Abstract
Background
Triple-negative breast cancer (TNBC) is a heterogeneous subtype of breast cancer that is prone to recurrence and metastasis with worse prognosis. Epirubicin-based chemotherapy is of great importance for patients with TNBC, but resistance to epirubicin severely limits the application of this drug and this has emerged as a major problem in the treatment of TNBC. The ubiquitin protein D (UBD) molecule has often been considered a tumor oncogene, and has been shown to promote the recurrence and metastasis of malignant tumor cells. Since the role of UBD in epirubicin resistance and its prognostic value in TNBC have not been reported, the study reported here was designed to identify the epirubicin-resistance molecule and clarify the related biomarker for TNBC prognosis.
Methods
UBD plasmid was transfected into MDA-MB-231 cells, and the cells were exposed to epirubicin to observe the ability of UBD in epirubicin resistance. UBD expression was also detected in 78 breast cancer tissues by immunohistochemistry. Statistical methods were used to study the relationship between UBD expression and epirubicin resistance in TNBC treatment. Kaplan–Meier survival analysis was used to determine the correlation between UBD expression and TNBC patients’ prognostic parameters.
Results
UBD expression was found increased in breast cancer tissues. Forced UBD expression was found to have a relationship with TNBC epirubicin resistance in vitro. High expression of UBD was found in TNBC, compared with in non-TNBC, and this played a positive role in epirubicin resistance and indicated the poor prognosis of TNBC treatment.
Conclusion
UBD may play an important role in epirubicin resistance in TNBC. UBD has the potential to be a novel biomarker in TNBC chemoresistance and may be a promising therapeutic target for TNBC patients.
Keywords: prognosis, chemoresistance, biomarker
Introduction
Breast cancer is a highly prevalent cancer in women around the world.1 According to gene expression, five main intrinsic molecular subtypes of breast cancer are known: luminal A, luminal B, human epidermal growth factor receptor 2 (HER2) enriched, basal like, and claudin low.2 Triple-negative breast cancer (TNBC) is defined by the lack of expression of the estrogen receptor, progesterone receptor, and HER2/neu.3 Due to the lack expression of hormone receptors and HER2, endocrine therapy and specific molecule target therapy are not available for TNBC. Further, TNBC is characterized by uncontrolled proliferation, and it easily invades and metastasizes to distant organ sites, such as the lung, liver, brain, and bone.4 Thus, TNBC patients frequently develop therapeutic resistance and rapid disease relapse, which bring a higher risk of death in these patients. Chemotherapy remains one of the most important anticancer methods in TNBC. Epirubicin is an antineoplastic agent belonging to the second-generation anthracycline antibiotics, which plays an antitumor role by binding to DNA, bursting and stabilizing the fragmentation process of double-stranded DNA.5 In clinic, epirubicin has been widely used in the treatment of breast, ovarian, bladder, lung, bronchus, pancreatic, stomach, esophagus, and liver cancers.4
The ubiquitin protein D (UBD) molecule belongs to the ubiquitin protein family, and the binding to its target protein will result in the protein degrading due to the proteasome.6 Through degradation of I kappa B alpha and the intrinsic immune activation induced by lipopolysaccharides (LPS),6 UBD participates in the process of maturing dendritic cells.7 Therefore, previous studies have focused on its role in the immune system. Research has revealed the important role of UBD expression in liver cancer. However, as far as we are aware, little research has been conducted exploring the role of UBD expression in TNBC chemoresistance and its relationship with the prognosis of patients with TNBC.
In the study reported here, we established the role of UBD in epirubicin resistance by studying the TNBC cell line MDA-MB-231. Further, we found enhanced UBD expression in breast cancer tissue and the clinical-pathological significance of this. We also studied the expression of UBD and its correlation with progression-free survival (PFS) in epirubicin-treated TNBC patients, and found that UBD plays an important role in epirubicin resistance in TNBC.
Materials and methods
Cell culture
The human TNBC cell line MDA-MB-231 was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum (FBS; Gibco®, Thermo Fisher Scientific, Waltham, MA, USA), and cultured at 37°C in humidified atmosphere of 95% air/5% CO2.
Plasmid
The plasmid pcDNA3-UBD and the scramble pcDNA3 were provided by the Laboratory of Signal Transduction, Eastern Hepatobiliary Surgery Institute, Second Military Medical University, Shanghai, People’s Republic of China.
