Abstract
Background
Children with Down syndrome (DS) present with delays in motor development. The reduced size of the cerebrum, brain maturation disorders, and pathophysiological processes lead to motor development delay. The aim of this study was to examine the gross motor function and estimate what motor abilities are significantly delayed in children with Down syndrome even if they attend physical therapy sessions. Another purpose of the study was to assess the functional balance.
Material/Methods
The study group consisted of 79 children with DS (42 boys, 37 girls), average age 6 years and 3 months ±4 years and 6 months. Participants were divided into 3 groups according to (i) age: <3 years old, 3–6 years old, and >6 years old; and (ii) motor impairment scale: mild (SNR 1), moderate (SNR 2), and severe (SNR 3). Children were assessed using the Gross Motor Function Measure-88 (GMFM-88) and Pediatric Balance Scale (PBS).
Results
None of the assessed children developed all the functions included in GMFM-88. The standing position was achieved at the specified age by 10% of children in the first age group (<3 years old) and 95% of children aged 3–6 years. Similarly, the walking ability was performed by 10% of children under 3 years old and by 95% of children aged 3–6 years. The median score of PBS was 50 points (min. 34 p. – max. 56 p.). There was a statistically significant correlation between PBS scores and GMFM-88 scores, r=0.7; p<0.0001, and between balance scores and GMFM – 88 E (walking, running, jumping) (r=0.64; p<0.0001).
Conclusions
Motor development, especially standing position and walking ability, is delayed in children with Down syndrome. Balance and motor functions are correlated with each other, so both aspects of development should be consider together in physical therapy of children with Down syndrome.
Keywords: Down Syndrome, Motor Skills, Neurotransmitter Agents
Background
Brain structure and function may influence the development of mental and motor abilities. Structural and functional disorders of the central nervous system may be influenced by genetic conditions. For instance, children with Down syndrome (DS) who have an extra chromosome 21 present with many brain disorders that cause retarded psychomotor development and problems with learning [1].
There are 3 groups of problems affecting the central nervous system that cause psychomotor dysfunctions in DS children:
Changes in the shape and number of neurons and changes in cerebrum size;
Disorders of the central nervous system maturation;
-
Pathophysiological processes:
Degenerative processes of the nervous system,
Disorders in neuronal apoptosis regulation,
Overexpression of genes that code beta amyloid precursor protein (APP)
Processes leading to decreased release of neurotransmitters.
Significant changes in cerebrum size appear after the 6th month of life [2] and deletions in motor development are also seen from the 6th month of life [3]. Volumetric neuroimaging studies have revealed smaller frontal, occipital, and temporal lobes with smaller hippocampal volume, reduced corpus callosum and cerebellum size, decreased superior temporal gyrus, and brainstem volume [3,4]. Such abnormalities in the brain lead to psychomotor dysfunctions among DS subjects. For instance, smaller frontal lobe volumes cause problems with voluntary activities, cognitive deficits, and gait quality, especially in adult life. Increasing age is associated with grey matter reduction in the frontal, parietal, and temporal cortex, similar to alterations in mild Alzheimer’s disease [4]. Hypoplasia of the cerebellum in DS children is a symptom caused by overexpression of the GART gene. Changes in the cerebellum involve the reduction of both white and grey matter [4]. A study using MR reported that granule cell density is reduced in DS children to approximately 70% of typically developing children [5,6]. Cerebellum hypoplasia is responsible for muscle hypotonia, problems with movement fluency and axial control (axial truncal muscle), and body balance, coordination, and speech disorders [7–10]. Corpus callosum size is also reduced in children with DS and is associated with mental retardation, problems with coordination, and atypical laterality [6].
Delayed myelination is another cerebral abnormality in DS [11]. Differences are seen from the 22 week of gestation but there are strong manifestations from the 6th month of life [11].
Pathophysiologic processes caused by overexpression of genes located in chromosome 21 lead to:
Degenerative processes of the nervous system caused by overexpression of genes coding peroxidase enzymes: Cu/Zn superoxide dismutase (Cu Zn-SOD; SOD -1),
Disorders in neuronal apoptosis regulation,
Overexpression of genes that code beta amyloid precursor protein (APP),
Processes leading to decreased ratio of neurotransmitters [12,13].
