Abstract
Background and Objectives
Fluorescence image-guided surgery (FIGS), with contrast provided by 5-ALA-induced-PpIX, has been shown to enable a higher extent of resection of high-grade gliomas. However, conventional FIGS with low-power microscopy lacks the sensitivity to aid in low-grade glioma (LGG) resection because PpIX signal is weak and sparse in such tissues. Intraoperative high-resolution microscopy of PpIX fluorescence has been proposed as a method to guide LGG resection, where sub-cellular resolution allows for the visualization of sparse and punctate mitochondrial PpIX production in tumor cells. Here, we assess the performance of three potentially portable high-resolution microscopy techniques that may be used for the intraoperative imaging of human LGG tissue samples with PpIX contrast: high-resolution fiber-optic microscopy (HRFM), high-resolution wide-field microscopy (WFM), and dual-axis confocal (DAC) microscopy.
Materials and Methods
Thick unsectioned human LGG tissue samples (n = 7) with ALA-induced-PpIX contrast were imaged using three imaging techniques (HRFM, WFM, DAC). The average signal-to-background ratio (SBR) was then calculated for each imaging modality (5 images per tissue, per modality).
Results
HRFM provides the ease of use and portability of a flexible fiber bundle, and is simple and inexpensive to build. However, in most cases (6/7), HRFM is not capable of detecting PpIX signal from LGGs due to high autofluorescence, generated by the fiber bundle under laser illumination at 405 nm, which overwhelms the PpIX signal and impedes its visualization. WFM is a camera-based method possessing high lateral resolution but poor axial resolution, resulting in sub-optimal image contrast.
Conclusions
Consistent successful detection of PpIX signal throughout our human LGG tissue samples (n = 7), with an acceptable image contrast (SBR > 2), was only achieved using DAC microscopy, which offers superior image resolution and contrast that is comparable to histology, but requires a laser-scanning mechanism to achieve optical sectioning.
Keywords: microendoscopy, endomicroscopy, glioma, extent of resection, intraoperative high-resolution microscopy, 5-ALA, PpIX, neurosurgery
INTRODUCTION
Mounting evidence suggests that the extent of neurosurgical resection of low- and high-grade gliomas correlates positively with patient prognosis: including incidence of recurrence [1–3], overall survival [4–12], and progression-free survival [4–6, 9, 10, 13]. Interestingly, a much higher rate of gross total resection, as assessed by post-operative MRI, (mean rate of 62.3% [14–18]) has been reported in patients with high-grade gliomas (HGGs) compared to low-grade gliomas (LGGs) (mean rate of 27.3% [19]). Reasons for this difference include the resemblance of LGGs to surrounding brain and the highly diffuse nature of LGGs, which result in a lack of contrast with methods such as intraoperative MRI (iMRI), intraoperative neuronavigation with pre-operative MRI, ultrasonography, computed tomography (CT) and fluorescence image-guided surgery (FIGS) with 5-ALA-induced-PpIX contrast.
In recent years, the use of FIGS with 5-ALA-induced-PpIX contrast for HGGs has demonstrated the ability to increase gross total resection (GTR) rates. This strategy has lower associated costs compared to most other intraoperative imaging modalities and can counteract the deleterious effects of intraoperative brain shift through its ability to continuously acquire images in real time. Importantly, studies have suggested a correlation between PpIX fluorescence intensities and biological metrics such as histopathological grade and cellular phenotypes [20, 21]. Although the use of FIGS has enabled maximal extent of resection for HGGs [22–26], it has not shown similar efficacy for LGGs due to the limited resolution of wide-field FIGS technologies that are unable to detect the weak and sparse sub-cellular production of PpIX by LGG cells. In short, since each pixel in a wide-field FIGS image represents the average signal from tens to hundreds of cells (tens to hundreds of microns), sparse sub-cellular foci of fluorescence are neither resolvable nor detectable.
