planning can reduce rates of hospital readmission,9 patient behavior is also potentially influential. For example, does the patient understand and have the capacity to follow the treatment plan, including self-care activities, such as taking prescribed medications, implementing recommended lifestyle changes, and attending scheduled clinic visits? Does the patient understand what early warning signs or symptoms to look for and when to promptly seek medical attention? Can the patient easily access appropriate clinical advice when needed? Clinical events attributed to individual clinicians are influenced positively or negatively by numerous factors, including patient behavior and the systems and supports that enable patients to effectively follow clinical recommendations and discharge plans.
4. Methodological Challenges
There are several important methodological considerations when both patient and clinician have shared accountability for PMs. These include designating the level of measurement, assigning patients to specific clinical care teams, specifying the episodes of care for longitudinal process measures and clinical outcomes, ensuring data validity, and applying appropriate risk-adjustment methodology.
4.1. Accountability and Attribution
4.1.1. Potential Levels of Aggregation
The level of aggregation should be carefully examined when shared accountability is considered for process and outcome PMs. Measures of clinician performance and outcomes may be reported at the level of individual clinicians, groups of practitioners organized into clinical practices or clinics, larger healthcare organizations (eg, provider groups, hospitals, healthcare delivery systems), health plans, employers, or communities. Similarly, metrics assessing patient performance (eg, adherence to medications) can be used to examine the individual’s behavior but could also be aggregated to the level of the healthcare plan or the employer. Such aggregation can assess the success of the organization or employer in achieving prevention goals for its enrollees or employees.
There are tradeoffs in selecting any particular level of attribution. Although measuring performance at an individual clinician level can have the most impact on individual patient and clinician behavior, such measurements can be unstable and unreliable because of small numbers of observations. Additionally, individual attribution does not account for the potential influence of the clinical environment. For example, if a clinic is poorly staffed or run, this could affect the quality of care provided by all of the clinicians working within that environment.
In contrast to individual-level measures, those aimed at evaluating a hospital or delivery system, such as an accountable care organization (ACO), can reflect care of the entire multidisciplinary team and its patients. This broader level of measurement can promote multidisciplinary, team-based, coordinated care and shared responsibility among healthcare clinicians. The challenge with organizational metrics is instilling a sense of ownership in all relevant individuals in the organization. An example is the National Database of Nursing Quality Indicators (www.nursingquality.org), which collects data at the hospital unit level to assess the functionality and quality of the team. This approach of using data to drive quality improvement is nonpunitive and gives the team ownership of the outcomes. Without this, clinicians might believe that any shortcomings are not their problem and must be the responsibility of “others.” Another challenge with this level of measurement is that it omits valuable information about the clinician-patient interaction, which can, in turn, influence patient experience, engagement, and outcomes.
4.1.2. Defining Patient Attribution
Regardless of the level of PM attribution selected, it is vital that the clinicians, clinics, larger healthcare organizations, health plans, employers, and communities involved be properly defined. Attribution is relatively straightforward when the level of assessment is the single clinician and the patient receives care from only that clinician; however, care from a single clinician is becoming somewhat rare in modern-day medical care. Attribution becomes more complex when a PM reflects patient care from more than one practitioner, medical group, or hospital system.10 In a recent study, it was found that within a single year, fee-for-service patients were seen by a median of 7 different physicians.11 Similarly, nearly half of Medicare patients change their primary care physician assignment over a 2-year period. Thus, the developers of clinician-level PMs must clearly define which patients are considered to be “within” a given clinician’s practice.
4.1.3. Defining Parties Responsible for a PM
One must also define and determine which party should take responsibility for which PMs. For example, it might not be appropriate to hold a subspecialist who sees a patient in consultation for a specific procedure responsible for that patient’s chronic prevention measures (eg, breast examination, diabetic retinal examination). Alternatively, multiple parties may be responsible for an outcome measure such as successful functional recovery after hip replacement surgery, which requires the collaboration of the surgeon, nurses, physical therapists, pharmacist, and social worker, as well as the cooperation and efforts of the patient to complete a rehabilitation program. The issue of attribution becomes less relevant when one is assessing the performance of the overall healthcare system.
In the Physician Quality Reporting System, the Centers for Medicare and Medicaid Services are evaluating provider-based quality measures by using administrative data.12 These measures include all Medicare beneficiaries for whom an eligible physician filed at least 1 professional claim and encompass all patients of a physician’s Medicare panel. Although the same beneficiary may be, and generally is, assigned to multiple physicians, all of these physicians are held accountable for all claims-based quality indicators applicable to that beneficiary in this voluntary program.
4.1.4. Defining Assessment Periods for PMs
Defining the appropriate period of evaluation is an important technical feature of PMs and should be meaningful from both patient and provider perspectives. PMs must define a discrete period (eg, within a 12-month period) of measurement consistent with the actual treatment goals for the measure. For example, an ACC/AHA PM for outpatient cardiac rehabilitation defines success on the basis of “referral to” such a program before hospital discharge. If the measure is made a shared PM, it can also assess whether the patient decided to participate and attended cardiac rehabilitation (or reasons for not attending) and whether it was within the appropriate time window (eg, within 3 months of an event).13 Measurement of patient performance could include whether the patient actually attended the first appointment for cardiac rehabilitation or, more importantly, whether the patient not only initiated attendance, but also maintained attendance and completed the entire program.
For outcome measures, selecting the longitudinal time period encompassed by the measure requires similar careful consideration. Outcome measures may include assessments of health status, symptoms, and function as shared-accountability measures of healthcare quality and can provide quantitative information on the variability in symptom control and quality of life over longitudinal periods of time. For example, the International Consortium for Health Outcomes Measurement has defined a set of recommended outcome measures, including health status, for patients with coronary artery disease.14 For risk adjustment of outcome measures, designation of an appropriate reference time, before which covariates are derived and after which outcomes are measured, is also important.