Proliferation assay
Cell proliferation was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded at a density of 6,000 cells per well in 96-well plates. After 24 hours, the cells were exposed to varied concentrations of epirubicin or equal volumes of sterile apyrogenic water for indicated time (0 hour, 36 hours, 72 hours, 96 hours).
Then the cells were incubated with 0.5 mg/mL MTT (Sigma-Aldrich Co, St Louis, MO, USA) reagent for 4 hours’ incubation at 37°C. Subsequently, the culture medium was removed and 150 μL of dimethyl sulfoxide was added to each well. The formazan crystals were solubilized by gently shaking the plates for 10 minutes and the absorbance was measured at 570 nm. Cell proliferation was calculated as a percentage of the control and the half maximal inhibitory concentration was determined from the dose-dependent growth inhibition data using GraphPad Prism (Version 5.01; GraphPad Software, Inc, San Diego, CA, USA).
Invasion assay
The invasive ability of cells was evaluated using 24-well Transwell® chambers containing an 8 μm pore polycarbonate filter (Corning Incorporated, Corning, NY, USA) coated with Matrigel™ (BD Biosciences, San Jose, CA, USA). The bottom chamber was filled with 600 μL standard medium containing 10% FBS. Cells were plated at a density of 5×104 in the upper chamber suspended in 200 μL serum-free medium supplemented with 20 nM Epirubicin. After incubation for 48 hours, the non-invaded cells were removed with a cotton-tip swab. Invaded cells that stuck to the lower surface of the membrane were fixed in methanol and stained with crystal violet. The cells were photographed and counted under a microscope at 200× magnification in six random fields from triplicate wells.
Tissue specimens and patient information
This study was approved by the Ethics Committee of the General Hospital of Shenyang Military Region. Tissue specimens were collected from 78 breast cancer patients treated in our hospital between 2006 and 2011. Pathological diagnosis was achieved by surgery. All the patients were recommended to receive epirubicin-based chemotherapy. Clinicopathologic staging was determined according to the AJCC Cancer Staging Manual criteria.8 Tissue specimens were fixed in 4% paraformaldehyde and embedded in paraffin for immunohistochemistry (IHC).
Detailed clinicopathologic characteristics of the patients are summarized in Table 1.
Table 1.
Clinical pathologic characteristics
| Variable | UBD high expression (n=39) | UBD low expression (n=39) | P |
|---|---|---|---|
| Median age, years (range) | 48 (37–69) | 51 (34–70) | 0.470 |
| Sex, n | |||
| Female | 39 | 39 | |
| Smoking, n (%) | |||
| Yes | 5 (12.80) | 7 (17.90) | 0.530 |
| No | 34 (87.20) | 32 (82.10) | |
| Alcohol history, n (%) | |||
| Yes | 7 (8.97) | 6 (7.69) | 0.760 |
| No | 32 (91.03) | 33 (92.31) | |
| Tumor stage, n (%) | |||
| T1/2 | 33 (84.60) | 36 (92.30) | 0.290 |
| T3/4 | 6 (15.40) | 3 (7.70) | |
| Nodal stage, n (%) | |||
| N0/1 | 26 (66.70) | 30 (76.90) | 0.310 |
| N2/3 | 13 (33.30) | 9 (23.10) | |
| Triple negative,* n (%) | |||
| Yes | 21 (53.80) | 7 (17.90) | 0.001 |
| No | 18 (46.20) | 32 (82.10) | |
| AJCC stage, n (%) | |||
| I–II | 20 (51.30) | 21 (53.80) | 0.820 |
| III | 19 (48.70) | 18 (46.20) | |
| Tumor diameter, n (%) | |||
| >5 cm | 9 (23.10) | 6 (15.40) | 0.390 |
| <5 cm | 30 (76.90) | 33 (84.60) |
Note:
Results of the analysis (P<0.05) are statistically significant (chi-square test).
Abbreviations: AJCC, American Joint Committee on Cancer; UBD, ubiquitin protein D.
Immunohistochemistry and analysis
Immunohistochemical analysis of tumor sections was performed using the anti-UBD antibody. Briefly, the sections were incubated with the primary antibody at 4°C overnight and then with horseradish peroxidase-conjugated secondary antibody at 37°C for 30 minutes. The sections were finally incubated with diaminobenzidine and counterstained with hematoxylin for detection. Assessment of the staining was based on the percentage of positively stained cells and the nuclear staining intensity evaluated by three pathology doctors. The procedure of human sample collection was approved by the ethical committee of the hospital.