In response to this unmet clinical need, the use of high-resolution intraoperative microscopy of 5-ALA-induced-PpIX has been proposed as an adjunct to optimize LGG resections [27–30]. 5-ALA is an orally administered pro-drug that is metabolized intracellularly (primarily by the mitochondria) to form the fluorescent molecule protoporphyrin IX (PpIX), where it has been shown that PpIX accumulates preferentially in glioma cells [21, 31–33]. Studies have indicated that an increase in PpIX production is specifically linked with increasing malignancy and proliferative index [20, 21, 34], a finding that supports the use of PpIX as a contrast agent to aid in glioma resections. As explained in the previous paragraph, the micron-scale resolution of a high-resolution microscope system is required to resolve the punctate expression of PpIX by sub-cellular mitochondria in LGG tissues.
In light of the potential advantages of intraoperative high-resolution microscopy for guiding LGG resections, we evaluated three potentially portable high-resolution microscopy techniques that could serve as adjunct tools to guide the final stages of low-grade glioma resections with 5-ALA-induced-PpIX contrast. We evaluated a relatively low-cost, easy-to-assemble, and increasingly popular option for in vivo cellular imaging: high-resolution fiber-optic microscopy (HRFM)[35, 36] and a similar modality, wide-field microscopy (WFM), which offers enhanced resolution. In addition, we evaluated an optical-sectioning technology being developed in our lab, the dual-axis confocal (DAC) microscope. These imaging techniques exhibit numerous tradeoffs in terms of sensitivity, resolution, contrast, imaging speed, cost, complexity, and size. We will summarize these differences and show imaging results from human LGG tissue specimens.
METHODS
LGG tissue samples (n = 7) with ALA-induced-PpIX contrast were collected from consenting patients at the Barrow Neurological Institute who were orally administered 5-ALA at a concentration of 20 mg/kg three hours prior to surgery. These tissues were fixed in 3% paraformaldehyde for 24 hours, and then stored in phosphate-buffered saline (PBS) at 4-deg C. The tissue samples, ranging in size from 0.5 cm2 to 2 cm2, were cut in half in order to prepare histology slides (PpIX fluorescence with counterstaining for nuclei and glial fibrillary acidic protein) from one half of the sample while the other half was imaged with our high-resolution microscopy devices without physical sectioning. Thick unsectioned tissues were imaged using three HR imaging techniques (HRFM, WFM and DAC) in a randomized order for each tissue sample to eliminate bias due to photobleaching (none was observed). A 405-nm fiber-coupled diode laser (Leading-Tech Laser, ADR-1805) was used as an excitation source for PpIX imaging and a 590-nm long-pass filter (Semrock, BLP01-594R-25) was used as a fluorescence emission filter. In order to perform a fair comparison of image contrast, roughly the same number of saturated pixels were collected during the imaging of all tissues with each device by adjusting detector gain and exposure settings. A laser power of 1 mW at the sample was utilized for DAC microscopy and WFM, while 2 mW was utilized for the HRFM. Imaging frame rates were kept consistently low (2 fps) for each modality. Detector background, measured in the absence of laser illumination, was negligible for all three imaging setups (< 1 gray level out of 256).
The HRFM system, described previously [35], consists of a wide-field microscope with 8x magnification that is relayed through a fiber bundle placed in contact with the sample (refer to Fig 1A). The HRFM system utilizes a CCD camera (Point Grey Grasshopper, GS3-U3-28S5M-C) for image detection. The fiber-optic bundle (Fujikura, FIGH-30-850N) is composed of 30,000 fibers with a center-to-center spacing of 4.4 μm and a field-of-view (active diameter of the fiber bundle) of 750 μm. There is no lens at the distal end of the fiber bundle. Therefore, the system resolution is determined by the fiber-to-fiber spacing of the bundle, which is 4.4 μm. This level of resolution is sufficient to resolve individual cell nuclei, though somewhat limited for the visualization of sub-cellular features. Nevertheless, it is still considered “high-resolution,” which we define as the ability to resolve individual cells (roughly 5- to 10-μm resolution or better). The acquisition frame rate used in this study was 2 fps but the imaging speed can be increased to video-rate (26fps) if the illumination intensity is increased and/or if the fluorescent features within the sample are sufficiently bright.
Figure 1.