Upregulation of SOD-1 in fetuses with DS leads to oxidative stress [13]. Increased SOD-1 activity in the mitochondrial intermembrane space is responsible for cognitive impairment and delays motor development by increased free radical generation and chronic oxidative stress [12].
DS subjects, mostly those above 40 years of age, present with progressive cognitive impairment resembling the cognitive profile of Alzheimer’s disease [3]. Overexpression of beta amyloid precursor protein (APP) initiates and stimulates neurodegenerative processes resulting in the occurrence of aggregated amyloid fibrils in the brain. It is believed that the overexpression of APP located in chromosome 21 leads to earlier neuronal apoptosis [14–16], which is why the occurrence of dementia symptoms or even Alzheimer’s disease is frequent in patients with DS, mostly as they become older [16]. Other neuropathological Alzheimer-type changes in DS patients are the reduced ratio of neurotransmitters such as N-acetylaspartate (NAA), choline (Cho), myoinositol (mI), gamma-amino butyric acid (GABA) in temporal lobes and reduced ratio of NAA, Cho, mI, glutamate – glutamine complex (Glx) in frontal lobes [14,16]. The reduced ratio of neurotransmitters such as Glx and NAA in frontal lobes and NAA, Cho, mI, GABA in temporal lobes and in the hippocampus in DS children may influence delay in developing motor abilities and may lead to problems associated with memorizing and learning [14,16,17].
Nowadays, the prognosis for children with DS is better than in previous years thanks to advanced medical treatment and educational opportunities. Although congenital heart diseases (in40–50% of children with DS) are still the main cause of death, the survival rate in DS has improved and is reported to be up to 91% at 1 year of age and 85% at age 10 [18]. Similarly, despite increased leukemia susceptibility in children with DS, overall survival rates are approximately 80% [19], which is why life expectancy has increased so much. All of the medical problems that may affect children with DS (e.g., thyroid problems, epilepsy, gastrointestinal defects, orthopedic problems such as atlantoaxial instability, and even leukemia) can be treated by specialists [12]. The purpose of therapy for children with DS is to help them stay healthy and increase their quality of life as early as possible, which is why there are diagnostic methods to determine as early as possible whether the child has DS. Some of these methods are prenatal, for example amniocentesis, chorionic villus sampling, pregnancy ultrasound, or non-invasive prenatal testing (NIPT), which is a molecular approach for assessing fetal aneuploidy using cell-free fetal deoxyribonucleic acid (cffDNA) from the plasma of pregnant women [12,20]. Others are performed after birth, such as fluorescent in situ hybridization followed by chromosomal karyotyping [12].
The sooner the diagnosis is made, the sooner intervention can begin. Knowing that the pathological changes in the number of neurons and changes in the cerebrum size, maturation disorders of the central nervous system, and pathophysiological processes lead to delays in motor development, especially from the 6th month of life, we speculated that motor abilities that children develop after the second half of infancy may develop later than other functions of motor development, in part because children with DS require more time and effort than typically developing children to acquire antigravitional skills, such as standing [21].
Aim
The aim of this study was to examine motor abilities and determine which are significantly delayed in DS children, even if they receive physical therapy. Another purpose of the study was to assess the functional balance as a feature of quality of movement.
Material and Methods
Ethics statement
The study was approved by the Bioethics Committee of the Poznan University of Medical Sciences in 2009 (consent ref. no. 23/10, dated 7 January 2010). Written consents were obtained from the parents of the children enrolled in the study and the consents were signed on behalf of the children enrolled.
