Abstract
Left ventricular assist devices (LVAD) are an increasingly implemented therapeutic intervention for patients with end-stage heart failure. A growing body of evidence, however, has shown an elevated risk of device thrombosis, a major complication jeopardizing the patient’s post-implantation survival. To date, multiple causative factors for LVAD thrombosis have been identified, such as internal shear stress, device material, infection, and inadequate anticoagulation.
Understanding the mechanisms leading to LVAD thrombosis will not only enable device optimization, but also allow for better patient handling, hence improving post-implantation outcome. In this review we highlight the most commonly identified factors leading to LVAD thrombosis and discuss their mechanisms.
Keywords: Embolism and Thrombosis, Heart-Assist Devices, Postoperative Complications
Background
Left ventricular assist devices (LVAD) are an increasingly implemented therapeutic intervention for patients with end-stage heart failure. A growing body of evidence, however, has shown an elevated risk of device thrombosis, a major complication jeopardizing the patient’s post-implantation survival [1]. The incidence of pump thrombosis has increased with increasing numbers of implantations, ranging from 0.014 to 0.05 events per patient-year [2–4]. To date, multiple causative factors for LVAD thrombosis have been identified, such as internal shear stress, device material, infection, and inadequate anticoagulation. Understanding the mechanisms leading to LVAD thrombosis will not only enable device optimization, but also allow for better patient handling, hence improving post-implantation outcome. In this review we highlight the most commonly identified factors leading to LVAD thrombosis and discuss their mechanisms.
Factors Leading to LVAD Thrombosis
The most common factors leading to LVAD thrombosis can be broken down into two main categories: device-related and non-device-related, the latter including patient and management.
Device-related factors can be further subdivided into those associated with the pump components and others related to the surgical implantation techniques [5,6]. The liability of pump components for de novo thrombosis, including inflow conduit, pump chassis, rotor, and outflow graft was among the early reported reasons prompting the need for pump replacement. The estimated probability for pump replacement need due to this was shown to increase with post-implantation time [7].
Device-Related Factors Leading to LVAD Thrombosis
According to the flow rheology, two main types of LVADs are utilized: continuous flow and pulsatile flow. Continuous flow devices, however, are more frequently implanted compared to pulsatile-flow ones due to their low complication profile. Continuous-flow LVAD has shown superior durability, less surgical trauma, higher energy efficiency, and lower thrombogenicity. The rotor is an integral part of the continuous flow pump and is primarily responsible for creating blood flow. Shear stress generated by rotation is the main reason for device-related thrombosis. In continuous-flow pumps (CF-pumps), two types of rotors are utilized: axial and centrifugal. In axial-flow rotors, the smaller the diameter, the higher the speed it needs to achieve physiological cardiac flow; however, this results in elevated shear stress, which increases the risk of both thrombosis and hemolysis [8]. Additionally, the heat generated from the pump rotors can contribute to thrombus formation, and thrombi are often encountered on the rotor or the inflow bearing, despite designs attempting to minimize the rotation-generated heat [9]. To eliminate the need for mechanical bearings, third-generation LVADs containing magnetically levitated rotors were developed, such as the HVAD® Pump (HeartWare International, Inc., MA, USA) (Figure 1), which adopts a centrifugal flow mechanism. By virtue of centrifugal flow, the third-generation LVADs can achieve the same amount of flow as axial devices, but at significantly lower rotational speed due to their large rotor diameter [8].
Figure 1.

The HVAD® Pump (HeartWare International, Inc., MA, USA). A third-generation, miniaturized, continuous-flow ventricular assist device.
With the application of recent developments and new systems, the incidence of pump thrombosis has decreased. Improvements of the device itself could reduce the risk of thrombosis (e.g., subsequent change in the impeller manufacturing process leading to significant reduction of thrombosis) from 26% to 8% [5]. Further design alternatives were also introduced to minimize the risk of device-related thrombosis; for example, a new version of the outflow graft bend relief portion was introduced, allowing for easier removal of air from the graft [10]. Additionally, graft gelatin seals were provided with the implantation kit as an alternative for pre-clotting [6].
Surgical Factors Leading to LVAD Thrombosis
Surgical implantation techniques, such as positioning of the inflow cannula and the size of the pump pocket, were shown to putatively contribute to thrombosis. In a study by Taghavi et al., a positive correlation between thrombus formation and the angulation of the inflow cannula has been reported [11]. Additionally, the depth of pump pocket was shown to be relatively lower in patients with reported pump thrombosis compared to thrombus-free ones. Thus, in order to maintain a stable inflow rheology in the pump and accordingly reduce the risk of thrombosis, precise positioning of the inflow cannula (Figure 2) and adequately sizing the pump pocket are imperative [10].
Figure 2.