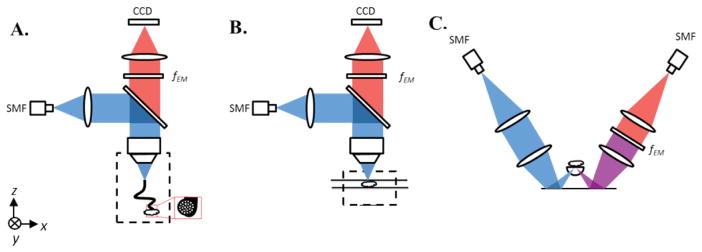
(A) HRFM setup. Excitation light from a 405-nm fiber-coupled diode laser is deflected by a dichroic beam splitter and focused into a coherent imaging fiber bundle. The fiber bundle transmits the light to the sample that is placed in contact with the distal end of the bundle. Emission light travels back from the sample through the bundle, is transmitted through the dichroic beam splitter and a long-pass emission filter (fEM), and is then imaged onto a CCD. (B) WFM setup. Excitation light travels the same path as in the HRFM setup, but is focused directly at the sample surface, instead of through a fiber bundle. Fluorescence emission is collected by a CCD detector array, as in the HRFM. (C) Dual-axis confocal (DAC) microscope setup. Excitation light from a 405-nm fiber-coupled diode laser is collimated and focused into tissue through a hemispherical fused-silica solid-immersion lens (SIL). Fluorescence emission is collected along an off-axis but symmetric optical path that includes a long-pass emission filter (fEM) and a single-mode collection fiber (SMF) that acts as a detection pinhole, followed by a PMT detector.
The WFM setup is largely identical to the HRFM, but uses a conventional microscope objective to image the sample directly onto the CCD (8x magnification) without relaying the image through a coherent fiber bundle (see Fig 1B). In this manner, image resolution is not limited by the center-to-center spacing of the fibers within the bundle. For imaging, the tissue is sandwiched between two glass slides in order to flatten the tissue surface and to maintain a constant working distance between the objective and the sample surface. For an intraoperative device, a glass window or prism could potentially be used to achieve a similar effect. For the WFM system used in this study, a field of view of 1.10 by 0.82 mm2 was obtained with a lateral resolution of ~2 μm. The acquisition frame rate was kept constant at 2 fps, though it is possible to increase the imaging frame rate to 26 fps, as with the HRFM.
The DAC system, described previously [37], is an optical-sectioning technology that consists of two off-axis low-NA beams intersecting at a 60° angle. The DAC microscope is a point-scanning technique that utilizes a galvanometric mirror for rapid laser scanning (~ 1 kHz) in one lateral direction (y direction, see Fig. 1C), and a piezo-electric actuator to scan the sample in the vertical direction (for vertical-sectioned imaging). Volume imaging is accomplished by translating the sample in the x direction. Signal detection is performed with an amplified photomultiplier tube. The DAC microscope used in this study acquires serial vertical image sections across a three-dimensional volume of tissue with a lateral field of view of 600 by 600 μm and a depth of up to 300 μm. The spatial resolution of the microscope is approximately 1 um in the lateral direction and 2 μm in the vertical direction, with image sections acquired at 2 fps with a 0.5-μm step size between serial vertical sections (Nyquist sampling).
RESULTS
HRFM is a low-cost system (<$5000) that offers the flexibility of a fiber bundle (~1-mm in diameter) that can be deployed in difficult-to-reach areas, such as deep sulci and cavities within the brain. Similarly to WFM, HRFM is capable of achieving video-rate imaging. However it exhibits limited resolution compared to WFM due to the spacing between fiber cores in the imaging bundle. A major drawback for the use of this system to image PpIX is a high level of autofluorescence generated by the fiber bundle itself at an excitation wavelength of 405 nm, which overwhelms the PpIX signal and impedes its visualization. In fact, previous studies have shown that the fiber-induced fluorescence from popular imaging bundles produce an autofluorescence emission peak that corresponds to the emission peak of PpIX near 625 nm [38]. Imaging fiber bundles from two different manufacturers were tested in this study with similar results: the Sumitomo IGN-08/30 and the Fujikura FIGH-30-850N. Due to fiber autofluorescence, HRFM was only able to detect PpIX fluorescence signal in one case where the PpIX signal was extremely strong and could overcome both the autofluorescence background as well as the fiber-bundle autofluorescence background (Fig. 2)
Figure 2.

