Abstract
This paper reviews state-of-the-art microwave ablation (MWA) of tumors. MWA is a novel method for treating inoperable tumors, ie, tumors that cannot be treated surgically. However, patients generally choose removal of the tumor by conventional techniques. A literature review of MWA for breast, liver, lung, and kidney tumors is reported here, with tabulation of our findings according to the type of technique used, with a detailed description of the time, type of microwave generator used, and number of patients treated with MWA. In some cases, the subjects were not human patients, but pig or bovine liver specimens. MWA is a technique that has proved to be promising and likely to be used increasingly in the ablation of cancerous tumors. However, MWA needs to be used more widely to establish itself as a common tool in the treatment of inoperable tumors.
Keywords: tumor, ablation, microwave, review
Introduction
A tumor is a swelling of a part of the body, generally without inflammation, caused by an abnormal growth of tissue, whether benign or malignant.1 Tumors can be cancerous (malignant) or non-cancerous (benign), and occur when cells multiply excessively in the body. Normally, the division and growth of cells occurs in a controlled manner. New cells are created all the time to replace old ones or to perform new functions. Cells that are damaged or are no longer needed die off and are replaced with fresh cells. If the balance of division and cell death is altered, a tumor may appear.2
According to 2012 World Health Organization statistics, the most frequent types of cancer worldwide (ranked according to number of deaths globally) are of the lung, prostate, colon, rectum, stomach, and liver (in men), and breast, colon, rectum, lung, cervix, and stomach (in women). There are several methods available to treat cancerous tumors, the most common being surgery, radiotherapy, and systemic chemotherapy.3 With early detection of tumors, it is possible to use a minimally invasive new technique that works by increasing the temperature inside the tumor hyperthermia, while avoiding damage to healthy adjacent tissue.4
Tumor ablation
Placement of a needle or catheter directly into a tumor and the use of heat, cold, or a chemical to destroy it is known as ablation. It is used most frequently to stop the spread of cancer to the bones or liver, although it can also be used in other organs. Ablation is usually employed when only a limited number of tumors are causing problems.
A common type of ablation, ie, radiofrequency ablation, uses a needle that carries an electric current. The end of the needle is placed into the tumor. Ultrasound or computed tomography can be used to ensure that the needle is correctly positioned. An electrical current is then passed through the needle, heating the tumor and destroying it. In general, radiofrequency ablation is done while the patient is under general anesthesia.9 Another type of ablation, known as cryoablation, uses a probe placed inside the tumor to freeze it, which destroys cancer cells.10 Other methods use heat (laser-induced interstitial thermotherapy) or alcohol to destroy cells.11
Microwave tumor ablation
Microwaves are being used increasingly in medicine for ablation of tumors, causing them to be burned and destroyed. Several prototype antennae have been used to perform microwave ablation (MWA). These antennae are designed to be as small as possible and with increasingly precise targeting power to prevent damage to healthy tissue.
The expectations generated by MWA have also sparked other investigations.24–27 Researchers are developing such antennae to generate heat via microwaves, as show in figure 1, and more patents are being granted for different antenna designs. The development of new software tools and other advances have led to the appearance of new methods for application of microwaves to ablate tumors.28–32 The figure 2 show the simulation of Microwave applicator inserted in breast tissue. Many research groups are investigating the effectiveness of MWA in the treatment of cancer. As they progress, there are more studies published on the evaluation, the comparison to other techniques and the application of ablation using microwaves.33–46 The tables included in this paper show the main features of some of the studies published in recent years.
Figure 1.

Applicator for microwave ablation.
Figure 2.