The study was conducted during 2009–2011. The study group consisted of 79 children with DS (42 boys, 37 girls), mean age: 6 years and 3 months ±4 years and 6 months. Participants were divided into 3 groups according to (i) age: <3 years old, 3–6 years old, >6 years old, and (ii) motor impairment rating scale: mild (SNR1), moderate (SNR2) and severe (SNR3). All children attended physical therapy sessions 1 time per week for 2 years. The therapy was conducted by NDT Bobath therapists and sensory integration therapists. The therapy was individually tailored for each child because children with DS have a wide variety of symptoms, although that all of them had laxity, low muscle tone, and psychomotor development deficits. The therapy for each child included developing psychomotor abilities according to individual motor skills assessed in each child. Therapy also focussed on developing good quality of motor function and normalization of muscle tone. Training balance reaction and postural maintenance and change were also addressed in each child’s therapy based on the knowledge of cerebellar hypoplasia, which presents in children with DS. Even children who were younger than 12 months were trained by NDT Bobath therapists, trying to normalize the muscle tone by influencing tone patterns, training protection reaction, and balance reaction appropriate to the age of the child and individual level of motor development. The aim of the therapy of the youngest research group was to facilitate standing position with good postural reactions.
The study took place in the greater Poland region, and involved patients with DS coming from towns and villages of the greater Poland region. Even if a child come from rural areas, she or he had the same therapists, and the same frequency of therapeutic meetings as a child who come from urban areas because each person in research group attended to Poznan Rehabilitation and Orthopedic Center, “YES” Association or to Leszno to Polish Association for Persons with Mental Disability “Kolo”. The economic status and education of parents and the influence of these factors on therapy were not considered. The study was approved by the Bioethics Committee of the Poznan University of Medical Sciences.
Children were also assessed using the Gross Motor Function Measure-88 (GMFM-88). The scale was first validated for children with cerebral palsy and is now also validated for children with DS [22–25]. There was no control group because the original validation sample of GMFM-88 included children aged from 5 months to 16 years, so the score of GMFM-88 is the percentage of the score of the original validation group [22–25]. A 5-year-old child without any motor disabilities should present all functions included on the GMFM-88 scale [22–25].
Gross Motor Function Measure-88 (GMFM-88)
GMFM-88 consists of motor functions grouped into 5 dimensions: 1) GMFM – 88 A: lying and rolling (17 items), 2) GMFM – 88 B: sitting (20 items), 3) GMFM – 88 C: crawling and kneeling (14 items), 4) GMFM – 88 D: standing (13), 5) GMFM – 88 E: walking, running, and jumping (24 items) [22]. According to the GMFM-88 guidelines formulated for the assessment of DS children, the environment should be as familiar for the children as possible to encourage the performance of activities [22]. Sometimes, several meetings were needed to assess a child because of the tendency of DS children to get distracted. Assessment of each child was completed within 1 week to avoid changes in motor functions which otherwise might have appeared due to child development. Each item was measured by observation and scored on a 4-point ordinal scale. The 0 value indicated that a child did not initiate the task, 1 point – the child performed less than 10% of a task, 2 points – the child partially completed an item (10% to <100%), 3 points – the child completed an activity (100%) [22–27].
Body balance was estimated by Pediatric Balance Scale (PBS) among children who were able to stand unsupported and who were older than 4 years old [28]. The scale has been created and validated by Franjoine. We assessed all 14 items of PBS on the criterion, based on a 0–4 scale [28]. For instance, retrieving an object from the floor or changing from a standing position to sitting with each test session lasting 10–20 minutes. A child who successfully completed all the tasks could gain a maximum of 56 points. The nearer to maximum the sore is, the better the functional balance in the context of everyday life.
Statistical analysis
Data were analyzed using STATISTICA 8.1 (StatSoft). Nonparametric tests were used to analyze differences between medians in ordinal scales. Mann-Whitney U test was used for comparing two independent samples. A few unrelated samples were tested using Kruskal-Wallis test with Dunn’s multiple comparison post-test. The correlation between samples was measured using Spearman’s rank correlation. P value 0.05 was considered statistically significant.
Results
The median GMFM-88 score for girls was 89.66% (5.1% –100%) and for boys: 91.11% (12.43% – 100%). There was no significant difference in either group divided by sex (p=0.73).