Representative images of human LGG tissue samples obtained with high-resolution fiber-optic microscopy (HRFM), wide-field microscopy (WFM), dual-axis confocal (DAC) microscopy, and fluorescence histology of PpIX (physically sectioned tissue).
WFM is capable of achieving high lateral resolution, with a demonstrated ability to achieve video-rate imaging at a low cost. However, WFM suffers from poor axial resolution, which translates to limited contrast when imaging LGG tissues with PpIX fluorescence due to the presence of tissue autofluorescence and PpIX signal collected from out-of-focus planes (i.e. high background). Because of this reduced contrast, the system’s effective sensitivity (i.e. the ability the visualize PpIX) also decreases, even though the actual sensitivity (ability to “collect” PpIX fluorescence photons) remains high. The variability in autofluorescence is high, both between various tumors as well as within a single tumor[39–43]. Therefore, WFM is occasionally able to detect PpIX signal at one tissue location but not at an adjacent spot (though PpIX is present in both regions).
As shown in Figure 2, DAC microscopy offers superior image resolution and contrast, comparable to histology. It is able to achieve this due to its optical-sectioning capability, characterized by a high axial resolution (2 – 3 μm FWHM) and efficient rejection of out-of-focus light [37, 44, 45]. Optical sectioning is particularly important when imaging samples with high autofluorescence background and weak signal [46], which is the case for LGG tissues with 5-ALA-induced PpIX contrast. These tissues exhibit an autofluorescence background that competes with PpIX fluorescence signals, resulting in deteriorated contrast if imaged using WFM. However, the drawbacks of DAC microscopy include a limited detection sensitivity due to low-NA collection optics, which often leads to a slow imaging rate (2 fps). In addition, miniature DAC microscopes, which have utilized micro-electro-mechanical systems (MEMS) scanners, are complex and have required a high cost of development.
An estimate of signal-to-background ratio was performed with images that were obtained with equivalent numbers of saturated pixels (see Methods section). The average background was calculated by averaging the intensity from ten 30-μm diameter regions of interest (ROIs) within the image corresponding to low-signal areas. The average signal-to-background ratio (SBR) was then calculated for each image (5 images per tissue, per modality) and plotted for all modalities. The box plots in Fig. 3 demonstrate the variability in contrast (signal-to-background ratio, or SBR) from imaging the tissue specimens with WFM and DAC microscopy. HRFM data is not shown since it could only detect PpIX signal in one of the tissue specimens.
Figure 3.

Variability in contrast, or signal-to-background ratio (SBR), when imaging multiple locations on each human tissue specimen from n=7 patients. SBR varies more for each patient sample in the WFM modality in comparison to the DAC modality. This variability is partially attributed to a fluctuation in autofluorescence within each tissue. WFM is more sensitive to these variations since it lacks optical sectioning and therefore collects autofluorescence from out-of focus regions. (A) SBR of images collected with WFM. (B) SBR of images collected with DAC microscopy.
Consistent successful detection of PpIX signal from all glioma tissue samples (n = 7), with an acceptable image contrast (SBR ≫ 2), was only achieved using the DAC system.
DISCUSSION AND CONCLUSIONS
We assessed the performance of three potentially portable high-resolution microscopy techniques that may be used for the intraoperative imaging of human LGG tissue samples with PpIX contrast: high-resolution fiber-optic microscopy (HRFM), high-resolution wide-field microscopy (WFM), and dual-axis confocal (DAC) microscopy (see Table 1 for summary of trade-offs). Results indicate that only DAC microscopy is able to consistently detect PpIX across all glioma tissue samples (n = 7 patients). The high- contrast imaging of PpIX exhibited by DAC microscopy supports its clinical utility and suggests that intraoperative optical-sectioning microscopy could serve as a valuable adjunct to wide-field imaging techniques for guiding the surgical resection of LGGs. We acknowledge that other optical-sectioning microscopy approaches (e.g. multiphoton, conventional confocal, structured illumination, etc.) should also exhibit similar advantages for image LGG tissues with high contrast. However, since the expression of PpIX in LGG tissues is sparse and sub-cellular in size, wide-field (low-resolution) imaging approaches often lack the sensitivity to detect such signals. Here we have corroborated previous reports showing that intraoperative high-resolution microscopy has the resolving power, and hence the sensitivity, to visualize PpIX expression in human LGG tissues [27–30].