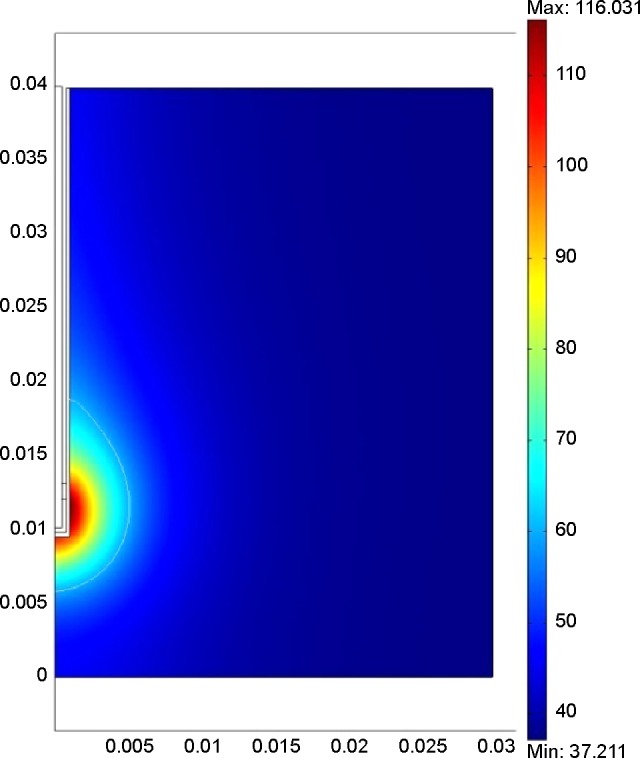
Simulation of microwave applicator inserted in breast tissue.
Table 1 shows that MWA offers a good ablation zone of approximately 3–4 cm (in one experiment, the ablation zone was 6.45 cm). In the last study mentioned in this table, the applicator was an array of three antennae, and the paper concludes that a single antenna can ablate tumors less than 4 cm in length and avoid damage to the lung.
Table 1.
Ablation of pig and bovine liver and lung tumors
| Microwave generator | Antenna | Time | Patients/tumors | Comments |
|---|---|---|---|---|
| 2,450 MHz 70–100 W5 | Microsulis Americas Inc, Waltham, MA, USA, 5.7 mm diameter | 4 minutes | Pig liver | Good in liver tissue. The ablation diameters were 3–6.45 cm. |
| 915 MHz 50–80 W6 | 1.9 mm diameter antenna | 10 minutes | Bovine liver | Two antennas were used to achieve a larger and more spherical zone of ablation and coagulation. More desirable coagulation geometry could be obtained by simultaneous application of double antennae at 70 W. |
| 2,450 MHz 60 or 80 W7 | 14 gauge antenna | 10 minutes | 8 pig livers | Effective control of the ablation zone was achieved. |
| 2,450 MHz 60 W8 | 14 gauge antenna | 10 minutes | 15 pigs with 56 tumors | Three different antenna arrays were compared. The three-antenna array was connected to the death of two pigs. |
The tables that follow show the results using different applicators to treat tumors in human patients. Many researchers have used the liver to test MWA because it is an organ that is difficult to operate on, and therefore alternative treatments, such as MWA, are appealing. Table 2 shows that MWA is an effective alternative treatment, with outcomes similar to those of surgery. The difference between the test frequencies was the therapy time; a frequency of 2,450 MHz allows more ablation than others. In further clinical trials conducted in 60 patients with 96 tumors measuring 1–8 cm, complete ablation was observed in 89 tumors.7
Table 2.
Ablation of liver tumors in patients
| Microwave generator | Antenna | Time | Patients/tumors | Comments |
|---|---|---|---|---|
| 915 MHz 40 W12 | 3.7 cm of effective radiation | 10 minutes | 6 patients with 16 liver metastases | They require improvements before MWA can be used on a routine basis. |
| 2,450 MHz 20–80 W13 | 14 gauge, Amica-Gen, HS Hospital Service SpA, Aprilia, Italy | 8–10 minutes | 6 patients | 5 patients showed disease-free survival. |
| 915 MHz, 2,450 MHz14 | 1–3 13 gauge antennae | 9.7 minutes with 915 MHz, 6.6 minutes with 2,450 MHz | 48 patients with 124 tumors, 72 with 915 MHz, 52 with 2,450 MHz | Both systems manage ablation of liver tumors but the 2,450 MHz system achieves more rapid and predictable ablations. |
| 2,450 MHz 100 W15 | 5 mm antenna | 4 minutes | 140 patients in 18 hospitals with 299 tumors | Morbidity was 8.3% and in-hospital mortality was 1.9%. |
| 902–928 MHz 10–32 W16 | 14 gauge antenna | 10 minutes | 10 tumors in 10 patients (5 male, 5 female) | 6 of <3 cm tumors showed complete necrosis and the rest had 50% partial necrosis. |
| 2,450 MHz 60–100 W17 | 14 or 16 gauge antenna | 5–15 minutes | 736 patients with 1,037 tumors in 14 hospitals | 22 major complications and 54 minor complications were observed, with no mortality. |
| 915 MHz 45 W18 | Not specified | 10 minutes | 87 patients with 224 tumors | The mortality rate was 2.3%, local recurrence occurred in 6 tumors and regional recurrence occurred in 37 tumors. |
| 915 MHz and 2,450 MHz19 | 14.5 gauge antenna for the 915 MHz generator and 14 gauge antenna for the 2,450 MHz generator | 10 minutes | 15 patients with 19 inoperable tumors | 100% success, with only 2 cases of complications at 8 months of follow-up. A 915 MHz generator was used in 11 patients and 2,450 MHz in the remaining 4. |
Abbreviation: MVA, microwave ablation.