4.2. Issues Relating to Patient Adherence and Self-Care
4.2.1. Defining Adherence and Self-Care
There are methodological and psychometric challenges specific to measuring patient adherence. Measuring patient adherence to medications involves common direct metrics (eg, direct observation and measurement of serum drug levels and biological markers) and indirect metrics (eg, electronic medication monitoring, pill counts, rates of prescription refills, and self- or proxy-[surrogate/clinician] reporting).15 Each metric has advantages, disadvantages, and arbitrary cutoffs to indicate adherence. For example, with regard to medication taking, serum drug levels can be influenced by metabolism; self-report measures can be biased by poor recall or inaccurate reporting resulting from patients’ desires to please clinicians; and pill counts do not reflect timing of medication taking. These measurement issues affect the validity of adherence measures. Generally, 80% has been considered an acceptable, albeit arbitrary, cutoff to indicate adherence to medications15,16; however, a cutoff of 80% may be too low for some diseases/treatments and medications (eg, immunosuppression after heart transplantation and in heart failure patients). Although defining taxonomy and measurement is an important first step, methods to measure adherence to taking medications, following clinician recommendations, and engaging in self-care behaviors need to be tested rigorously to determine reliability, validity, and sensitivity to change over time.
4.2.2. Challenges With Handling Patient Treatment Refusals
PMs involving adherence must also account for when patients decline therapy. Currently, patients who opt out of evidence-based treatments are generally not counted in the denominator of the PM. This approach inherently overestimates actual performance and negates the potential impact that a clinician may have on patients’ acceptance of treatment plans. When a shared-accountability framework is used, patients who refuse treatment or tests would also be considered in the denominator. With this approach, the patient’s control over the decision to adhere is acknowledged and patient autonomy is respected, while it is also recognized that the success or failure to take a medication can be affected by the clinician and system of care. Adding patients who decline treatment back into the equation (ie, in the denominator) also supports shared care planning, as well as innovative strategies to encourage shared decision making and longitudinal patient engagement in the patient’s health. Nevertheless, this approach may have potential unintended adverse consequences if, because of the incentives generated by accountability, clinicians or healthcare systems exclude patients who are nonadherent. Education geared toward clinicians and the healthcare system about the value of expanding the pool of included patients is crucial to successful implementation of shared PMs.
It may also be valuable to determine the level at which the problem occurs (eg, the clinician who prescribes the medication, the patient who decides not to take the medication, the patient who fills the prescription but does not take the medication). Thus, expanding inclusion criteria (by including patients who decline therapy) as an alternative to excluding such patients could provide clinicians with a more “real-world” view of their overall rates of success for a given shared PM. Such an inclusive metric can also encourage development of strategies to improve these metrics in the future.
4.3. Adjusting for Patient Case Mix
4.3.1. Psychosocial Factors Impacting Patient Case Mix
It is clear that, to be meaningful, PMs of outcomes require risk adjustment for patient case mix. As was noted previously, many existing performance metrics may be influenced by patients. Patients, however, can differ in their baseline likelihood of being adherent to therapeutic recommendations or participating actively in self-care strategies. Factors that can affect self-care include patient preferences, values, culture, religion, and socioeconomic status (ie, education, income, and occupation); psychological factors (eg, depression); behavioral factors (eg, substance abuse); cognitive factors (eg, health literacy, dementia); and environmental factors (eg, social support).17 Collecting information on these factors may be challenging if they are not readily available in electronic health records. Thus, when comparing clinicians or healthcare systems, it is important, if possible, to adjust for some of these factors; however, it also must be recognized that the adjustment of PMs for socioeconomic status may obscure important failures to provide the best care to patients with low socioeconomic status.18,19
5. Factors Impeding or Facilitating the Adoption of Shared-Accountability PMs
5.1. Health Information Systems
If PMs evolve to incorporate the concept of shared accountability, there will need to be a way to track patients, care processes, and ultimately outcomes longitudinally across multiple healthcare settings. For example, measuring adherence to medications after an acute event requires access to information from the discharging institution (discharge instructions and medications), pharmacy refill information, and, ideally, follow-up ambulatory clinic notes (to determine medication changes or discontinuation by the outpatient care team). Although electronic health records are being adopted in many of these settings, electronic health records often do not collect standardized information, nor do they allow for easy interoperable sharing or merging of information across settings. As a result, creating comprehensive patient care records needed for measuring longitudinal shared-accountability PMs will be challenging in the current system.
In the near future, it is hoped that healthcare systems and the government can work together on health information exchanges that will support development of standard nomenclature and facilitate data mapping and sharing of information between disparate healthcare information systems, while maintaining the meaning of the information being exchanged. Furthermore, in Stage 3 of the meaningful use criteria (the Centers for Medicare and Medicaid Services Electronic Health Record Incentive Programs), it has been proposed that there will be patient-collected data, which could further support the development and use of shared PMs20 through clinician access to comprehensive patient- reported outcome data and patient access to self-management tools.
