Abstract
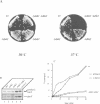
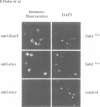
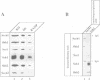
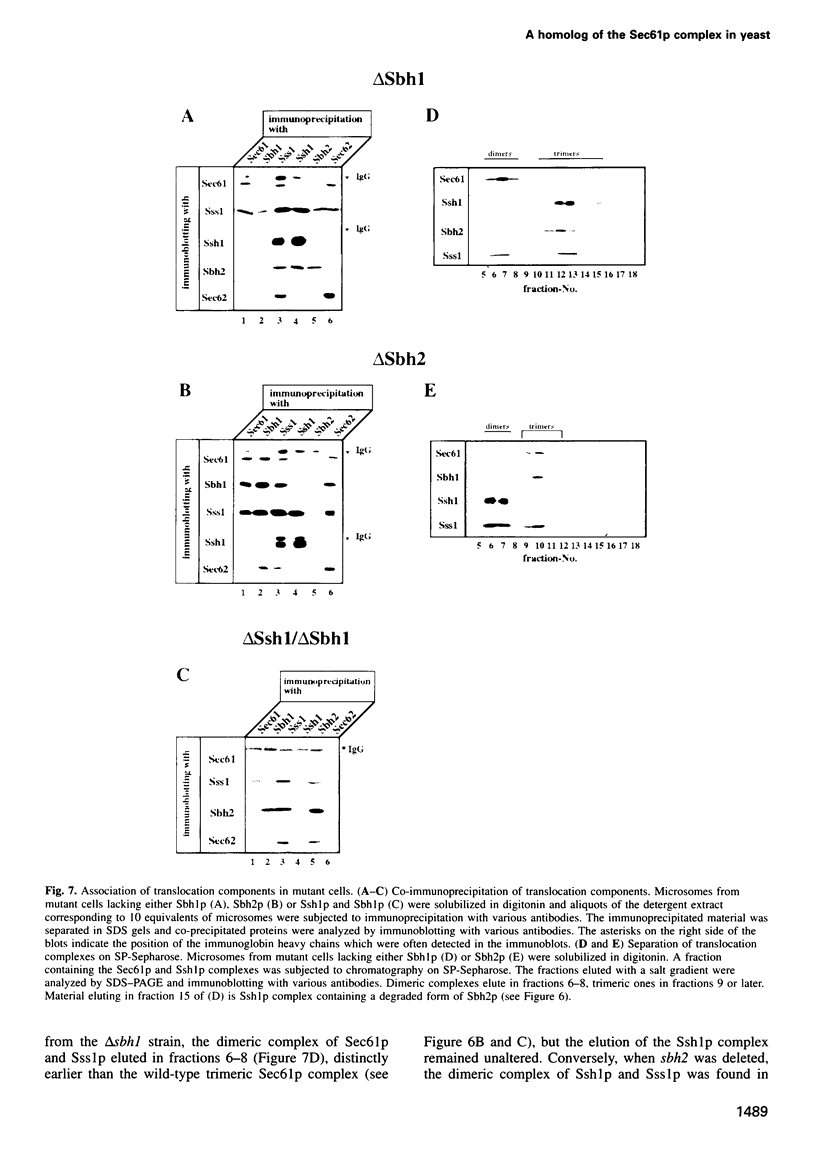
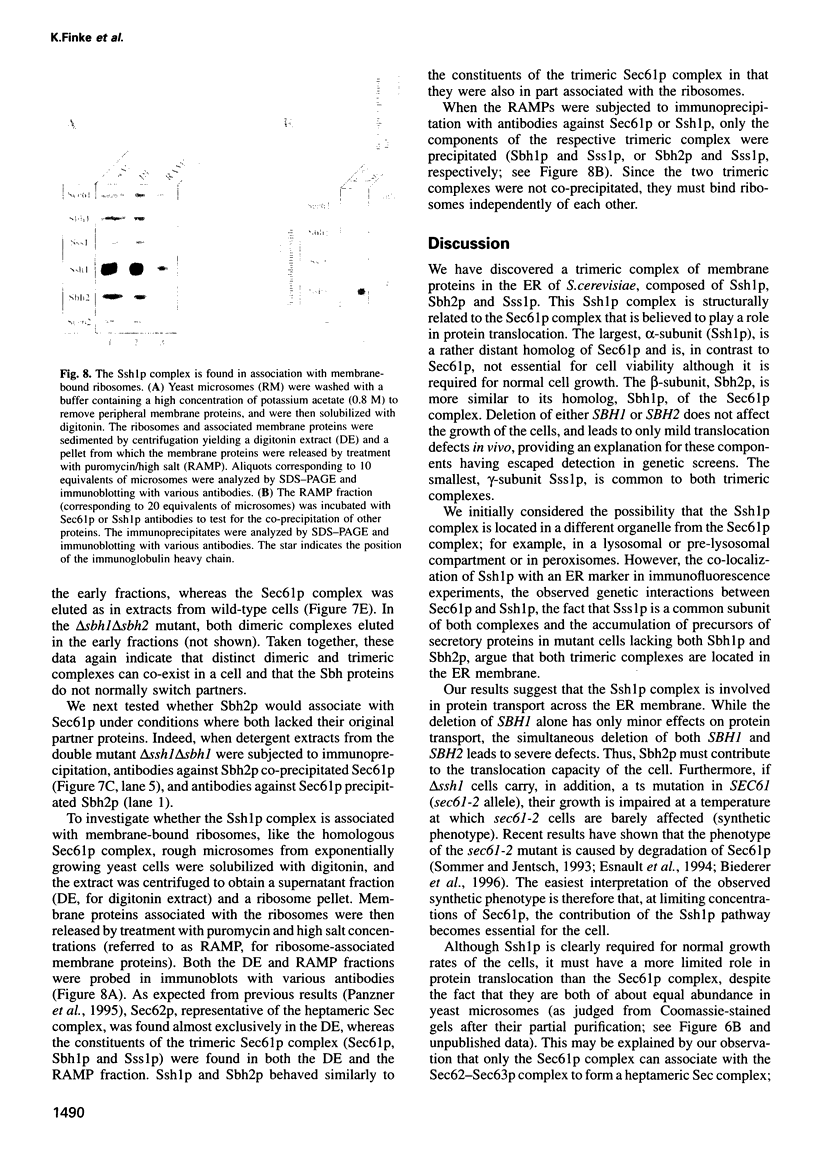
Yeast microsomes contain a heptameric Sec complex involved in post-translational protein transport that is composed of a heterotrimeric Sec61p complex and a tetrameric Sec62-Sec63 complex. The trimeric Sec61p complex also exists as a separate entity that probably functions in co-translational protein transport, like its homolog in mammals. We have now discovered in the yeast endoplasmic reticulum membrane a second, structurally related trimeric complex, named Ssh1p complex. It consists of Ssh1p1 (Sec sixty-one homolog 1), a rather distant relative of Sec61p, of Sbh2p, a homolog of the Sbh1p subunit of the Sec61p complex, and of Sss1p, a component common to both trimeric complexes. In contrast to Sec61p, Ssh1p is not essential for cell viability but it is required for normal growth rates. Sbh1p and Sbh2p individually are also not essential, but cells lacking both proteins are impaired in their growth at elevated temperatures and accumulate precursors of secretory proteins; microsomes isolated from these cells also exhibit a reduced rate of post-translational protein transport. Like the Sec61p complex, the Ssh1p complex interacts with membrane-bound ribosomes, but it does not associate with the Sec62-Sec63p complex to form a heptameric Sec complex. We therefore propose that it functions exclusively in the co-translational pathway of protein transport.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akimaru J., Matsuyama S., Tokuda H., Mizushima S. Reconstitution of a protein translocation system containing purified SecY, SecE, and SecA from Escherichia coli. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6545–6549. doi: 10.1073/pnas.88.15.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky J. L., Schekman R. A Sec63p-BiP complex from yeast is required for protein translocation in a reconstituted proteoliposome. J Cell Biol. 1993 Dec;123(6 Pt 1):1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Collins P., Gilmore R. Access of proteinase K to partially translocated nascent polypeptides in intact and detergent-solubilized membranes. J Cell Biol. 1989 Feb;108(2):299–307. doi: 10.1083/jcb.108.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley K. S., Liao S., Worrell V. E., Reinhart G. D., Johnson A. E. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 1994 Aug 12;78(3):461–471. doi: 10.1016/0092-8674(94)90424-3. [DOI] [PubMed] [Google Scholar]