The conclusion of Tables 3 and 4 is similar to that for liver tumors, ie, MWA is a viable treatment option for lung and kidney tumors, with results similar to those of surgery when patients are followed up at 1, 3, and 6 months for lung and kidney tumors, and complete necrosis were seen in most patients.
Table 3.
Ablation of lung tumors in patients
| Microwave generator | Antenna | Time | Patients/tumors | Comments |
|---|---|---|---|---|
| 915 MHz 45 W20 | 14.5 gauge antenna | 10 minutes | 24 patients with 26 inoperable tumors | Technical success in 100%, without major complications. 1, 3, and 6 months and annually follow, was observed complete necrosis in 61.6% of lesions. Partial necrosis in 30.8% and progression of disease in only one case. |
| 2,450 MHz 120 W or 180 W21 | 1.8 mm diameter antenna | 180 W: 2 minutes in <2 cm tumors, 3.5 minutes in 2–3 cm tumors, 4–6 minutes in 3–5 cm tumors 120 W: 1 minute for 1 cm tumors, 8 minutes for 2.4 cm tumors | 23 patients with 29 tumors | Recurrence was assessed at 1, 3, and 6 months after ablation. In 93% of patients ablation was successful, 6 months of local recurrence was identified in 3 of 26 lesions, giving a local control rate of 88%. |
| 902–928 MHz 10–32 W22 | 14 gauge antenna | 10 minutes | 10 patients | 5 of 10 specimens were clearly measurable with a maximum diameter of ablation of 4.8 cm and volume of zone of ablation was on average 15.1 cm. |
| 915 MHz 45 W23 | 14.5 gauge antenna | 10 minutes | 9 patients with 10 tumors | Patients were followed up at 1, 3, and 6 months, concluding that MWA is a valid alternative to other techniques of ablation. |
Abbreviation: MVA, microwave ablation.
Table 4.
Ablation of kidney tumors in patients
| Microwave generator | Antenna | Time | Patients/tumors | Comments |
|---|---|---|---|---|
| 915 MHz 45 W47 | 14.5 gauge antenna | 10 minutes | 12 patients | Patients had a 3–14-month follow-up to observe the therapeutic effects and complications. There were no serious complications or unexpected side effects after ablation. |
| 2,450 MHz 50 W48 | 15 gauge antenna coated with ethylene polytetrafluoride | 8 minutes | 48 patients with MWA, 54 patients with partial nephrectomy | 3-year survival was 91.3% for MWA and 96% for partial nephrectomy. |
Abbreviation: MVA, microwave ablation.
There are presently no data available on the use of MWA in breast cancer. However, in 2011, Cepeda suggested using MWA for this type of cancer. Figure 3 shows the injury caused by the applicator, which was used in the ex vivo test. In his doctoral thesis, he elaborated the design of an applicator for minimally invasive MWA, performing a computational analysis by finite element modeling, and then validated the model with experiments on phantoms and ex vivo porcine tissue breast.49
Figure 3.
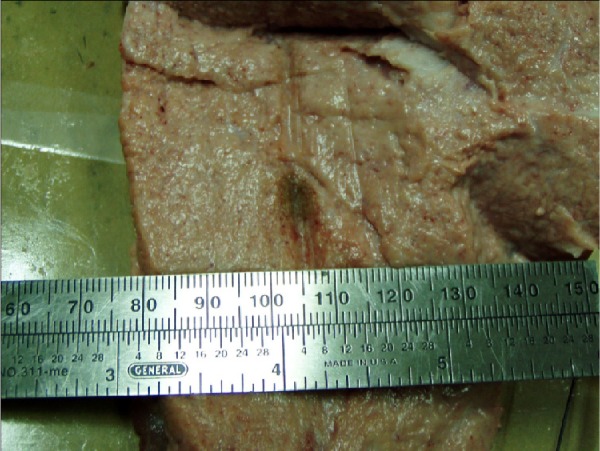
Ablation of breast tissue ex vivo at 2,450 MHz and 10 W.