5.2. Payment Reforms, Healthcare Ownership, and ACOs
Current payment reform policies support the adoption of concepts behind shared-accountability PMs. ACOs are an assembly of clinicians (eg, hospitals, health systems, physicians, nurses, pharmacists) responsible for improving care for individuals and the health of the population. Their goals also include reducing the rate of growth in healthcare expenditures while advancing outcomes and reducing costs across the healthcare continuum, including acute, ambulatory-care, and extended-care settings. The Affordable Care Act authorized the Centers for Medicare and Medicaid Services to contract with ACOs to provide health care to Medicare beneficiaries under a Shared Savings Program that began in January 2012.21
ACOs often emphasize new models of healthcare financial reimbursement. Rather than receiving traditional fee-for-service reimbursement (which emphasizes that the more one does, the more one is paid), ACOs are often reimbursed for providing comprehensive coverage for a patient (capitated care) or specific payment for the care of a particular disease condition for a particular period of time (bundled payment). Although these latter models can facilitate streamlining of care and avoiding excessive interventions, concern also exists that they may lead to undertreatment of patients. Given that one of the main goals of ACOs is to improve the health of individual patients and populations, longitudinal shared-accountability PMs may provide a mechanism for promoting and improving the quality of patient care under these new healthcare reimbursement and organizational schemes.22 Finally, standardizing accountability PMs may affect compensation under government and insurance reimbursement programs; this will require aligning both federal and state laws, which are currently complex.
6. Special Issues Relating to Patient Accountability Metrics
6.1. Patient PM and Accountability
A step toward developing shared accountability for quality is the development of patient PMs. If patient-specific PMs are developed (eg, did the patient lose weight, quit smoking, come in for routine follow-up care, or take his or her medications?), there will be questions about how these PMs are used and how to integrate patient-specific PMs into a shared-accountability framework. Resources such as the AHA’s “Life’s Simple 7”23 program or the ACC’s CardioSmart Web sites24 provide patients with online tools to help them identify modifiable risk factors for coronary artery disease, understand why the risk factors are important, and learn how to improve those risk factors. The AHA’s Heart360 program25 provides an online tool that allows patients to track progress toward controlling their BP, lipid, weight, and glucose levels. Although these programs provide educational information, consideration will need to be given to how to motivate patients to change their behavior to achieve their health goals.
Incentives, positive or negative, have also been used to help patients achieve PM targets. Currently, some employers penalize employees for exhibiting behaviors that negatively impact the company’s health plan expenses.26 Individuals who contribute to their own negative health outcomes by smoking, being overweight or obese, using alcohol to excess, or engaging in drug abuse have been shown to miss more days of work, spend more time at clinicians ‘offices or treatment sites, and often do less work when they are on the job.27,28 These employees may be coached and given opportunities to make lifestyle changes geared toward healthy behaviors. The Affordable Care Act allows 50% higher premiums for patients who continue to practice adverse health behaviors such as smoking, and some companies have begun to charge higher health insurance premiums to employees for continuing to engage in behaviors that adversely affect their health.
Alternatively, positive incentives can be implemented to encourage patients to improve their health behaviors. For example, American Express Company paid thousands of employees to exercise during the summer of 2011, giving each $200 toward their healthcare expenses simply for walking 2.5 miles per day. Similarly, a health insurance company, Humana Inc., established a program called HumanaVitality29 to offer incentive prizes, such as camping gear, cameras, and Caribbean hotel rooms, to their customers who see their provider and undergo tests to manage BP and cholesterol. Both financial (eg, lower insurance rates) and nonfinancial (eg, preferred appointment times or passes for a reserved parking area) benefits can be offered to incentivize specified healthy behaviors.
Although extension of accountability to the patient is an interesting and potentially exciting opportunity to improve care, the field of behavioral economics in medicine is quite young.30–32 It must be acknowledged that, to date, there has been limited research on the effectiveness or durability of these incentive programs with regard to patients’ treatment adherence or lifestyle modification. Furthermore, achieving meaningful behavioral change is difficult, and the possibility remains that any such system of reward or penalty could lead to unanticipated adverse consequences. Therefore, we strongly encourage that novel programs that test these strategies also include a thorough evaluation program.
6.2. Patient Financial Incentives and Unintended Consequences
No mechanism currently exists for aligning financial incentives for the patient and clinician. Even if alignment were achievable, it could impact the clinician-patient relationship adversely. For example, financial rewards might motivate clinicians or patients to try medications, diagnostic tests, or treatments for which the evidence base on improving patient outcomes is weak. A related concern is that the financial incentive may be so significant for patients that it would lead them to press their clinicians for treatments that have an unfavorable balance of benefits and risks. In contrast, in circumstances in which no financial incentive is involved, the clinician might not recommend the treatment approach, or the patient might decline to participate in the approach. Another serious concern is that financial incentives and penalties could disproportionately impact certain patient populations and ultimately create additional barriers to their getting needed care or achieving better outcomes. It will be important to conduct surveillance after the implementation of shared PMs to identify unintended consequences of shared PMs, including potential adverse effects on the patient–clinician relationship. Finally, it will be important to align the financial interests of patients and the healthcare system toward the common goals of the shared PMs.
7. Conclusion and Key Recommendations
Shared-accountability measures should be formulated with recognition of joint ownership of care processes and outcomes by patients, clinicians, and the healthcare system. This approach implies that accountability for good performance must be “owned” by multiple parties, including not only clinicians and systems that influence care, but also patients and their caregivers. Explicit acknowledgment of shared accountability changes the perception and definition of many existing PMs and supports analyses of performance that are based on all of the factors that can impact affect decision making and clinical outcomes. In the development of any performance measure, consideration should be given to patient preferences in the evaluation of the outcomes of any shared PM. We have outlined key concepts, measurements, and considerations that need to be borne in mind when shared-accountability PMs are developed and implemented. The following are recommendations from the present statement:
The principles of shared accountability should be considered during the process of developing, analyzing, reporting, and interpreting PMs.
Measures of treatment and outcomes ideally should be longitudinal in nature and should focus on evidence-based therapies.
Purchasers and payers should work with stakeholders to determine ways to apply principles of shared accountability.