Real-time polymerase chain reaction
Quantitative polymerase chain reaction (PCR) was performed using an Applied Biosystems SYBR® Green PCR Kit and Applied Biosystems 7900HT Fast Real-Time PCR System (both Thermo Fisher Scientific). The mRNA level of specific genes was normalized against 18S. The primer sequences used in this study are listed in Table 2.
Table 2.
Sequence of primers for real-time polymerase chain reaction
| Primer | Sequence (5′ to 3′) |
|---|---|
| UBD forward primer | TCATATGGGTTGGCATCAAA |
| UBD reverse primer | TGTCTGCAGAGATGGCTCC |
| 18s forward primer | CGGCTACCACATCCAAGGAA |
| 18s reverse primer | GCTGGAATTACCGCGGCT |
Abbreviation: UBD, ubiquitin protein D.
Western blot
The cell extract was separated on polyacrylamide-sodium dodecyl sulfate gels, transferred, and probed with a specific primary antibody. The protein band, specifically bound to the primary antibody, was detected using an IRDye® 800CW-conjugated secondary antibody and LI-COR imaging system (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
All data in this study were analyzed with SPSS software (v 18.0; IBM Corporation, Armonk, NY, USA). Reports were presented as mean values ± standard error of the mean. The difference between two groups or paired samples was analyzed by Student’s t-test. The differences of quantitative variables between groups were analyzed by one-way analysis of variance. Correlation analysis was performed using Pearson’s correlation test. Survival curves were calculated using the Kaplan–Meier method and compared with a log-rank test. All statistical differences were two-sided, and P<0.05 was considered statistically significant.
Results
High expression of UBD enhanced the epirubicin resistance in TNBC cell line
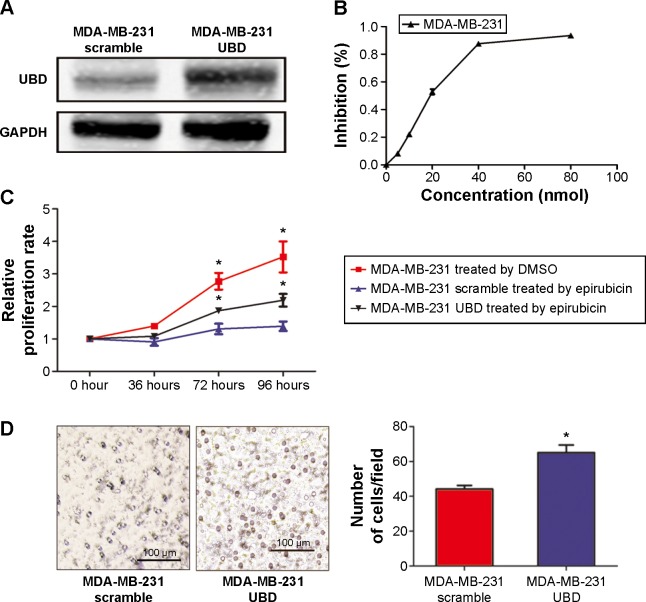
To identify the effect of UBD on epirubicin resistance in a TNBC cell line, MDA-MB-231 cells were transfected with UBD plasmid and the control scramble (Figure 1A). To determine the concentration of the epirubicin effect on the MDA-MB-231 cells, the dose-dependent growth inhibition data of MDA-MB-231 were evaluated and results show that 20 nM is close to the cell half maximal inhibitory concentration (Figure 1B). As shown in Figure 1B, UBD overexpression remarkably suppressed the proliferation of MDA-MB-231 cells treated with 20 nM epirubicin. Invasion chamber assay was used to investigate whether UBD upregulation exerted inhibitory effects on cell migration and invasion. As shown in Figure 1C, the migration ability of MDA-MB-231 cells treated with 20 nM epirubicin was significantly enhanced by highly expressed UBD.
Figure 1.