Table 1.
Comparison of high-resolution microscopy techniques
| Image Contrast | Resolution | Imaging Rate | Potential Portability | Cost | |
|---|---|---|---|---|---|
| HRFM | Poorest | High lateral & poor axial | High (2–30 Hz) | Excellent | Moderate |
| WFM | Poor | High lateral & poor axial | High (2–30 Hz) | Good | Low |
| DAC | Excellent | High lateral & axial | Low to moderate (2–8 Hz) | Good | High |
While DAC microscopy offers superior image quality when imaging sub-cellular PpIX expression in LGG tissues as compared to WFM and HRFM, a drawback of DAC microscopy is its increased complexity and cost, as well as its slow imaging frame rate. A slow imaging rate makes an in vivo microscope system vulnerable to motion artifacts during intraoperative handheld use. In order to address this limitation, our lab is developing a line-scanned DAC microscope system with imaging performance that is comparable to conventional point-scanned DAC microscopy at shallow depths [47]. While the contrast of an LS-DAC microscope is somewhat deteriorated due to a loss of confocality along one dimension, we are also exploring the use of deconvolution methods with detector arrays to mitigate this effect [48]. Additional oncological utility may also be derived from incorporation of software enabling quantification of tumor density per high-powered field, which would complement the current standard of extent-of-resection evaluation based on macroscopic magnetic resonance imaging.
Acknowledgments
We would like to acknowledge funding support from the National Institute for Biomedical Imaging and Bioengineering: R00 EB008557 (Liu), the National Institute of Dental and Craniofacial Research: R01 DE023497 (Liu), the National Institute of Neurological Diseases and Stroke: R01 NS082745 (Sanai), and the National Cancer Institute: R01 CA175391 (Liu and Sanai).
Footnotes
AUTHOR CONTRIBUTIONS
DM, DW, SB, and NS performed the research. DM and JTCL designed the research study. DM, JTCL, and NS analyzed the data. DM, JTCL, YW, and NS wrote the paper. All authors contributed to the final manuscript.
The authors declare no conflicts of interest.
References
- 1.Shaw EG, Berkey B, Coons SW, Bullard D, Brachman D, Buckner JC, Stelzer KJ, Barger GR, Brown PD, Gilbert MR, Mehta M. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. J Neurosurg. 2008;109(5):835–41. doi: 10.3171/JNS/2008/109/11/0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nava F, Tramacere I, Fittipaldo A, Bruzzone MG, Dimeco F, Fariselli L, Finocchiaro G, Pollo B, Salmaggi A, Silvani A, Farinotti M, Filippini G. Survival effect of first- and second-line treatments for patients with primary glioblastoma: a cohort study from a prospective registry, 1997–2010. Neuro Oncol. 2014 doi: 10.1093/neuonc/not316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pang BC, Wan WH, Lee CK, Khu KJ, Ng WH. The role of surgery in high-grade glioma--is surgical resection justified? A review of the current knowledge. Ann Acad Med Singapore. 2007;36(5):358–63. [PubMed] [Google Scholar]
- 4.Ahmadi R, Dictus C, Hartmann C, Zurn O, Edler L, Hartmann M, Combs S, Herold-Mende C, Wirtz CR, Unterberg A. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir (Wien) 2009;151(11):1359–65. doi: 10.1007/s00701-009-0435-x. [DOI] [PubMed] [Google Scholar]