There was no significant difference between motor functions in DS children in different groups divided according to motor impairment rating scale, p=0.56 (Table 1). However, when both age and motor impairment scale were taken into account, a significant difference between groups was observed (Table 2). Two groups with moderate (SNR 2) and severe (SNR3) motor impairment were combined because of the small number of children with severe motor impairment.
Table 1.
Median GMFM-88 score based on motor impairment scale and age.
| GMFM-88 [%] median (minimum – maximum) | P | |||
|---|---|---|---|---|
| Age <3 years old | Age 3–6 years old | Age >6 years old | ||
| SNR 1 | 44.57 (5.1–87.73) | 91.73 (78–98.46) | 98.99 (89.7–100) | <0.0001 |
| SNR 2 – SNR 3 | 27.68 (12.57–43.37) | 89.06 (64.25–98.3) | 92.71 (82.73–100) | 0.0005 |
Kruskal-Wallis’s Test with Dunn’s multiple comparison post-test.
Table 2.
Median GMFM-88 scores based on motor impairment rating scale.
| Motor impairment rating scale (SNR) | P | |||
|---|---|---|---|---|
| SNR 1 | SNR 2 | SNR 3 | ||
| GMFM-88 [%] median (minimum–maximum) | 89.7 (5.1–100) | 92.11 (27.68–100) | 89.42 (12.57–98.06) | 0.56 |
Kruskal-Wallis Test.
Many functions included in GMFM-88 were considered with respect to the typical age when they should be achieved. The standing position was achieved by 10% of children in the first age group (<3 years old) and 95% of children aged 3 to 6 years. Similarly, the walking ability was performed by 10% of children under 3 years old and by 95% of children aged 3 to 6 years.
Functional balance was assessed in 44 children older than 4 years in the research group (26 girls and 18 boys) by Pediatric Balance Scale (PBS). The median score of PBS was 50 points (min. 34 p. – max. 56 p.). There was not any significant correlation between balance score and gender (p=0.7).
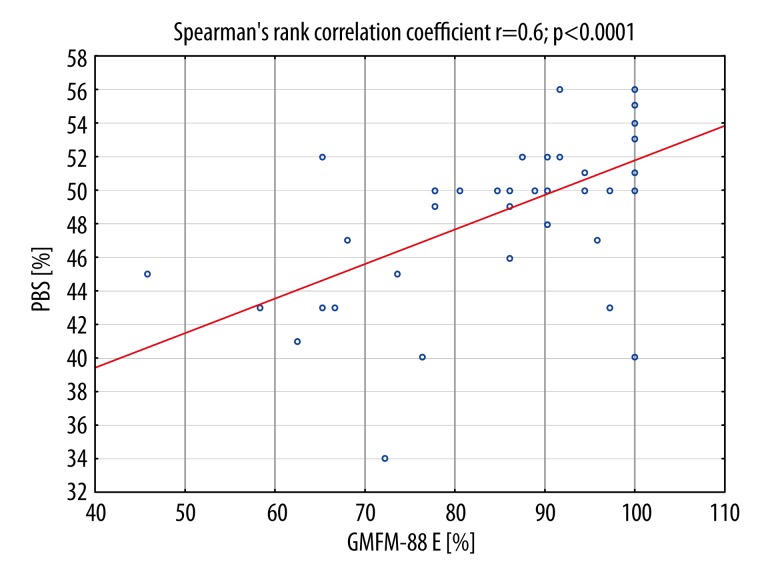
There was a statistically significant correlation between PBS scores and GMFM-88 scores, r=0.7; p<0.0001, especially between balance scores and GMFM – 88 E (walking, running, jumping) (r=0.64; p<0,0001 (Figure 1).
Figure 1.
Positive correlation between Gross Motor Function Measure-88 E (GMFM– 88 E walking, running, jumping) scores and balance score (Pediatric Balance Scale, PBS) in children with Down syndrome.