Ubiquitin protein D (UBD) overexpression enhanced the resistance of epirubicin in triple-negative breast cancer cell lines. (A) Representative Western blot showing the overexpression of UBD in the MDA-MB-231 cell line transfected by UBD plasmid. (B) The inhibition rate of MDA-MB-231 cells treated with different concentrations of epirubicin by MTT, and the half maximal inhibitory concentration of MDA-MB-231 is about 20 nM. (C) The relative proliferation of UBD and its control cells was evaluated by MTT assay in MDA-MB-231 cell lines overexpressing UBD and their control plasmids treated with epirubicin (20 nM). The relative proliferation of blank triple-negative breast cancer cells, which were not treated with a chemotherapy drug, was also measured by MTT. (D) Migration assays were undertaken in MDA-MB-231 cells overexpressing UBD and their control plasmids treated with epirubicin (20 nM).
Notes: The lines above the bars indicated Standard Deviation. The asterisk (*) indicated P<0.05. The length of the scale bar indicated 100 μm.
Abbreviations: DMSO, dimethyl sulfoxide; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
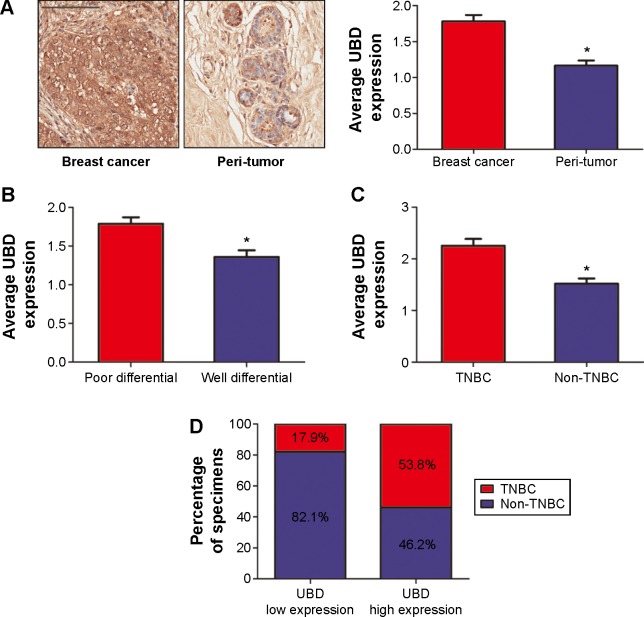
UBD expression was elevated in breast cancer and correlated with TNBC
To explore the function of UBD expression in breast cancer, we examined UBD expression in 78 human breast cancer tissues. As shown in Figure 2A, UBD was significantly increased in breast cancer tissues compared with in the corresponding tissues, as determined by immunostaining analysis. Of note, breast cancer with poor prognosis exhibited higher expression levels of UBD (Figure 2B). Further, it was revealed that the majority of patients with a high expression of UBD were prone to have TNBC (Figure 2C), and UBD was detected at higher amounts in TNBC than in non-TNBC (Figure 2D). Based on immunostaining results, the 78 human breast cancer patient tissues were divided into two groups: high UBD expression group (n=39) and low UBD expression group (n=39). Tissues in the UBD high group were prone to having TNBC compared with those in the non-TNBC group (P<0.05; Table 1).
Figure 2.
Ubiquitin protein D (UBD) expression is elevated in breast cancer and has a closed relationship with triple-negative breast cancer (TNBC). (A) The average UBD density in 78 paired breast cancer tissue samples was measured using immunohistochemical (IHC) staining – a representative view is shown. (B) Comparison of UBD expression in 78 different differentiated human breast cancer tissues using IHC staining. (C) Comparison of UBD expression in TNBC and non-TNBC by IHC staining. (D) The percentage of TNBC and non-TNBC in UBD overexpression and low expression tissues.
Notes: (A) Scale bar: 100 μm. (B, C) Data were analyzed by t-test (*P<0.05).
High expression of UBD indicated the poor prognosis of TNBC patients treated with epirubicin
In our cohort, 28 patients were diagnosed as having TNBC by pathology. Twenty-one patients had a higher UBD expression and seven patients had a lower UBD expression. Epirubicin-based adjuvant chemotherapy was administrated to the patients and the PFS was observed. In patients with a higher expression of UBD, the median PFS was 23 months. In contrast, the patients with a lower expression of UBD had a median PFS of 36 months. A trend toward poor PFS was found in patients with higher expression levels of UBD compared with those with lower UBD expression levels (P<0.05; Figure 3A). This possibly implies that a high expression of UBD enhanced the chemotherapy resistance in epirubicin-based adjuvant chemotherapy and mediated the poor prognosis of TNBC.