When considering shared accountability in PMs, one must carefully consider the level of analysis (eg, individual, practice, system), the timeframe of the analysis, the attribution of subjects to a denominator and definitions for the numerator (ie, what constitutes success), and the different care settings (eg, inpatient, outpatient, home care).
It is important to consider examining process PMs that retain in the denominator patients who decline or are unable to adhere to treatment recommendations, in addition to examining rates that exclude such patients (inasmuch as the former are more reflective of actual care success rates and may be informative with regard to clinician and system factors influencing these rates).
Comparisons of shared-accountability PMs should account for factors that affect the patient’s ability to implement treatment recommendations and manage self-care, such as patient preferences, culture and beliefs, demographics, clinical characteristics, and socioeconomic factors (eg, education, income, occupation); psychological, behavioral, cognitive, and environmental factors; and community resources that support clinician and patient efforts to achieve desired health outcomes.
As a principle of shared accountability, performance on these measures should be reported back to both clinicians and patients in a timely fashion to facilitate shared care management and achievement of best outcomes.
Reward or penalty incentives attached to PMs should account for all factors that influence the measure, including clinician and system performance and patient ability to implement treatment recommendations. Ideally, the reward would be given to the healthcare system, with the system then sharing the reward with the multiple individuals on the team (eg, patients and clinicians) contributing to success.
Care must be taken and strategies must be implemented to monitor the impact of shared-accountability measures to ensure that implementation does not lead to adverse patient selection by clinicians or decreased access to care.
The goals of patient-based performance measurement should be to enhance patient and family engagement and achieve better outcomes and care experience. Future research should both examine how the design and implementation of these programs influences their effectiveness and assess for potential unintended consequences.
Supplementary Material
Staff
American College of Cardiology
Patrick T. O’Gara, MD, FACC, ACC President
Shalom Jacobovitz, Chief Executive Officer
William J. Oetgen, MD, MBA, FACC, FACP, Senior Vice President, Science and Quality
Lara Slattery, MHS, Senior Director, ACC Scientific Reporting
Jensen S. Chiu, MHA, Team Lead, Quality Measurement
Amelia Scholtz, PhD, Publications Manager, Clinical Policy and Pathways
American College of Cardiology/American Heart Association
Naira Tahir, MPH, Associate, Quality Measurement
American Heart Association
Elliott Antman, MD, FAHA, AHA President
Nancy Brown, Chief Executive Officer
Rose Marie Robertson, MD, FAHA, Chief Science Officer
Gayle R. Whitman, PhD, RN, FAHA, FAAN, Senior Vice President, Office of Science Operations
Melanie B. Turner, MPH, Science and Medicine Advisor, Office of Science Operations
Jody Hundley, Production Manager, Scientific Publications, Office of Science Operations
American Association of Cardiovascular and Pulmonary Rehabilitation
Megan Cohen, Executive Director
American Academy of Family Physicians
Heidy Robertson-Cooper, MPA, Senior Strategist, Health Care Quality
American Medical Association–Physician Consortium for Performance Improvement
Mark Antman, DDS, MBA, Director, Measure Development Operations
Samantha Tierney, MPH, Project Manager II, Measure Development Operations
American Nurses Association
Maureen Dailey, DNSc, RN, CWOCN, Senior Policy Fellow
American Society of Health-System Pharmacists
Shekhar Mehta, PharmD, MS, Director of Clinical Guidelines and Quality Improvement
National Committee for Quality Assurance
Bob Rehm, MBA, Assistant Vice President of Performance Measurement
Appendix 1. Author Relationships With Industry and Other Entities (Relevant)
| Committee Member |
Employer/Title | Consultant | Speakers Bureau |
Ownership/ Partnership/ Principal |
Personal Research |
Institutional, Organizational, or Other Financial Benefit |
Expert Witness |
|---|---|---|---|---|---|---|---|
| Eric D. Peterson (Co-Chair) |
Duke Clinical
Research Institute—Executive Director; Fred Cobb, MD, Distinguished Professor of Medicine |
None | None | None | None | None | None |
| P. Michael Ho (Co-Chair) |
Denver VA Medical
Center—Staff Cardiologist; University of Colorado Denver—Associate Professor of Medicine |
None | None | None | None | None | None |
| Mary Barton | National Committee for
Quality Assurance—Vice President, Performance Measurement |
None | None | None | None | None | None |
| Craig Beam | American Heart Association | None | None | None | None | None | None |
| L. Hayley Burgess | HCA Clinical Services Group—Director of Medication Safety and System Innovations |
None | None | None | None | None | None |
| Donald E. Casey, Jr. | NYUPN Clinically
Integrated Network—Chief Medical Officer |
None | None | None | None | None | None |
| Joseph P. Drozda, Jr. | Mercy Health System—Director, Outcomes Research |
None | None | None | None | None | None |
| Gregg C. Fonarow | UCLA Medical Center—Professor of Medicine |
None | None | None | None | None | None |
| David Goff, Jr. | Colorado School of Public Health—Dean and Professor |
None | None | None | None | None | None |
| Kathleen L. Grady | Northwestern University Feinberg School of Medicine—Associate Professor; Bluhm Cardiovascular Institute of Northwestern Memorial Hospital—Administrative Director, Center for Heart Failure |
None | None | None | None | None | None |
| Dana E. King | West Virginia University—Professor and Chair, Department of Family Medicine |
None | None | None | None | None | None |
| Marjorie L. King | Helen Hayes Hospital—Director, Cardiac Rehabilitation |
None | None | None | None | None | None |
| Frederick A. Masoudi | University of Colorado at
Denver— Professor of Medicine, Cardiology |
None | None | None | None | None | None |
| David R. Nielsen | American Academy
of Otolaryngology—Chief Executive Officer and Executive Vice President |
None | None | None | None | None | None |
| Stephen Stanko | The Mended Hearts,
Inc—Chair, Catheterization Patient Outreach |
None | None | None | None | None | None |
This table represents the relationships of committee members with industry and other entities that were reported by authors to be relevant to this document. These relationships were reviewed and updated in conjunction with all meetings and/or conference calls of the Writing Committee during the document development process. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of 5% or more of the voting stock or share of the business entity, or ownership of $10 000 or more of the fair market value of the business entity, or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. A relationship is considered to be modest if it is less than significant under the preceding definition. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted.