Discussion
It is common knowledge that DS children develop slowly, but it may not be obvious how slowly their development progresses. None of the DS children in the age group 3 to 6 years old developed 100% of the motor functions evaluated by GMFM-88. It is important to notice that all GMFM-88 functions should be performed by typically developing children at age 5 [29]. There are other studies showing that 6-year-old children with DS do not develop functions typical for 5-year-old children who develop normally [23,26]. The difference in developing motor abilities increases with age and it is approximately 2 years in 5-year old DS children [23]
Retarded motor functions may cause delay in acquiring abilities in areas of development such as mental, emotional, and social [30]. Children explore the world by reaching objects, tasting them, crawling to them [31]. The ability to stand and walk makes their hands free, which enables them to hold an object. It also allows children to better see things because the head is higher than in the earlier stages of motor development. The ability to stand enables a child to explore the world more independently. However, the pathophysiological processes in the brain, changes in the cerebrum size and maturation disorders of the central nervous system, observed in DS children especially from the 6 month of life, cause dysfunctions in motor development. For this reason, psychomotor development is thought to be delayed. When the development of the central nervous system is delayed and the musculoskeletal system is disordered because of low muscle tone, laxity of tendons, and instability of articulations, then the motor development may be delayed. The majority of DS children (95%) in the present study achieved the ability to stand upright at between 3 and 6 years of age. Only 10% of children younger than 3 years old could stand. Children without any disabilities acquire the ability to stand when they are 9–10 months old. The results of the present study that the standing position is the most difficult for infants with Down syndrome to develop in the first year of life, corroborate those obtained by the aforementioned author as well as Piper (2010) and Pereira (2013). The standing position is achieved after acquiring postural alignment between the head, torso, and hip [32,33]. The ability to stand is difficult for children with an additional 21st chromosome because it engages both the flexor and extensor of the trunk. DS children very often present with primary muscle synergies because of muscle hypotonia. This is why children with DS should attend physiotherapy sessions to improve postural alignment, as well as proper distribution of muscle tone and symmetry, thus minimizing psychomotor development delay [33]. In addition, to maintain the standing position children have to be able to keep their bodies balanced. Because DS children have cerebellar hypoplasia, their balance reaction can be disordered.
Walking is another motor ability which DS children develop later than typically developing infants. Most DS children included in the study began walking when they were older than 3 years. The result is similar to the period of developing the walking ability shown by Melyn and White [34]. In his study, Palisano described the 3rd year of life as when children with DS develop the ability to walk [25]. Typically developing children learn to walk during their 1st year of life and sometimes during the 2nd year of life [35]. Walking ability is an example of a motor function that gives children independence, and affects cognitive, social, and subsequent motor development. Childcare starts to be easier when they begin to walk because there is no need to constantly lift and hold infants to change their position. Walking may be difficult for children with DS because it requires good balance. Another reason for delay in developing walking is inherent joint laxity and muscle hypotonia of individuals with DS [12,36]. The low muscle tone and postural abnormalities seen in DS children delay the development of body balance and disorders the balance reactions in the upright position, which may delay walking ability. Infants with DS begin to walk on average about 1 year later than normal infants (ND) [37]. In addition, more individuals with DS appear to have greater instability during walking, particularly in the mediolateral direction and have increased energetic cost [37].
Delays in acquiring motor abilities such as independent standing and walking are also affected by pathophysiologic processes and the shape and volume of the brain, especially the cerebellum, caused by the additional chromosome 21 [2–4]. At present there is no radiological examination we could have used in this study to measure the size of the brain, including the cerebellum. Because there was no indication to perform an examination such as MR or CT, we did not perform any radiological measurement of the cerebellum in the present study. In addition, some children would have needed anesthesia during MR or CT, which may be problematic for them and their parents. Knowing that the cerebellum is responsible for maintaining balance [7], PBS was performed to show cerebellar function. In the measurement of balance, various other methods may be used, including observation of oculovestibular reflexes, vestibulospinal reflexes, and tests using posturography [38]. However, PBS seemed to be the simplest, quickest, and cheapest examination.