- Crowley K. S., Reinhart G. D., Johnson A. E. The signal sequence moves through a ribosomal tunnel into a noncytoplasmic aqueous environment at the ER membrane early in translocation. Cell. 1993 Jun 18;73(6):1101–1115. doi: 10.1016/0092-8674(93)90640-c. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Sanders S. L., Feldheim D. A., Schekman R. Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature. 1991 Feb 28;349(6312):806–808. doi: 10.1038/349806a0. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol. 1987 Aug;105(2):633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2653–2664. doi: 10.1083/jcb.109.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault Y., Blondel M. O., Deshaies R. J., Scheckman R., Képès F. The yeast SSS1 gene is essential for secretory protein translocation and encodes a conserved protein of the endoplasmic reticulum. EMBO J. 1993 Nov;12(11):4083–4093. doi: 10.1002/j.1460-2075.1993.tb06092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault Y., Feldheim D., Blondel M. O., Schekman R., Képès F. SSS1 encodes a stabilizing component of the Sec61 subcomplex of the yeast protein translocation apparatus. J Biol Chem. 1994 Nov 4;269(44):27478–27485. [PubMed] [Google Scholar]
- Fang H., Green N. Nonlethal sec71-1 and sec72-1 mutations eliminate proteins associated with the Sec63p-BiP complex from S. cerevisiae. Mol Biol Cell. 1994 Sep;5(9):933–942. doi: 10.1091/mbc.5.9.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D., Schekman R. Sec72p contributes to the selective recognition of signal peptides by the secretory polypeptide translocation complex. J Cell Biol. 1994 Aug;126(4):935–943. doi: 10.1083/jcb.126.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D., Yoshimura K., Admon A., Schekman R. Structural and functional characterization of Sec66p, a new subunit of the polypeptide translocation apparatus in the yeast endoplasmic reticulum. Mol Biol Cell. 1993 Sep;4(9):931–939. doi: 10.1091/mbc.4.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H., Aigle M., Aljinovic G., André B., Baclet M. C., Barthe C., Baur A., Bécam A. M., Biteau N., Boles E. Complete DNA sequence of yeast chromosome II. EMBO J. 1994 Dec 15;13(24):5795–5809. doi: 10.1002/j.1460-2075.1994.tb06923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N., Fang H., Walter P. Mutants in three novel complementation groups inhibit membrane protein insertion into and soluble protein translocation across the endoplasmic reticulum membrane of Saccharomyces cerevisiae. J Cell Biol. 1992 Feb;116(3):597–604. doi: 10.1083/jcb.116.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D., Prehn S., Hartmann E., Kalies K. U., Rapoport T. A. A mammalian homolog of SEC61p and SECYp is associated with ribosomes and nascent polypeptides during translocation. Cell. 1992 Oct 30;71(3):489–503. doi: 10.1016/0092-8674(92)90517-g. [DOI] [PubMed] [Google Scholar]