- 5.Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–45. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 6.Majchrzak K, Kaspera W, Bobek-Billewicz B, Hebda A, Stasik-Pres G, Majchrzak H, Ladzinski P. The assessment of prognostic factors in surgical treatment of low-grade gliomas: a prospective study. Clin Neurol Neurosurg. 2012;114(8):1135–44. doi: 10.1016/j.clineuro.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 7.Janny P, Cure H, Mohr M, Heldt N, Kwiatkowski F, Lemaire JJ, Plagne R, Rozan R. Low grade supratentorial astrocytomas. Management and prognostic factors. Cancer. 1994;73(7):1937–45. doi: 10.1002/1097-0142(19940401)73:7<1937::aid-cncr2820730727>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Claus EB, Horlacher A, Hsu L, Schwartz RB, Dello-Iacono D, Talos F, Jolesz FA, Black PM. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–33. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 9.Jung TY, Jung S, Moon JH, Kim IY, Moon KS, Jang WY. Early prognostic factors related to progression and malignant transformation of low-grade gliomas. Clin Neurol Neurosurg. 2011;113(9):752–7. doi: 10.1016/j.clineuro.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Hervey-Jumper SL, Berger MS. Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol. 2014;16(4):284. doi: 10.1007/s11940-014-0284-7. [DOI] [PubMed] [Google Scholar]
- 11.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 12.Snyder LA, Wolf AB, Oppenlander ME, Bina R, Wilson JR, Ashby L, Brachman D, Coons SW, Spetzler RF, Sanai N. The impact of extent of resection on malignant transformation of pure oligodendrogliomas. J Neurosurg. 2014;120(2):309–14. doi: 10.3171/2013.10.JNS13368. [DOI] [PubMed] [Google Scholar]
- 13.Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52(4):371–9. doi: 10.1016/s0090-3019(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 14.Brown PD, Maurer MJ, Rummans TA, Pollock BE, Ballman KV, Sloan JA, Boeve BF, Arusell RM, Clark MM, Buckner JC. A prospective study of quality of life in adults with newly diagnosed high-grade gliomas: the impact of the extent of resection on quality of life and survival. Neurosurgery. 2005;57(3):495–504. doi: 10.1227/01.neu.0000170562.25335.c7. discussion 495–504. [DOI] [PubMed] [Google Scholar]
- 15.Chaichana KL, Kosztowski T, Niranjan A, Olivi A, Weingart JD, Laterra J, Brem H, Quiñones-Hinojosa A. Prognostic significance of contrast-enhancing anaplastic astrocytomas in adults. J Neurosurg. 2010;113(2):286–92. doi: 10.3171/2010.2.JNS091010. [DOI] [PubMed] [Google Scholar]
- 16.Chaichana KL, Halthore AN, Parker SL, Olivi A, Weingart JD, Brem H, Quinones-Hinojosa A. Factors involved in maintaining prolonged functional independence following supratentorial glioblastoma resection. Clinical article. J Neurosurg. 2011;114(3):604–12. doi: 10.3171/2010.4.JNS091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stark AM, Nabavi A, Mehdorn HM, Blömer U. Glioblastoma multiforme-report of 267 cases treated at a single institution. Surg Neurol. 2005;63(2):162–9. doi: 10.1016/j.surneu.2004.01.028. discussion 169. [DOI] [PubMed] [Google Scholar]
- 18.Ushio Y, Kochi M, Hamada J-i, Kai Y, Nakamura H. Effect of surgical removal on survival and quality of life in patients with supratentorial glioblastoma. Neurol Med Chir (Tokyo) 2005;45(9):454–60. doi: 10.2176/nmc.45.454. discussion 460–1. [DOI] [PubMed] [Google Scholar]
- 19.El-Hateer H, Souhami L, Roberge D, Maestro RD, Leblanc R, Eldebawy E, Muanza T, Melançon D, Kavan P, Guiot MC. Low-grade oligodendroglioma: an indolent but incurable disease? Clinical article. J Neurosurg. 2009;111(2):265–71. doi: 10.3171/2008.11.JNS08983. [DOI] [PubMed] [Google Scholar]