Dysfunction in balance may lead to problems in psychomotor abilities [39,40], especially those that are more advanced in childhood motor development, classified as GMFM – 88 E (walking, running, and jumping) [41]. The study showed that there is a correlation between psychomotor ability and GMFM – 88, especially GMFM- 88 E. Balance functions play an important role in development of motor abilities. The cerebellum is important not only for balance but also limb coordination and locomotion [42], and it is well known that the cerebellum is involved in developing motor function at each level of learning motor abilities [10,43]. This is why the therapy for children with DS should consider developing motor skills and improving balance [41,44].
Study limitations
The limitations of the study are associated with the expensive MR examination, 1H-MRS; therefore, information about brain disorders was derived from research literature available in PubMed.
Conclusions
Psychomotor development, especially standing and walking ability, is delayed in DS children even if they attend physical therapy sessions. Functional balance should be considered in therapy of children with DS because balance may influence development of motor abilities, especially those that are developed in childhood.
Abbreviations
- DS
Down syndrome
- MR
magnetic resonance
- GMFM-88
Gross Motor Function Measure-88l
- SNR 1
mild motor impairment
- SNR 2
moderate motor impairment
- SNR 3
severe motor impairment
- NAA
N-acetylaspartate
- Cho
choline
- mI
myoinositol
- GABA
gamma-amino butyric acid
- Glx
glutamate-glutamine complex
- APP
beta amyloid precursor protein
- Cu Zn-SOD, SOD-1
Cu/Zn superoxide dismutase
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Florin T, Ludwig S, Aronson P. Werner H Netter’s Pediatrics. Elsevier; Atlanta: 2011. [Google Scholar]
- 2.Rondal JA, Perera J. Neurobehavioural Specificity. John Wiley and Sons Ltd; West Sussex: 2006. Down syndrome. [Google Scholar]
- 3.Teipel SJ, Alexander GE, Schapiro MB. Age related cortical grey matter reduction in non demented Down’s syndrome adults determined by MRI with voxel – based morphometry. Brain. 2004;127:811–24. doi: 10.1093/brain/awh101. [DOI] [PubMed] [Google Scholar]
- 4.Pinter JD, Eliez S, Schmitt JE, et al. Neuroanatomy of Down’s syndrome: a high-resolution MRI study. Am J Psychiatry. 2001;158:1659–65. doi: 10.1176/appi.ajp.158.10.1659. [DOI] [PubMed] [Google Scholar]
- 5.Baxter LL, Moran TH, Richtsmeier JT, et al. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum Mol Genet. 2000;9:195–202. doi: 10.1093/hmg/9.2.195. [DOI] [PubMed] [Google Scholar]
- 6.Roubertoux PL, Bichler Z, Pinoteau W. Functional analysis of genes implicated in Down syndrome: 2. laterality and corpus callosum size in mice transpolygenic for Down syndrome chromosomal region-1 (DCR-1) Behav Genet. 2005;35:333–41. doi: 10.1007/s10519-005-3225-0. [DOI] [PubMed] [Google Scholar]
- 7.Singer HS, Mink JW, Gilberg DL, Jankovic J. Movements disorders in childhood. Philadelphia: Saunders Elsevier; 2010. [Google Scholar]
- 8.Menkes JH, Sarnat HB, Maria BL. Child Neurology. Philadelphia: Lippincott Williams Wilkins; 2006. [Google Scholar]
- 9.Saavedra S, Joshi A, Woollacott M, van Donkelar P. Eye hand coordination in children with cerebral palsy. Exp Brain Ress. 2009;192(2):155–65. doi: 10.1007/s00221-008-1549-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sveljo O, Culic M, Koprivesk K, Lucic M. The functional neuroimaging evidence of cerebellar involvement in the simple cognitive tasks. Brain Imaging Behav. 2014;8(4):480–86. doi: 10.1007/s11682-014-9290-3. [DOI] [PubMed] [Google Scholar]