Figure 3.
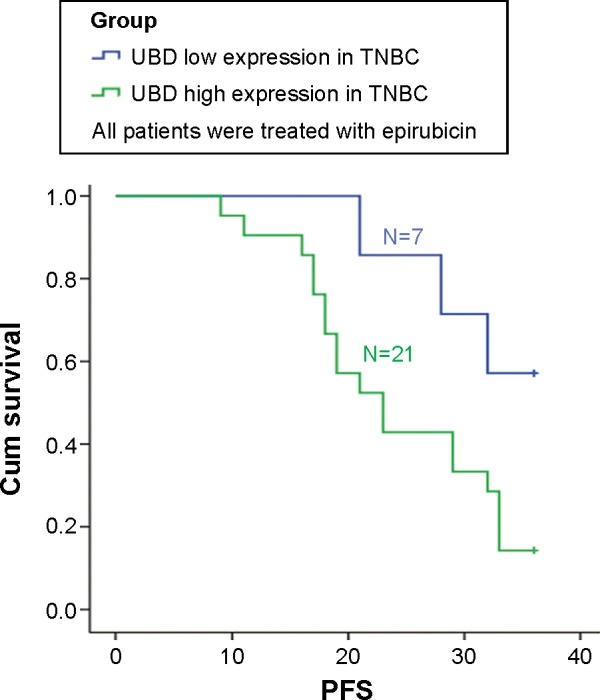
Ubiquitin protein D (UBD) expression has a closed relationship with the prognosis of triple-negative breast cancer (TNBC) patients treated with epirubicin chemotherapy. Disease-free survival analyses of 28 TNBC patients treated with epirubicin were compared between the UBD low expression (below the median value) and UBD high expression (above the median value) groups.
Note: The units for the values on x-axis indicated months.
Abbreviations: PFS, progression-free survival; cum, cumulative.
Discussion
Breast cancer accounts for 30%–40% of malignant tumors in women. It is responsible for the second highest rate of cancer-related mortality in the USA, with an estimated 39,620 deaths in 2013.9,10 Along with the public’s great concern, studies have confirmed that the prognosis is associated with the molecular subtypes of patients with breast cancer. TNBC, a heterogeneous subtype of breast cancer, is particularly prone to recurrence, metastasis, and chemotherapy resistance. So far, no effective molecular targeted drug has been made available for TNBC. Surgical resection and chemotherapy are the therapeutic means in clinic. According to the National Comprehensive Cancer Network guideline, epirubicin-based adjuvant chemotherapy is recommended and the curative effect will directly influence the patient’s prognosis.11 However, resistance to epirubicin is very common in TNBC patients and the mechanism is still unclear. Gyémánt et al explored the synergistic effect between flavonoids and epirubicin on multidrug-resistant cancer cells.12 They found that chrysin and amorphigenin synergistically enhanced the effect of epirubicin on the mouse lymphoma cell line and formononetin did the same on the MDA-MB-231 cell line.
UBD has been reported a tumor oncogene that promotes tumor cells’ malignant biological behaviors, such as recurrence and metastasis, via multiple signaling pathways including phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (Akt), Wnt/beta-catenin, and nuclear factor kappa-B.13–16 Given the positive connection between UBD activation and tumor progression, it was predicted to be of importance in breast cancer development. However, there have been limited reports in support of this theory. In the study reported here, in vitro experiments were conducted and clinical breast cancer samples were involved. We found that UBD expression correlated with epirubicin resistance in MDA-MB-231 cells and that the level of UBD was significantly increased in breast cancer tissues. Also, we found that the majority of patients with high expression levels of UBD were prone to be hormone-receptor negative, Her2 negative, and to have cancer with a poor prognosis. Further analysis revealed that the TNBC patients with an overexpression of UBD obtained worse clinical benefit and a higher recurrence rate from epirubicin-based chemotherapy than those with a low level of UBD expression. The findings imply that UBD overexpression enhanced the chemotherapy resistance of epirubicin-based adjuvant chemotherapy.