- 20.Valdes PA, Kim A, Brantsch M, Niu C, Moses ZB, Tosteson TD, Wilson BC, Paulsen KD, Roberts DW, Harris BT. delta-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: the need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro Oncol. 2011;13(8):846–56. doi: 10.1093/neuonc/nor086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbs SL, Chen B, O’Hara JA, Hoopes PJ, Hasan T, Pogue BW. Protoporphyrin IX level correlates with number of mitochondria, but increase in production correlates with tumor cell size. Photochem Photobiol. 2006;82(5):1334–41. doi: 10.1562/2006-03-11-RA-843. [DOI] [PubMed] [Google Scholar]
- 22.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 23.Della Puppa A, Ciccarino P, Lombardi G, Rolma G, Cecchin D, Rossetto M. 5-aminolevulinic Acid fluorescence in high grade glioma surgery: surgical outcome, intraoperative findings, and fluorescence patterns. Biomed Res Int. 2014;2014:232561. doi: 10.1155/2014/232561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao H, Noguchi M, Maruyama T, Muragaki Y, Kobayashi E, Iseki H, Sakuma I. An integrated diagnosis and therapeutic system using intra-operative 5-aminolevulinic-acid-induced fluorescence guided robotic laser ablation for precision neurosurgery. Med Image Anal. 2012;16(3):754–66. doi: 10.1016/j.media.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, Pichlmeier U, Mehdorn M. Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery. 2009;65(6):1070–6. doi: 10.1227/01.NEU.0000360128.03597.C7. discussion 1076–7. [DOI] [PubMed] [Google Scholar]
- 26.Tonn JC, Stummer W. Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: practical use, risks, and pitfalls. Clin Neurosurg. 2008;55:20–6. [PubMed] [Google Scholar]
- 27.Liu JT, Meza D, Sanai N. Trends in fluorescence image-guided surgery for gliomas. Neurosurgery. 2014;75(1):61–71. doi: 10.1227/NEU.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanai N, Snyder LA, Honea NJ, Coons SW, Eschbacher JM, Smith KA, Spetzler RF. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J Neurosurg. 2011;115(4):740–8. doi: 10.3171/2011.6.JNS11252. [DOI] [PubMed] [Google Scholar]
- 29.Zehri AH, Ramey W, Georges JF, Mooney MA, Martirosyan NL, Preul MC, Nakaji P. Neurosurgical confocal endomicroscopy: A review of contrast agents, confocal systems, and future imaging modalities. Surg Neurol Int. 2014;5:60. doi: 10.4103/2152-7806.131638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martirosyan NL, Georges J, Eschbacher JM, Cavalcanti DD, Elhadi AM, Abdelwahab MG, Scheck AC, Nakaji P, Spetzler RF, Preul MC. Potential application of a handheld confocal endomicroscope imaging system using a variety of fluorophores in experimental gliomas and normal brain. Neurosurg Focus. 2014;36(2):E16. doi: 10.3171/2013.11.FOCUS13486. [DOI] [PubMed] [Google Scholar]
- 31.Duffner F, Ritz R, Freudenstein D, Weller M, Dietz K, Wessels J. Specific intensity imaging for glioblastoma and neural cell cultures with 5-aminolevulinic acid-derived protoporphyrin IX. J Neurooncol. 2005;71(2):107–11. doi: 10.1007/s11060-004-9603-2. [DOI] [PubMed] [Google Scholar]
- 32.Stummer W, Stepp H, Möller G, Ehrhardt A, Leonhard M, Reulen HJ. Technical principles for protoporphyrin-IX-fluorescence guided microsurgical resection of malignant glioma tissue. Acta Neurochir (Wien) 1998;140(10):995–1000. doi: 10.1007/s007010050206. [DOI] [PubMed] [Google Scholar]
- 33.Olivo M, Wilson BC. Mapping ALA-induced PPIX fluorescence in normal brain and brain tumour using confocal fluorescence microscopy. Int J Oncol. 2004;25(1):37–45. [PubMed] [Google Scholar]
- 34.Johansson A, Palte G, Schnell O, Tonn JC, Herms J, Stepp H. 5-Aminolevulinic acid-induced protoporphyrin IX levels in tissue of human malignant brain tumors. Photochem Photobiol. 2010;86(6):1373–8. doi: 10.1111/j.1751-1097.2010.00799.x. [DOI] [PubMed] [Google Scholar]