- 11.Nadel L. Down’s syndrome: a genetic disorder in biobehavioral perspective. Genes Brain Behav. 2003;2:156–66. doi: 10.1034/j.1601-183x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- 12.Skallerup SJ. Babies with Down syndrome A new parents’s guide. Bathesda: Woodbine Hause; 2008. [Google Scholar]
- 13.Helguera P, Pelsman S, Pigino G, et al. ETS-2 and Neurodegeneration in Down’s Syndrome. J Neurosci. 2005;2(25):2295–303. doi: 10.1523/JNEUROSCI.5107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Śmigielska-Kuzia J, Boćkowski L, Sobaniec W, et al. Amino-acid metabolic processes in the temporal lobes assessed by proton magnetic resonance spectroscopy (1HMRS) in children with Down syndrome. Pharmacol Rep. 2010;62:1070–77. doi: 10.1016/s1734-1140(10)70369-8. [DOI] [PubMed] [Google Scholar]
- 15.Malak R, Kotwicka M, Krawczyk-Wasielewska A, et al. Motor skills, cognitive development and balance functions of children with Down syndrome. Ann Agric Environ Med. 2013;20(4):803–6. [PubMed] [Google Scholar]
- 16.Śmigielska-Kuzia J, Sobaniec W. Brain metabolic profile obtained by proton magnetic resonance spectroscopy HMRS in children with Down syndrome. Adv Med Sci. 2007;52(1):183–87. [PubMed] [Google Scholar]
- 17.Arcos-Burgos M, Londoño AC, Pineda DA, et al. Analysis of brain metabolism by Proton – Magnetic –Resonance – Spectroscopy (1H-MRS) in attention deficit hyperactivity disorders suggests a generalized differentia ontogenic pattern from controls. Atten Defic Hyperact Disord. 2012;4(4):205–12. doi: 10.1007/s12402-012-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layangool T, Sangtawesin C, Kirawittaya T, et al. Survival analysis of Down syndrome with congenital heart disease: a 5-years registry at QSNICH. Med Assoc Thai. 2014;97(Suppl 6):108–14. [PubMed] [Google Scholar]
- 19.Caldwell JT, Ge Y, Taub JW. Prognosis and management of acute myeloid leukemia in patients with Down syndrome. Expert Rev Hematol. 2014;7(6):831–40. doi: 10.1586/17474086.2014.959923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Non-invasive Prenatal Testing [editorial] A Review of the Cost Effectiveness and Guidelines Canadian Agency for Drugs and Technologies in Health [serial online] 2014;Feb [cited 2014 Feb 10]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK274056/ [PubMed] [Google Scholar]
- 21.Tudella E, Pereira K, Basso RP, Savelsbergh GJ. Description of the motor development of 3–12 month old infants with Down syndrome: the influence of the postural body position. Res Dev Disabil. 2011;32(5):1514–20. doi: 10.1016/j.ridd.2011.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Russel DJ, Rosenbaum PL, Avery LM, Lane M. Gross Motor Function Measure (GMFM-6 & GMFM-88) user’s manual. London: Mac Keith Press; 2002. [Google Scholar]
- 23.Russel D, Palisano R, Walter S, et al. Evaluating motor function in children with Down syndrome: validity of GMFM. Dev Med Child Neurol. 1998;40:693–701. doi: 10.1111/j.1469-8749.1998.tb12330.x. [DOI] [PubMed] [Google Scholar]
- 24.Connolly B, Michael B. Performed of retarded children with and without Down syndrome, on the Bruininks – Oseretksy Test of Motor proficiency. Phys Ther. 1986;66:344–48. doi: 10.1093/ptj/66.3.344. [DOI] [PubMed] [Google Scholar]
- 25.Palisano RJ, Walter SD, Russell DJ, et al. Gross Motor Function of children with Down syndrome: creation of motor growth curves. Arch Phys Med Rehabil. 2001;82:494–500. doi: 10.1053/apmr.2001.21956. [DOI] [PubMed] [Google Scholar]