Conclusion
Epirubicin-based chemotherapy is of great importance in improving the prognosis of patients with TNBC, but the resistance to epirubicin severely limits the application of this drug. Identifying the epirubicin-resistance molecule and clarifying the related biomarker for TNBC prognosis could enable the development of successful strategies to circumvent this issue. In the study reported here, we demonstrated that UBD upregulation is involved in the process of epirubicin resistance in TNBC patients, which was confirmed by experiments in vitro. It is worth exploring whether inhibition of UBD might be a potential therapeutic approach in the treatment of epirubicin resistance in TNBC.
Acknowledgments
The authors thank Dr Di Chen for his kind help and guidance. This work was supported by the Key Project of the National 12th Five-Year Research Program of China (number 2012ZX0903016-002). The funders had no role in the design of the study, data collection and analysis, decision to publish, or preparation of the manuscript for publication.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen VN. Divide and conquer: the genetic basis of molecular subclassification of breast cancer. EMBO Mol Med. 2011;3(4):183–185. doi: 10.1002/emmm.201100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maire V, Némati F, Richardson M, et al. Polo-like kinase 1: a potential therapeutic option in combination with conventional chemotherapy for the management of patients with triple-negative breast cancer. Cancer Res. 2013;73(2):813–823. doi: 10.1158/0008-5472.CAN-12-2633. [DOI] [PubMed] [Google Scholar]
- 4.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer. 2009;9(Suppl 2):S73–S81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forrest RA, Swift LP, Evison BJ, et al. The hydroxyl epimer of doxorubicin controls the rate of formation of cytotoxic anthracycline-DNA adducts. Cancer Chemother Pharmacol. 2013;71(3):809–816. doi: 10.1007/s00280-012-2049-x. [DOI] [PubMed] [Google Scholar]
- 6.Hofmann K. Ubiquitin-binding domains and their role in the DNA damage response. DNA Repair (Amst) 2009;8(4):544–556. doi: 10.1016/j.dnarep.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Bates EE, Ravel O, Dieu MC, et al. Identification and analysis of a novel member of the ubiquitin family expressed in dendritic cells and mature B cells. Eur J Immunol. 1997;27(10):2471–2477. doi: 10.1002/eji.1830271002. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 9.Toriola AT, Colditz GA. Trends in breast cancer incidence and mortality in the United States: implications for prevention. Breast Cancer Res Treat. 2013;138(3):665–673. doi: 10.1007/s10549-013-2500-7. [DOI] [PubMed] [Google Scholar]
- 10.Pujol H. Trends in breast cancer incidence, survival, and mortality. Lancet. 2000;356(9229):591–952. doi: 10.1016/S0140-6736(05)73968-4. author reply 593. [DOI] [PubMed] [Google Scholar]
- 11.Joerger M, Ess S, Dehler S, et al. A population-based study on the patterns of use of different chemotherapy regimens in Swiss patients with early breast cancer. Swiss Med Wkly. 2012;142:w13571. doi: 10.4414/smw.2012.13571. [DOI] [PubMed] [Google Scholar]
- 12.Gyémánt N, Tanaka M, Antus S, et al. In vitro search for synergy between flavonoids and epirubicin on multidrug-resistant cancer cells. In Vivo. 2005;19(2):367–374. [PubMed] [Google Scholar]
- 13.Lee CG, Ren J, Cheong IS, et al. Expression of the FAT10 gene is highly upregulated in hepatocellular carcinoma and other gastrointestinal and gynecological cancers. Oncogene. 2003;22(17):2592–2603. doi: 10.1038/sj.onc.1206337. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Dong Z, Liang J, et al. As an independent prognostic factor, FAT10 promotes hepatitis B virus-related hepatocellular carcinoma progression via Akt/GSK3β pathway. Oncogene. 2014;33(7):909–920. doi: 10.1038/onc.2013.236. [DOI] [PubMed] [Google Scholar]
- 15.Gong P, Canaan A, Wang B, et al. The ubiquitin-like protein FAT10 mediates NF-kappaB activation. J Am Soc Nephrol. 2010;21(2):316–326. doi: 10.1681/ASN.2009050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan R, Wang K, Hu J, et al. Ubiquitin-like protein FAT10 promotes the invasion and metastasis of hepatocellular carcinoma by modifying β-catenin degradation. Cancer Res. 2014;74(18):5287–5300. doi: 10.1158/0008-5472.CAN-14-0284. [DOI] [PubMed] [Google Scholar]