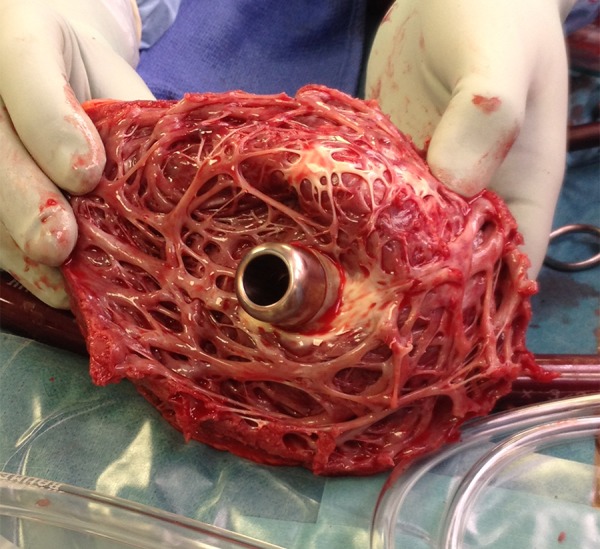
Intraoperative picture showing the precisely positioned inflow cannula of the HVAD® Pump (HeartWare International, Inc., MA, USA) in the left ventricular apex during explantation of the device for transplant.
Non-Device-Related Factors Leading to LVAD Thrombosis
Before discussing the non-device-related factors which might predispose to LVAD thrombosis, it might be helpful to briefly highlight the most prominent hemodynamic features in heart failure (HF) and the underlying molecular background that prompts thrombotic reactions.
Abnormal coagulation is notorious in patients with heart failure [12]. Poor myocardial contractility along with dilated cardiac chambers is the most prominent hallmark of heart failure, which results in low cardiac output and abnormal blood flow. Furthermore, patients with heart failure (HF) demonstrate a dysfunctional endothelium that normally serves as an anticoagulant barrier, as well as elevated plasma levels of certain clotting factors such as von Willebrand factor (vWf), adhesion molecules such as P-selectin, growth factors such as vascular endothelial growth factor (VEGF), and inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1 (IL-1) [13]. Adding to the increased risk of infection, this all serves as a perfect pro-thrombotic milieu, making HF patients highly prone to conditions such as stroke and thromboembolism, which are being seen in almost one-third of patients [14]. In light of this, non-device-related factors which might predispose to LVAD thrombosis can be subdivided into pre-pump, post-pump, and systemic factors.
Pre-pump clots can be generated as a result of the pro-thrombotic environment characterizing HF; such clots can then be sucked into the pump conduit. Adequate anticoagulation and antiplatelet therapy are indispensible to prevent pre-pump clots in the situation of HF [15] (Figure 3). The International Society for Heart and Lung Transplantation has also developed guidelines for mechanical circulatory support that include anticoagulation recommendations [16]. The choice of medications and intensity of antithrombotic therapy varies in adults depending on patient factors, risk of device thrombosis, and institutional preference. The general target is INR value of 2 to 3 [5].
Figure 3.
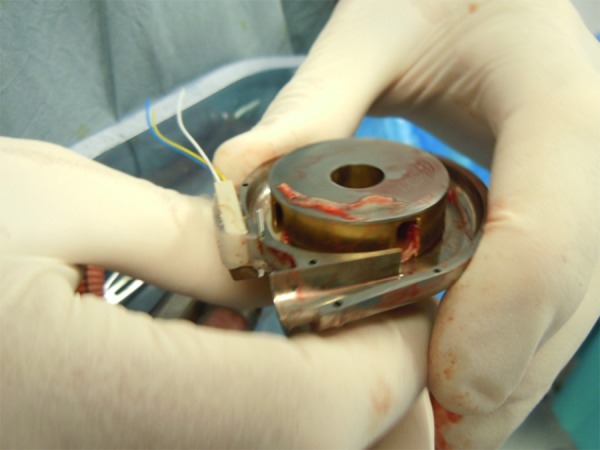
Intraoperative image depicting severe pump thrombosis of a patient with inadequate anticoagulation regime.
Post-pump LVAD thrombosis includes those reported to occur in the aortic root, as well as the aortic valves due to the positioning of the inflow cannula [17,18]. Interestingly, continuous-flow pumps were shown to promote LVAD thrombosis preferably in areas where blood flow stasis is expected, such as the carotid bulb [19]. Finally, infections were reported to confer higher risk of thrombosis and related complications such as neurological dysfunctions in LVAD patients [20].
Short Overview of the Therapeutic Strategies
Thrombus evasion and/or treatment strategies should be evaluated by experts in order to prevent further aggravation of the patient’s state. Systemic and local thrombolytic therapies are the major endeavors employed; however, surgical interventions to exchange the device are in some cases required. Growing evidence suggests the effectiveness of thrombolytic therapy applied directly in the left ventricle or in the device itself. In fact, targeted therapy was shown to significantly reduce systemic bleeding, hypercoagulopathy, and thrombus recurrence. Another advantage of this strategy is the reduced dose of thrombolytic agent required. The time needed for resolving the thrombus ranges between 27 and 37 minutes. Finally, device exchange can be the last resort, especially in cases where high risk of bleeding and/or mortality is suspected, and is usually preformed through medial sternotomy [5].