- 26.Connolly B, Morgan S, Russell F. A Longitudinal study of children with Down syndrome who experienced early intervention programming. Phys Ther. 1993;73:170–81. doi: 10.1093/ptj/73.3.170. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd MC, Ulrich DA. The use of the Kick and Drive Gym to increase kicking in infants with Down syndrome. J Sport Exerc Psychol. 2006;28:121. [Google Scholar]
- 28.Franjoine MR, Gunther JS, Taylor MJ. Pediatric balance scale: a modified version of the berg balance scale for the school-age child with mild to moderate motor impairment. Pediatr Phys Ther. 2003;15(2):114–28. doi: 10.1097/01.PEP.0000068117.48023.18. [DOI] [PubMed] [Google Scholar]
- 29.Myrelid A, Gustafsson J, Olars B. Growth charts for Down’s syndrome from birth to 18 years of age. Arch Dis Chold. 2002;87:97–103. doi: 10.1136/adc.87.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang PP, Doherty S, Rouke SB, Bellugi U. Unique profile in visuo-perceptual skills in a genetic syndrome. Brain Cognition. 1995;29:54–65. doi: 10.1006/brcg.1995.1267. [DOI] [PubMed] [Google Scholar]
- 31.Jurkowska M. Biomedical science in the era of complete sequence of human genome. Med Wieku Rozwoj. 2001;5(3):197–212. [in Polish] [PubMed] [Google Scholar]
- 32.Piper MC, Darrah J. Motor assessment of the developing infant. Philadelphia: PA: W.B. Saunders Company; 2010. (1994) [Google Scholar]
- 33.Pereira K, Basso RP, Lindquist ARR. Infants with Down syndrome: Percentage and age for acquisition of gross motor skills. Res Dev Disabil. 2013;34:894–901. doi: 10.1016/j.ridd.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Melyn MA, White DT. Mental and developmental milestones of noninstitutionalized Down’s syndrome children. Pediatrics. 1973;52:542–45. [PubMed] [Google Scholar]
- 35.Brazelton TB, Sparrow JS. Touch points Birth to three. Cambridge Massachusetts: Da Capo Press; 2006. [Google Scholar]
- 36.Agiovlasitis S, McCubbin JA, Yun J, et al. Effects of Down syndrome on three-dimensional motion during walking at different speeds. Gait Posture. 2009;30(3):345–50. doi: 10.1016/j.gaitpost.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Ulrich DA, Ulrich BD, Angulo-Kinzler RM. Treadmill training of infants with Down syndrome: evidence-based developmental outcomes. Pediatrics. 2001;108(5):E84. doi: 10.1542/peds.108.5.e84. [DOI] [PubMed] [Google Scholar]
- 38.Mańko G, Kruczkowski D, Niźnikowski T. The effect of programed physical activity measured with levels of body balance maintenance. Med Sci Monit. 2014;20:1841–49. doi: 10.12659/MSM.889521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gowen E, Miall RC. Behavioural aspects of cerebellar function in adults with Asperger syndrome. Cerebellum. 2005;4:1–11. doi: 10.1080/14734220500355332. [DOI] [PubMed] [Google Scholar]
- 40.Bernard JA, Mittal VA. Cerebellar-motor dysfunction in schizophrenia and psychosis-risk: the importance of regional cerebellar analysis approaches. Front Psychiatry. 2014;25(5):160. doi: 10.3389/fpsyt.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davies PL, Rose JD. Motor skills of typically developing adolescents awkwardness of improvement? Phys Occup Ther Pediatr. 2000;20:19–42. [PubMed] [Google Scholar]
- 42.Morton SM, Bastian AJ. Relative contributions of balance and voluntary leg-coordination deficits to cerebellar gait ataxia. J Neurophysiol. 2003;89:1844–56. doi: 10.1152/jn.00787.2002. [DOI] [PubMed] [Google Scholar]
- 43.Blumenfeld H. Neuroanatmony through clinical cases. Sunderland: Sinauer Associates; 2010. [Google Scholar]
- 44.Kwon JY, Jung Chang H, Young Lee J. Effects of hippotherapy on gait paramaters in children with bilateral spastic cerebral palsy. Arch Phys Med Rehabil. 2001;92:774–79. doi: 10.1016/j.apmr.2010.11.031. [DOI] [PubMed] [Google Scholar]