HCA indicates Hospital Corporation of America; NYUPN, New York University Physicians Network; UCLA, University of California, Los Angeles; and VA, Veterans Affairs.
Appendix 2. Reviewer Relationships With Industry and Other Entities (Relevant)
| Peer Reviewer | Representation | Consultant | Speakers Bureau |
Ownership/ Partnership/ Principal |
Personal Research |
Institutional, Organizational, or Other Financial Benefit |
Expert Witness |
|---|---|---|---|---|---|---|---|
| Mazen Abu-Fadel | Content Reviewer—ACC Councils and Sections |
None | None | None | None | None | None |
| Cynthia Arslanian-Engoren |
Content Reviewer—AHA Committee: CV and Stroke Nursing Council |
None | None | None | None | None | None |
| Brian Carlin | Official Reviewer—AACVPR | None | None | None | None | None | None |
| Lola Coke | Official Reviewer—ANA | None | None | None | None | None | None |
| Eileen Collins | Official Reviewer—AACVPR | None | None | None | None | None | None |
| Silvana Lawrence | Content Reviewer—ACC Councils and Sections |
None | None | None | None | None | None |
| Maulik D. Majmudar, | Content Reviewer—ACC Patient-Centered Care Committee |
None | None | None | None | None | None |
| Ariane Marelli | Content Reviewer—ACC Councils and Sections |
None | None | None | None | None | None |
| Sara Paul | Content Reviewer—AHA Committee: CV and Stroke Nursing Council |
None | None | None | None | None | None |
| Robert Piana | Content Reviewer—ACC Councils and Sections |
None | None | None | None | None | None |
| Susan Pressler | Content Reviewer—AHA Committee: CV and Stroke Nursing Council |
None | None | None | None | None | None |
| Michael Rich | Content Reviewer—ACC Councils and Sections | ||||||
| Barbara Riegel | Official Reviewer—AHA | None | None | None | None | None | None |
| William Roach | Official Reviewer—AHA | None | None | None | None | None | None |
| Darryl Roberts | Official Reviewer—ANA | None | None | None | None | None | None |
| Joachim Roski | Official Reviewer—AMA-PCPI | None | None | None | None | None | None |
| Amy Schneider | Official Reviewer—AAFP | None | None | None | None | None | None |
| Chittur Sivaram | Content Reviewer—ACC Councils and Sections |
None | None | None | None | None | None |
| Karen Walker | Content Reviewer—ACC Councils and Sections |
None | None | None | None | None | None |
| Minnow Walsh | Content Reviewer—ACC CQC Committee and ACC Patient- Centered Care Committee |
None | None | None | None | None | None |
This table represents the relevant relationships with industry and other entities that were disclosed at the time of peer review. It does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of 5% or more of the voting stock or share of the business entity, or ownership of $10 000 or more of the fair market value of the business entity, or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. A relationship is considered to be modest if it is less than significant under the preceding definition. Relationships that exist with no financial benefit are also included for the purposes of transparency. Relationships in this table are modest unless otherwise noted.
AACVPR indicates American Association of Cardiovascular and Pulmonary Rehabilitation; AAFP, American Academy of Family Physicians; ACC, American College of Cardiology; AHA, American Heart Association; AMA-PCPI, American Medical Association-Physician Consortium for Performance Improvement; ANA, American Nurses Association; CQC: Clinical Quality Committee; CV, cardiovascular; and NCQA, National Committee for Quality Assurance.
Appendix A: Comprehensive Author Relationships With Industry and other entities—ACC/AHA/AACVPR/AAFP/ANA The Concepts for Clinician–Patient Shared Accountability in Performance Measures
| Name | Employment | Consultant | Speaker | Ownership/ Partnership/ Principal |
Research | Institutional, Organizational, or Other Financial Benefit |
Expert Witness |
|---|---|---|---|---|---|---|---|
| Eric. D. Peterson, Chair |
Executive Director, Duke Clinical Research Institute |
|
None | None |
|
None | |
| Mary Barton | National Committee for Quality Assurance, Vice President |
None | None | None | None | None | |
| Craig Beam | Medical
Development Specialists—Senior Vice President |
None | None | None | None | None | None |
| L. Hayley Burgess | HCA Clinical Services Group—Director of Medication Safety and System Innovations |
None | None | None | None | None | None |
| Donald E. Casey, Jr. |
Atlantic Health— Chief Medical Officer |
None | None | None | None | None | None |
| Joseph P. Drozda, Jr. |
Mercy Health— Director, Outcomes Research |
None | None | None |
|
None | |
| Gregg C. Fonarow | Ahmanson-UCLA Cardiomyopathy Center—Director, Division of Cardiology |
|
None | None | None | ||
| David Goff, Jr. | Wake
Forest University—Professor of Medicine |
None | None | None | None | None | |
| Kathleen L. Grady | Bluhm
Cardiovascular Institute— Administrative Director, Center for Heart Failure |
None | None | None |
|
|
None |
| P. Michael Ho | Stanford University School of Medicine— Professor of Medicine |
|
None | None | None | None | None |
| Dana King | Medical University of South Carolina— Professor & Vice Chair, Department of Family Medicine |
|
None | None | None | None | None |
| Marjorie L. King | Columbia University (Helen Hayes Hospital)—Director, Cardiac Rehabilitation |
None | None | None | None | None | None |
| Frederick A. Masoudi | University of Colorado at Denver— Associate Professor of Medicine, Division of Cardiology |
None | None | None |
|
|
|
| David R. Nielsen | The American Academy of Otolaryngology— Chief Executive Officer & Executive Vice President |
None | None | None | None | None | |
| Stephen Stanko | Mended Hearts— Chair, Catherization Patient Outreach |
None | None | None | None | None | None |
This table represents all relationships of committee members with industry and other entities that were reported by authors, including those not deemed to be relevant to this document, at the time this document was under development. A person is deemed to have a significant interest in a business if the interest represents ownership of 5% or more of the voting stock or share of the business entity, or ownership of $ 10 000 or more of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person’s gross income for the previous year. A relationship is considered to be modest if it is less than significant under the preceding definition. Relationships that exist with no financial benefit are also included for the purposes of transparency. Relationships in this table are modest unless otherwise noted.