Conclusion
MWA has proven to be a successful therapeutic tool in the treatment of cancer. Its use is expected to increase in frequency for ablation of tumors. Although many successful tests have been done, MWA has not progressed to widespread use in dedicated cancer treatment centers, in particular for inoperable tumors (even though most of the clinical studies have been done in people who could not be treated by surgery). The advantages of MWA need to be recognized more widely, given that it has been proven to be effective. Hospitals should consider acquiring the expertise and infrastructure needed to establish MWA for the treatment of cancers, and thus offer patients an alternative treatment option.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.The Oxford English Dictionary. Oxford, UK: Oxford University Press; 2010. [Google Scholar]
- 2.National Service of Medicine of the United States [Accessed January, 2014]. Available from: www.nlm.nih.gov.
- 3.World Health Organization GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [Accessed May 6, 2015]. Available from: http://www.who.int/cancer/en/
- 4.Storm FK.Hyperthermia Paper presented at the Microwave Symposium Digest, IEEE MTT-S InternationalJune 15–19, 1981Los Angeles, CA, USA. [Google Scholar]
- 5.Garrean S, Hering J, Saied A, et al. Ultrasound monitoring of a novel microwave ablation (MWA) device in porcine liver: lessons learned and phenomena observed on ablative effects near major intrahepatic vessels. J Gastrointest Surg. 2009;13(2):334–340. doi: 10.1007/s11605-008-0715-4. [DOI] [PubMed] [Google Scholar]
- 6.Shi W, Liang P, Zhu Q, et al. Microwave ablation: results with double 915 MHz antennae in ex vivo bovine livers. Eur J Radiol. 2011;79(2):214–217. doi: 10.1016/j.ejrad.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Jiao D, Qian L, Zhang Y, et al. Microwave ablation treatment of liver cancer with 2,450-MHz cooled-shaft antenna: an experimental and clinical study. J Cancer Res Clin Oncol. 2010;136(10):1507–1516. doi: 10.1007/s00432-010-0808-9. [DOI] [PubMed] [Google Scholar]
- 8.Planché O, Teriitehau C, Boudabous S, et al. In vivo evaluation of lung microwave ablation in a porcine tumor mimic model. Cardiovasc Interv Radiol. 2013;36(1):221–228. doi: 10.1007/s00270-012-0399-8. [DOI] [PubMed] [Google Scholar]
- 9.Tomas V, Jan V. Ablation applicator for destructive hyperthermia treatment. J Phys Conf Ser. 2011;329(1):012035. [Google Scholar]
- 10.Shinohara K. Cryotherapy. Int J Clin Oncol. 2007;12(6):416–426. doi: 10.1007/s10147-007-0708-4. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society [Accessed January 14, 2014]. Available from: www.cancer.org.
- 12.Hompes R, Fieuws S, Aerts R, Thijs M, Penninckx F, Topal B. Results of single-probe microwave ablation of metastatic liver cancer. Eur J Surg Oncol. 2010;36(8):725–730. doi: 10.1016/j.ejso.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 13.Zanus G, Boetto R, Gringeri E, et al. Microwave thermal ablation for hepatocarcinoma: six liver transplantation cases. Transplant Proc. 2011;43(4):1091–1094. doi: 10.1016/j.transproceed.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Simo KA, Tsirline VB, Sindram D, et al. Microwave ablation using 915-MHz and 2.45-GHz systems: what are the differences? HPB (Oxford) 2013;15(12):991–996. doi: 10.1111/hpb.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd DM, Lau KN, Welsh F, et al. International multicentre prospective study on microwave ablation of liver tumours: preliminary results. HPB (Oxford) 2011;13(8):579–585. doi: 10.1111/j.1477-2574.2011.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratanaprasatporn L, Charpentier KP, Resnick M, Lu S, Dupuy D. Intra-operative microwave ablation of liver malignancies with tumour permittivity feedback control: a prospective ablate and resect study. HPB (Oxford) 2013;15(12):997–1001. doi: 10.1111/hpb.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livraghi T, Meloni F, Solbiati L, Zanus G. Complications of microwave ablation for liver tumors: results of a multicenter study. Cardiovasc Interv Radiol. 2012;35(4):868–874. doi: 10.1007/s00270-011-0241-8. [DOI] [PubMed] [Google Scholar]