- Görlich D., Rapoport T. A. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 1993 Nov 19;75(4):615–630. doi: 10.1016/0092-8674(93)90483-7. [DOI] [PubMed] [Google Scholar]
- Hartmann E., Sommer T., Prehn S., Görlich D., Jentsch S., Rapoport T. A. Evolutionary conservation of components of the protein translocation complex. Nature. 1994 Feb 17;367(6464):654–657. doi: 10.1038/367654a0. [DOI] [PubMed] [Google Scholar]
- High S., Andersen S. S., Görlich D., Hartmann E., Prehn S., Rapoport T. A., Dobberstein B. Sec61p is adjacent to nascent type I and type II signal-anchor proteins during their membrane insertion. J Cell Biol. 1993 May;121(4):743–750. doi: 10.1083/jcb.121.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High S., Görlich D., Wiedmann M., Rapoport T. A., Dobberstein B. The identification of proteins in the proximity of signal-anchor sequences during their targeting to and insertion into the membrane of the ER. J Cell Biol. 1991 Apr;113(1):35–44. doi: 10.1083/jcb.113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnickel B., Rapoport T. A. A posttargeting signal sequence recognition event in the endoplasmic reticulum membrane. Cell. 1995 Jul 28;82(2):261–270. doi: 10.1016/0092-8674(95)90313-5. [DOI] [PubMed] [Google Scholar]
- Kalies K. U., Görlich D., Rapoport T. A. Binding of ribosomes to the rough endoplasmic reticulum mediated by the Sec61p-complex. J Cell Biol. 1994 Aug;126(4):925–934. doi: 10.1083/jcb.126.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellaris K. V., Bowen S., Gilmore R. ER translocation intermediates are adjacent to a nonglycosylated 34-kD integral membrane protein. J Cell Biol. 1991 Jul;114(1):21–33. doi: 10.1083/jcb.114.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T., Silver P. Suppression of a sec63 mutation identifies a novel component of the yeast endoplasmic reticulum translocation apparatus. Mol Biol Cell. 1993 Sep;4(9):919–930. doi: 10.1091/mbc.4.9.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes W., Prehn S., Rapoport T. A. Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J. 1994 Sep 1;13(17):3973–3982. doi: 10.1002/j.1460-2075.1994.tb06713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicchitta C. V., Murphy E. C., 3rd, Haynes R., Shelness G. S. Stage- and ribosome-specific alterations in nascent chain-Sec61p interactions accompany translocation across the ER membrane. J Cell Biol. 1995 May;129(4):957–970. doi: 10.1083/jcb.129.