- 35.Shin D, Pierce MC, Gillenwater AM, Williams MD, Richards-Kortum RR. A fiber-optic fluorescence microscope using a consumer-grade digital camera for in vivo cellular imaging. PLoS One. 2010;5(6):e11218. doi: 10.1371/journal.pone.0011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung EL, Schnitzer MJ. Fiber-optic fluorescence imaging. Nature methods. 2005;2(12):941–950. doi: 10.1038/nmeth820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu JT, Mandella MJ, Crawford JM, Contag CH, Wang TD, Kino GS. Efficient rejection of scattered light enables deep optical sectioning in turbid media with low-numerical-aperture optics in a dual-axis confocal architecture. J Biomed Opt. 2008;13(3):034020. doi: 10.1117/1.2939428. [DOI] [PubMed] [Google Scholar]
- 38.Udovich JA, Kirkpatrick ND, Kano A, Tanbakuchi A, Utzinger U, Gmitro AF. Spectral background and transmission characteristics of fiber optic imaging bundles. Appl Opt. 2008;47(25):4560–8. doi: 10.1364/ao.47.004560. [DOI] [PubMed] [Google Scholar]
- 39.Croce AC, Fiorani S, Locatelli D, Nano R, Ceroni M, Tancioni F, Giombelli E, Benericetti E, Bottiroli G. Diagnostic potential of autofluorescence for an assisted intraoperative delineation of glioblastoma resection margins. Photochem Photobiol. 2003;77(3):309–18. doi: 10.1562/0031-8655(2003)077<0309:dpoafa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Saraswathy A, Jayasree RS, Baiju KV, Gupta AK, Pillai VP. Optimum wavelength for the differentiation of brain tumor tissue using autofluorescence spectroscopy. Photomed Laser Surg. 2009;27(3):425–33. doi: 10.1089/pho.2008.2316. [DOI] [PubMed] [Google Scholar]
- 41.Pascu A, Romanitan MO, Delgado JM, Danaila L, Pascu ML. Laser-induced autofluorescence measurements on brain tissues. Anat Rec (Hoboken) 2009;292(12):2013–22. doi: 10.1002/ar.21034. [DOI] [PubMed] [Google Scholar]
- 42.Siebert R, Vu Thi MH, Jean F, Charon Y, Collado-Hilly M, Duval MA, Mandat T, Menard L, Palfi S, Tordjmann T. Development of a new autofluorescence probe for the analysis of normal and tumour brain tissues. 2008 [Google Scholar]
- 43.Butte PV, Mamelak AN, Nuno M, Bannykh SI, Black KL, Marcu L. Fluorescence lifetime spectroscopy for guided therapy of brain tumors. Neuroimage. 2011;54(Suppl 1):S125–35. doi: 10.1016/j.neuroimage.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu JT, Mandella MJ, Friedland S, Soetikno R, Crawford JM, Contag CH, Kino GS, Wang TD. Dual-axes confocal reflectance microscope for distinguishing colonic neoplasia. J Biomed Opt. 2006;11(5):054019. doi: 10.1117/1.2363363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Wang D, Liu JT. Assessing the tissue-imaging performance of confocal microscope architectures via Monte Carlo simulations. Opt Lett. 2012;37(21):4495–7. doi: 10.1364/OL.37.004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray JM, Appleton PL, Swedlow JR, Waters JC. Evaluating performance in three-dimensional fluorescence microscopy. J Microsc. 2007;228(Pt 3):390–405. doi: 10.1111/j.1365-2818.2007.01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang D, Chen Y, Wang Y, Liu JT. Comparison of line-scanned and point-scanned dual-axis confocal microscope performance. Opt Lett. 2013;38(24):5280–3. doi: 10.1364/OL.38.005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, Meza D, Wang Y, Gao L, Liu J. Sheet-scanned dual-axis confocal microscopy using Richardson–Lucy deconvolution. Optics Letters. 2014;39(18):5431–5434. doi: 10.1364/OL.39.005431. [DOI] [PMC free article] [PubMed] [Google Scholar]