- 18.Iannitti DA, Martin RCG, Simon CJ, et al. Hepatic tumor ablation with clustered microwave antennae: the US Phase II Trial. HPB (Oxford) 2007;9(2):120–124. doi: 10.1080/13651820701222677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veltri A, Gazzera C, Rotondella C, Camerano F, Busso M, Gandini G. Image-guided microwave ablation of hepatic tumours: preliminary experience. Radiol Med. 2012;117(3):378–392. doi: 10.1007/s11547-011-0745-y. [DOI] [PubMed] [Google Scholar]
- 20.Carrafiello G, Mangini M, Fontana F, et al. Microwave ablation of lung tumours: single-centre preliminary experience. Radiol Med. 2014;119(1):75–82. doi: 10.1007/s11547-013-0301-z. [DOI] [PubMed] [Google Scholar]
- 21.Little M, Chung D, Boardman P, Gleeson F, Anderson E. Microwave ablation of pulmonary malignancies using a novel high-energy antenna system. Cardiovasc Interv Radiol. 2013;36(2):460–465. doi: 10.1007/s00270-012-0465-2. [DOI] [PubMed] [Google Scholar]
- 22.Wolf FJ, Aswad B, Ng T, Dupuy DE. Intraoperative microwave ablation of pulmonary malignancies with tumor permittivity feedback control: ablation and resection study in 10 consecutive patients. Radiology. 2012;262(1):353–360. doi: 10.1148/radiol.11110015. [DOI] [PubMed] [Google Scholar]
- 23.Carrafiello G, Mangini M, De Bernardi I, et al. Microwave ablation therapy for treating primary and secondary lung tumours: technical note. Radiol Med. 2010;115(6):962–974. doi: 10.1007/s11547-010-0547-7. [DOI] [PubMed] [Google Scholar]
- 24.Cavagnaro M, Amabile C, Bernardi P, Pisa S, Tosoratti N. A minimally invasive antenna for microwave ablation therapies: design, performances, and experimental assessment. IEEE Trans Biomed Eng. 2011;58(4):949–959. doi: 10.1109/TBME.2010.2099657. [DOI] [PubMed] [Google Scholar]
- 25.Prakash P, Converse MC, Webster JG, Mahvi DM. An optimal sliding choke antenna for hepatic microwave ablation. IEEE Trans Biomed Eng. 2009;56(10):2470–2476. doi: 10.1109/TBME.2009.2025264. [DOI] [PubMed] [Google Scholar]
- 26.Keangin P, Rattanadecho P, Wessapan T. An analysis of heat transfer in liver tissue during microwave ablation using single and double slot antenna. Int Comm Heat Mass Transfer. 2011;38(6):757–766. [Google Scholar]
- 27.He N, Wang W, Ji Z, Li C, Huang B. Microwave ablation: an experimental comparative study on internally cooled antenna versus non-internally cooled antenna in liver models. Acad Radiol. 2010;17(7):894–899. doi: 10.1016/j.acra.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Phasukkit P, Sanpanich A, Tungjitkusolmun S, Hamamoto K. Effect of phase difference in multi-antenna microwave thermal ablation for breast cancer treatment. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:3718–3721. doi: 10.1109/EMBC.2013.6610351. [DOI] [PubMed] [Google Scholar]
- 29.Sindram D, Swan RZ, Lau KN, McKillop IH, Iannitti DA, Martinie JB. Real-time three-dimensional guided ultrasound targeting system for microwave ablation of liver tumours: a human pilot study. HPB (Oxford) 2011;13(3):185–191. doi: 10.1111/j.1477-2574.2010.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Top CB, Gençer NG. Harmonic motion microwave Doppler imaging: a simulation study using a simple breast model. IEEE Trans Med Imaging. 2014;33(2):290–300. doi: 10.1109/TMI.2013.2284234. [DOI] [PubMed] [Google Scholar]
- 31.Kurumi Y, Tani T, Naka S, et al. MR-guided microwave ablation for malignancies. Int J Clin Oncol. 2007;12(2):85–93. doi: 10.1007/s10147-006-0653-7. [DOI] [PubMed] [Google Scholar]
- 32.Keangin P, Rattanadecho P. Analysis of heat transport on local thermal non-equilibrium in porous liver during microwave ablation. Int Comm Heat Mass Transfer. 2013;67:46–60. [Google Scholar]