Conclusions
To sum up, LVADs are lifesaving surgical implants for the management of end-stage HF, which can serve either as a bridge to heart transplantation or as destination therapy. LVAD provides adequate circulatory support and mechanical unloading, which can even reverse the progression of heart failure and improve myocardial function [21]. However, LVAD-associated risk of thrombosis is a critical matter which can dramatically affect patient survival. Factors which influence LVAD thrombosis can be attributed to both the device itself and the disease. Excellent engineering is essential to improve pump mechanics and minimize the implant-associated risks. Equally important, vigilant anti-thrombotic measures are crucial at both pre- and post-operative stages to overcome the fragile hemodynamic physiology of heart failure.
Footnotes
Source of support: Self financing
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370(1):33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013;32:675–83. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein DJ, John R, Salerno C, et al. Algorithm for the diagnosis and management of suspected pump thrombus. J Heart Lung Transplant. 2013;32:667–70. doi: 10.1016/j.healun.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med. 2014;370:33–40. doi: 10.1056/NEJMoa1313385. [DOI] [PubMed] [Google Scholar]
- 5.Sabashnikov A, Mohite PN, Simon AR, Popov AF. HeartWare miniaturized intrapericardial ventricular assist device: advantages and adverse events in comparison to contemporary devices. Expert Rev Med Devices. 2013;10(4):441–52. doi: 10.1586/17434440.2013.811851. [DOI] [PubMed] [Google Scholar]
- 6.Mehra MR, Stewart GC, Uber PA. The vexing problem of thrombosis in long-term mechanical circulatory support. J Heart Lung Transplant. 2014;33(1):1–11. doi: 10.1016/j.healun.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration. Thoratec Corporation issues worldwide medical device correction of HeartMate II® Left Ventricular Assist System. [Accessed December 4, 2013]. Available from: http://www.fda.gov/Safety/Recalls/ArchiveRecalls/2008/ucm112525.htm.
- 8.Birschmann I, Dittrich M, Eller T, et al. Ambient hemolysis and activation of coagulation is different between Heart Mate II and HeartWare left ventricular assist devices. J Heart Lung Transplant. 2014;33(1):80–87. doi: 10.1016/j.healun.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Uriel N, Han J, Morrison KA, et al. Device thrombosis in Heart Mate II continuous-flow left ventricular assist devices: A multifactorial phenomenon. J Heart Lung Transplant. 2014;33(1):51–59. doi: 10.1016/j.healun.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration. Thoratec Corporation, Heart Mate II Left Ventricular Assist System (LVAS): class 1 recall- outflow graft may kink or deform. [Accessed December 4, 2013]. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm298710.htm.
- 11.Taghavi S, Ward C, Jayarajan SN, et al. Surgical technique influences HeartMate II left ventricular assist device thrombosis. Ann Thorac Surg. 2013;96(4):1259–65. doi: 10.1016/j.athoracsur.2013.05.081. [DOI] [PubMed] [Google Scholar]
- 12.Belov D, Lyubarova R, Fein S, Torosoff M. Disseminated intravascular coagulation with congestive heart failure and left ventricular thrombus: A case report with literature review of 7 cases. Am J Case Rep. 2015;16:53–56. doi: 10.12659/AJCR.892380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chong AY, Lip GY. Viewpoint: the prothrombotic state in heart failure: a maladaptive inflammatory response? Eur J Heart Fail. 2007;9(2):124–28. doi: 10.1016/j.ejheart.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow’s triad revisited. J Am Coll Cardiol. 1999;33(5):1424–26. doi: 10.1016/s0735-1097(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 15.Baumann Kreuziger LM. Management of anticoagulation and antiplatelet therapy in patients with left ventricular assist devices. J Thromb Thrombolysis. 2015;39(3):337–44. doi: 10.1007/s11239-014-1162-6. [DOI] [PubMed] [Google Scholar]
- 16.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 international society for heart and lung transplantation guidelines for mechanicalcirculatory support: executive summary. J Heart Lung Transplant. 2013;32(2):157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Freed BH, Jeevanandam V, Jolly N. Aortic root and valve thrombosis after implantation of a left ventricular assist device. J Invasive Cardiol. 2011;23(4):E63–65. [PubMed] [Google Scholar]
- 18.Demirozu ZT, Frazier OH. Aortic valve noncoronary cusp thrombosis after implantation of a nonpulsatile, continuous-flow pump. Tex Heart Inst J. 2012;39(5):618–20. [PMC free article] [PubMed] [Google Scholar]
- 19.Reul JT, Reul GJ, Frazier OH. Carotid-bulb thrombus and continuous-flow left ventricular assist devices: a novel observation. J Heart Lung Transplant. 2014;33(1):107–9. doi: 10.1016/j.healun.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Shah MR, Naftel DC, Miller MA, et al. Thrombotic complications increase after percutaneous site/pocket infection in patients with left ventricular assist devices: An INTERMACS analysis. J Heart Lung Transplant. 2003;32:S84–85. [Google Scholar]
- 21.Song Z, Gu K, Gao B, et al. Hemodynamic effects of various support modes of continuous flow LVADs on the cardiovascular system: A numerical study. Med Sci Monit. 2014;20:733–41. doi: 10.12659/MSM.890824. [DOI] [PMC free article] [PubMed] [Google Scholar]