No financial relationship
Significant (greater than $10 000) relationship.
AAO indicate American Academy of Otolaryngology; AACVPR indicates American Association of Cardiovascular and Pulmonary Rehabilitation; AAFP, American Academy of Family Physicians; ACC, American College of Cardiology; ACTION-GWTG; ACTION Get with the Guidelines; AHA, American Heart Association, AHRQ, Agency for Healthcare Research and Quality; AMA-PCPI, American Medical Association—Physician Consortium for Performance Improvement; ANA, American Nurses Association; ASHP, American Society of Health-System Pharmacists; CMSS, Council of Medical Specialty Societies; DCRI, Duke Clinical Research Institute; HCA, Hospital Corporation of America; IMPROVE HF, Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting; NHLBI, National Heart, Lung and Blood Institute; NIAID, National Institute of Allergy and Infectious Diseases; NIH, National Institutes of Health; and PRT, Pharmaceutical Roundtable; UCLA, University of California, Los Angeles.
DCRI has numerous grants and contracts sponsored by industry. These include the following: Aastrom Biosciences†; Abbott†; Abiomed†; Acom Cardiovascular†; Adolor Corp.†; Advanced Cardiovascular Systems†; Advanced Stent Technologies†; Adynnx; Aijnomoto†; Allergan†; Amgen†; Alnylam Pharma†; Alpharma†; Amylin Pharmaceuticals†; Anadys†; Anesiva†; Angel Medical Systems†; ANGES MG†; Angiomedtrix†; APT Nidus Center†; ASCA Biopharma†; Astellas Pharma†; Asklepios†; AstraZeneca†; Atritech†; Attention Therapeutics†; Aventis†; Baxter†; Bayer†; Berlex†; BG Medicine†; Biogen†; Biolex Therapeutics†; Biomarker Factory†; Biosite†; Boehringer Ingelheim Biogen†; Boston Scientific†; Bristol-Myers Squibb†; BMS Pfizer†; Carbomed†; CardioDx†; CardioKinetix†; Cardiovascular Systems†; Cardiovax†; Celsion Corp.†; Centocor†; Cerexa†; Chase Medical†; Conatus Pharmaceuticals†; Conor Medsystems†; Cortex†; Corgentech†; CSL Behring†; CV Therapeutics†; Daiichi Pharmaceuticals†; Daiichi-Sankyo†; Daiichi-Sankyo Lilly†; Datascope; Dendreon†; Dainippon†; Dr. Reddy’s Laboratories; Eclipse Surgical Technologies†; Edwards Lifesciences†; Eisai†; Endicor†; EnteroMedics†; Enzon Pharmaceuticals†; Eli Lilly†; Ethicon†; Ev3†; Evalve†; F2G†; Flow Cardia†; Fox Hollow Pharmaceuticals†; Fujisawa†; Genetech†; General Electric†; General Electric Co.†; General Electric Healthcare†; General Electric Medical Systems†; Genzyme Corp.†; Genome Canada†; Gilead Sciences†; GlaxoSmithKline†; Guidant Corp.†; Heartscape Technologies†; Hoffman-LaRoche†; Hospira†; Idera Pharmaceuticals†; Ikaria†; Imcor Pharmaceuticals†; Immunex†; INFORMD†; Inimex†; Inspire Pharmaceuticals†; Ischemix†; Janssen†; Johnson and Johnson†; Jomed†; Juventus Therapeutics†; KAI Pharmaceuticals†; King Pharmaceuticals†; Kyowa Pharma†; Luitpold†; Mardil†; MedImmune†; Medscape†; Medtronic Diabetes†; Medtronic†; Medtronic Vascular†; Merck Group†; MicroMed Technology†; Millennium Pharmaceuticals†; Mitsubishi Tanabe†; Momenta†; Nabriva†; Neuron Pharmaceuticals†; NitroMed; NovaCardia Inc†; Novartis AG Group†; Novartis Pharmaceuticals†; Oncura†; Orexigen†; Ortho-McNeil-Janssen†; OSI Eyetech†; OSI Pharmaceuticals†; Pfizer†; Pharmacyclics†; Pharmasset†; Pharmos†; Phyxius Pharmaceuticals; Pharsight†; Pluristen Therapeutics†; Portola Pharmaceuticals†; Proventys†; Radiant†; Regado Biosciences†; Rengeneron Pharmaceuticals†; Roche Molecular Systems†; Roche Group†; Roche Diagnostic†; Salix Pharmaceuticals†; Sanofi-Pasteur, Inc; Sanofi-aventis†; Santaris Pharmaceuticals†; Schering-Plough†; Scios†; Siemens†; Southwest Oncology Group†; Spectranetics†; Summit†; Sunovion Pharmaceuticals†; TAP Pharmaceutical Products†; Tengion†; The Medicines Company†; Theravance†; TherOx†; Tethys Bioscience†; Theregen†; Three Rivers Pharmaceuticals†; The EMMES Corporation†; UCB†; Valentis†; Valleylab†; Vertex†; Viacor† and Wyeth†.