- 33.Wu H, Exner AA, Krupka TM, Weinberg BD, Patel R, Haaga JR. Radiofrequency ablation: post-ablation assessment using CT perfusion with pharmacological modulation in a rat subcutaneous tumor model. Acad Radiol. 2009;16(3):321–331. doi: 10.1016/j.acra.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denys A, Lachenal Y, Duran R, Chollet-Rivier M, Bize P. Use of high-frequency jet ventilation for percutaneous tumor ablation. Cardiovasc Intervent Radiol. 2014;37(1):140–146. doi: 10.1007/s00270-013-0620-4. [DOI] [PubMed] [Google Scholar]
- 35.Swan R, Sindram D, Martinie J, Iannitti D. Operative microwave ablation for hepatocellular carcinoma: complications, recurrence, and long-term outcomes. J Gastrointest Surg. 2013;17(4):719–729. doi: 10.1007/s11605-013-2164-y. [DOI] [PubMed] [Google Scholar]
- 36.Simo KA, Sereika SE, Newton KN, Gerber DA. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;104(7):822–829. doi: 10.1002/jso.21933. [DOI] [PubMed] [Google Scholar]
- 37.Skonieczki BD, Wells C, Wasser EJ, Dupuy DE. Radiofrequency and microwave tumor ablation in patients with implanted cardiac devices: is it safe? Eur J Radiol. 2010;79(3):343–346. doi: 10.1016/j.ejrad.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Brannan JD. Electromagnetic measurement and modeling techniques for microwave ablation probes. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3076–3078. doi: 10.1109/IEMBS.2009.5332536. [DOI] [PubMed] [Google Scholar]
- 39.Martin RG, Scoggins C, McMasters K. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17(1):171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 40.Carrafiello G, Mangini M, Fontana F, et al. Complications of microwave and radiofrequency lung ablation: personal experience and review of the literature. Radiol Med. 2012;117(2):201–213. doi: 10.1007/s11547-011-0741-2. [DOI] [PubMed] [Google Scholar]
- 41.Lubner MG, Brace CL, Hinshaw JL, Lee FT., Jr Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010;21(8):S192–S203. doi: 10.1016/j.jvir.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka T, Westphal S, Isfort P, et al. Microwave ablation compared with radiofrequency ablation for breast tissue in an ex vivo bovine udder model. Cardiovasc Interv Radiol. 2012;35(4):914–920. doi: 10.1007/s00270-011-0253-4. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y, Yang J. Microwave endometrial ablation for endometrial protection in women with breast cancer on adjuvant tamoxifen. J Obstet Gynaecol Res. 2013;39(9):1411–1414. doi: 10.1111/jog.12072. [DOI] [PubMed] [Google Scholar]
- 44.Wolf FJ, Dupuy DE, Machan JT, Mayo-Smith WW. Adrenal neoplasms: effectiveness and safety of CT-guided ablation of 23 tumors in 22 patients. Eur J Radiol. 2012;81(8):1717–1723. doi: 10.1016/j.ejrad.2011.04.054. [DOI] [PubMed] [Google Scholar]
- 45.Lehman D, Landman J. Cryoablation and radiofrequency for kidney tumor. Curr Urol Rep. 2008;9(2):128–134. doi: 10.1007/s11934-008-0024-1. [DOI] [PubMed] [Google Scholar]
- 46.Siperstein A, Garland A, Engle K, et al. Local recurrence after laparoscopic radiofrequency thermal ablation of hepatic tumors. Ann Surg Oncol. 2000;7(2):106–113. doi: 10.1007/s10434-000-0106-x. [DOI] [PubMed] [Google Scholar]
- 47.Carrafiello G, Mangini M, Fontana F, et al. Single-antenna microwave ablation under contrast-enhanced ultrasound guidance for treatment of small renal cell carcinoma: preliminary experience. Cardiovasc Interv Radiol. 2010;33(2):367–374. doi: 10.1007/s00270-009-9745-x. [DOI] [PubMed] [Google Scholar]
- 48.Guan W, Bai J, Liu J, et al. Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol. 2012;106(3):316–321. doi: 10.1002/jso.23071. [DOI] [PubMed] [Google Scholar]
- 49.Cepeda MFJ. Estudio y Desarrollo de Aplicadores Coaxiales Tipo Slot de Ablación por Microondas para el Tratamiento Mínimamente Invasivo del Cáncer de Mama. México: Bioelectronica, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional; 2011. [Google Scholar]