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K., Hanada M., Tokuda H. Disruption of the gene encoding p12 (SecG) reveals the direct involvement and important function of SecG in the protein translocation of Escherichia coli at low temperature. EMBO J. 1994 Jul 15;13(14):3272–3277. doi: 10.1002/j.1460-2075.1994.tb06628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K., Mizushima S., Tokuda H. A novel membrane protein involved in protein translocation across the cytoplasmic membrane of Escherichia coli. EMBO J. 1993 Sep;12(9):3409–3415. doi: 10.1002/j.1460-2075.1993.tb06015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzner S., Dreier L., Hartmann E., Kostka S., Rapoport T. A. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995 May 19;81(4):561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Rose M. D., Misra L. M., Vogel J. P. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989 Jun 30;57(7):1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Deshaies R. J., Sanders S. L., Daum G., Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989 Dec;109(6 Pt 1):2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler I., Chiang A., Kurihara T., Rothblatt J., Way J., Silver P. A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2665–2675. doi: 10.1083/jcb.109.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders S. L., Whitfield K. M., Vogel J. P., Rose M. D., Schekman R. W. Sec61p and BiP directly facilitate polypeptide translocation into the ER. Cell. 1992 Apr 17;69(2):353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Schatz P. J., Beckwith J. Genetic analysis of protein export in Escherichia coli. Annu Rev Genet. 1990;24:215–248. doi: 10.1146/annurev.ge.24.120190.001243. [DOI] [PubMed] [Google Scholar]
- Scidmore M. A., Okamura H. H., Rose M. D. Genetic interactions between KAR2 and SEC63, encoding eukaryotic homologues of DnaK and DnaJ in the endoplasmic reticulum. Mol Biol Cell. 1993 Nov;4(11):1145–1159. doi: 10.1091/mbc.4.11.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. M., Blobel G. A protein-conducting channel in the endoplasmic reticulum. Cell. 1991 May 3;65(3):371–380. doi: 10.1016/0092-8674(91)90455-8. [DOI] [PubMed] [Google Scholar]
- Simon S. M., Peskin C. S., Oster G. F. What drives the translocation of proteins? Proc Natl Acad Sci U S A. 1992 May 1;89(9):3770–3774. doi: 10.1073/pnas.89.9.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer T., Jentsch S. A protein translocation defect linked to ubiquitin conjugation at the endoplasmic reticulum. Nature. 1993 Sep 9;365(6442):176–179. doi: 10.1038/365176a0. [DOI] [PubMed] [Google Scholar]
- Stirling C. J., Rothblatt J., Hosobuchi M., Deshaies R., Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992 Feb;3(2):129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]