Appendix B: Comprehensive Reviewer Relationships With Industry and other entities – ACC/AHA/AACVPR/AAFP/ANA The Concepts for Clinician–Patient Shared Accountability in Performance Measures
| Peer Reviewer | Representation | Consultant | Speaker | Ownership/ Partnership/ Principal |
Research | Institutional, Organizational or Other Financial Benefit |
Expert Witness |
|---|---|---|---|---|---|---|---|
| Mazen Abu-Fadel | Content Reviewer- ACC Councils and Sections |
None |
|
None | None | None | None |
| Cynthia Arslanian- Engoren |
Content Reviewer- AHA Committee: CV and Stroke Nursing Council |
None | None | None | None | None | None |
| Brian Carlin | Official Reviewer- AACVPR |
|
None | None | None |
|
None |
| Lola Coke | Official Reviewer- ANA | None | None | None | None | None | None |
| Eileen Collins | Official Reviewer- AACVPR | None | None | None | None | None | None |
| Silvana Lawrence | Content Reviewer- ACC Councils and Sections |
None | None | None | None | None | None |
| Maulik D. Majmudar, | Content reviewer- ACC Patient-Centered Care Committee |
None | None | None | None | None | None |
| Ariane Marelli | Content Reviewer- ACC Councils and Sections |
None | None | None | None | None | None |
| Robert Piana | Content Reviewer- ACC Councils and Sections |
|
None | None |
|
|
|
| Sara Paul | Content Reviewer- AHA Committee: CV and Stroke Nursing Council |
None | None | None | None |
|
None |
| Susan Pressler | Content Reviewer- AHA Committee: CV and Stroke Nursing Council |
None | None | None | None |
|
None |
| Michael Rich | Content Reviewer- ACC Councils and Sections |
None | None | None | None | None | None |
| Barbara Riegel | Official Reviewer- AHA |
None | None | None | None |
|
None |
| William Roach | Official Reviewer- AHA |
None | None | None | None |
|
None |
| Darryl Roberts | Official Reviewer- ANA |
None | None | None | None | None | None |
| Joachim Roski | Official Reviewer- AMA-PCPI |
None | None | None | None | None | None |
| Amy Schneider | Official Reviewer- AAFP |
None | None | None | None | None | None |
| Chittur Sivaram | Content Reviewer- ACC Councils and Sections |
|
None | None | None | None | None |
| Karen Walker | Content Reviewer- ACC Councils and Sections |
None | None | None | None | None | None |
| Minnow Walsh | Content Reviewer- ACC CQC Committee and ACC Patient-Centered Care Committee |
|
None | None | None | None | None |
This table represents the relevant relationships with industry and other entities that were disclosed at the time of peer review. It does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of 5% or more of the voting stock or share of the business entity, or ownership of $10 000 or more of the fair market value of the business entity, or if funds received by the person from the business entity exceed 5% of the person's gross income for the previous year. A relationship is considered to be modest if it is less than significant under the preceding definition. Relationships that exist with no financial benefit are also included for the purposes of transparency. Relationships in this table are modest unless otherwise noted.
AACVPR indicates American Association of Cardiovascular and Pulmonary Rehabilitation; AAFP, American Academy of Family Physicians; ACC, American College of Cardiology; AHA, American Heart Association; AMA-PCPI, American Medical Association–Physician Consortium for Performance Improvement; ANA, American Nurses Association; ASHP, American Society of Health-System Pharmacists; CQC: Clinical Quality Committee; NCQA, National Committee for Quality Assurance; NIH, National Institute of Health.
Footnotes
This document was approved by the American College of Cardiology Board of Trustees and the American Heart Association Science Advisory and Coordinating Committee in May 2014.
The online-only Comprehensive RWI Data Supplement table is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIR.0000000000000139/-/DC1.
Permissions: Multiple copies, modification, alteration, enhancement, and/or distribution of this document are not permitted without the express permission of the American Heart Association. Instructions for obtaining permission are located at http://www.heart.org/HEARTORG/General/Copyright-Permission-Guidelines_UCM_300404_Article.jsp. A link to the “Copyright Permissions Request Form” appears on the right side of the page.’
References
- 1.Centers for Medicare and Medicaid Services. Hospital Quality Initiatives: Outcome Measures. [Accessed November 15, 2013]; Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/OutcomeMeasures.html.
- 2.National Committee for Quality Assurance. [Accessed May 1, 2013]; Available at: http://www.ncqa.org/HomePage.aspx.
- 3.Richard AA, Shea K. Delineation of self-care and associated concepts. J Nurs Scholarsh. 2011;43:255–264. doi: 10.1111/j.1547-5069.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine (US) Committee on Public Health Strategies to Improve Health. For the Public’s Health: The Role of Measurement in Action and Accountability. Washington, DC: National Academies Press; 2011. [Accessed May 1, 2013]. Available at: http://www.ncbi.nlm.nih.gov/books/NBK209716/. [PubMed] [Google Scholar]
- 5.Chassin MR, Loeb JM, Schmaltz SP, et al. Accountability measures— using measurement to promote quality improvement. N Engl J Med. 2010;363:683–688. doi: 10.1056/NEJMsb1002320. [DOI] [PubMed] [Google Scholar]
- 6.Spertus JA, Eagle KA, Krumholz HM, et al. American College of Cardiology and American Heart Association methodology for the selection and creation of performance measures for quantifying the quality of cardiovascular care. Circulation. 2005;111:1703–1712. doi: 10.1161/01.CIR.0000157096.95223.D7. [DOI] [PubMed] [Google Scholar]
- 7.Kramer JM, Hammill B, Anstrom KJ, et al. National evaluation of adherence to beta-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J. 2006;152:454.e1–454.e8. doi: 10.1016/j.ahj.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 8.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 9.Gonseth J, Guallar-Castillón P, Banegas JR, et al. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. Eur Heart J. 2004;25:1570–1595. doi: 10.1016/j.ehj.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg MW, Damberg CL. Methodological Considerations in Generating Provider Performance Scores for Use in Public Reporting: A Guide for Community Quality Collaboratives. Rockville, MD: Agency for Healthcare Research and Quality; 2011. AHRQ Pub. No. 11-0093. [Google Scholar]
- 11.Pham HH, Schrag D, O’Malley AS, et al. Care patterns in Medicare and their implications for pay for performance. N Engl J Med. 2007;356:1130–1139. doi: 10.1056/NEJMsa063979. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Medicare and Medicaid Services. [Accessed May 1, 2013];Physician Feedback Program: 2010 Individual Physician Quality and Resource Use Reports: Feedback Session 2. 2012 Apr 5; Available at: http://www.cms.gov/Outreach-and-Education/Outreach/NPC/Downloads/QRUR-Feedback-Session2.pdf.
- 13.Thomas RJ, King M, Lui K, et al. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services. Circulation. 2010;122:1342–1350. doi: 10.1161/CIR.0b013e3181f5185b. [DOI] [PubMed] [Google Scholar]
- 14.The International Consortium for Health Outcomes Measurement (ICHOM) [Accessed May 19, 2014]; Available at: http://www.ichom.org.
- 15.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 16.Wu J-R, Moser DK, De Jong MJ, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. Am Heart J. 2009;157:285–291. doi: 10.1016/j.ahj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 18.Ross JS, Bernheim SM, Lin Z, et al. Based on key measures, care quality for Medicare enrollees at safety-net and non-safety-net hospitals was almost equal. Health Aff (Millwood) 2012;31:1739–1748. doi: 10.1377/hlthaff.2011.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall L, Harbin V, Hooker J, et al. Safety net hospital performance on national quality of care process measures. J Healthc Qual. 2012;34:21–31. doi: 10.1111/j.1945-1474.2011.00186.x. [DOI] [PubMed] [Google Scholar]
- 20.Department of Health and Human Services, Office of the National Coordinator for Health Information Technology, HIT Policy Committee. [Accessed July 13, 2013];Request for Comment Regarding the Stage 3 Definition of Meaningful Use of Electronic Health Records (EHRs) Available at: http://www.healthit.gov/sites/default/files/hitpc_stage3_rfc_final.pdf.
- 21.Patient Protection and Affordable Care Act. [Accessed October 13, 2013];Public Law 111–148. 124 Stat. 119. 2010 Mar 23; Available at: http://www.gpo.gov/fdsys/pkg/PLAW-111pub1148/pdf/PLAW-111pub1148.pdf.
- 22.Berwick DM. Launching accountable care organizations—the proposed rule for the Medicare Shared Savings Program. N Engl J Med. 2011;364:e32. doi: 10.1056/NEJMp1103602. [DOI] [PubMed] [Google Scholar]
- 23.American Heart Association. [Accessed August 15, 2014];Life’s Simple 7. Available at: http://mylife-check.heart.org/.
- 24.American College of Cardiology. [Accessed August 15, 2014];CardioSmart. Available at: https://www.cardiosmart.org.
- 25.American Heart Association. [Accessed August 15, 2014];Heart360. Available at: https://www.heart360.org/.
- 26.Poor employee health habits drive lost productivity according to major new study of nearly 20,000 American workers. [Accessed October 22, 2013];Business Wire. 2012 Aug 6; Available at: http://www.businesswire.com/news/home/20120806006042/en/Poor-Employee-Health-Habits-Drive-Lost-Productivity#.
- 27.Osilla KC, dela Cruz E, Miles JNV, et al. Exploring productivity outcomes from a brief intervention for at-risk drinking in an employee assistance program. Addict Behav. 2010;35:194–200. doi: 10.1016/j.addbeh.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvey SB, Glozier N, Carlton O, et al. Obesity and sickness absence: results from the CHAP study. Occup Med (Lond) 2010;60:362–368. doi: 10.1093/occmed/kqq031. [DOI] [PubMed] [Google Scholar]
- 29.Humana Inc. [Accessed October 22, 2013];Humana Vitality. Available at: https://www.humana.com/vitality/.
- 30.Loewenstein G, Asch DA, Volpp KG. Behavioral economics holds potential to deliver better results for patients, insurers, and employers. Health Aff (Millwood) 2013;32:1244–1250. doi: 10.1377/hlthaff.2012.1163. [DOI] [PubMed] [Google Scholar]
- 31.Kimmel SE, Troxel AB, Loewenstein G, et al. Randomized trial of lottery-based incentives to improve warfarin adherence. Am Heart J. 2012;164:268–274. doi: 10.1016/j.ahj.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel MS, Volpp KG. Leveraging insights from behavioral economics to increase the value of healthcare service provision. J Gen Intern Med. 2012;27:1544–1547. doi: 10.1007/s11606-012-2050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.