Abstract
Background
Working memory is a domain of ‘executive function.’ Delayed nonmatching-to-sample (DNMTS) procedures are commonly used to examine working memory in both human laboratory and preclinical studies.
New Method
The aim was to develop an automated DNMTS procedure maintained by food pellets in rhesus monkeys using a touch-sensitive screen attached to the housing chamber. Specifically, the DNMTS procedure was a 2-stimulus, 2-choice recognition memory task employing unidimensional discriminative stimuli and randomized delay interval presentations.
Results
DNMTS maintained a delay-dependent decrease in discriminability that was independent of the retention interval distribution. Eliminating reinforcer availability during a single delay session or providing food pellets before the session did not systematically alter accuracy, but did reduce total choices. Increasing the intertrial interval enhanced accuracy at short delays. Acute Δ9-THC pretreatment produced delay interval-dependent changes in the forgetting function at doses that did not alter total choices. Acute methylphenidate pretreatment only decreased total choices.
Comparison with existing methods
All monkeys were trained to perform NMTS at the 1 s training delay within 60 days of initiating operant touch training. Furthermore, forgetting functions were reliably delay interval-dependent and stable over the experimental period (~6 months).
Conclusions
Consistent with previous studies, increasing the intertrial interval improved DNMTS performance, whereas Δ9-THC disrupted DNMTS performance independent of changes in total choices. Overall, the touchscreen-based DNMTS procedure described provides an efficient method for training and testing experimental manipulations on working memory in unrestrained rhesus monkeys.
Keywords: Touchscreen, Rhesus monkey, Working memory, delayed nonmatching to sample, Δ9-Tetrahydrocannabinol, Methylphenidate
1. Introduction
The neuropsychological construct ‘executive function’ has been broadly defined as self-directed behavior that alters future consequences (Barkley, 1997; Bickel et al., 2012). The executive functions consist of a number of related neurobiological and behavioral processes such as working memory, attention, behavioral inhibition/impulsivity and behavioral flexibility closely associated with frontal cortical function (Robbins, 1996). Preclinical research stems from interest in treating mental health disorders and diseases that impact working memory (Buccafusco, 2008). Some examples include attention deficit hyperactivity disorder (Alderson, 2013), schizophrenia (Lett, 2014), and Alzheimer’s (Jahn, 2013). In particular, drug addiction has been suggested to impair working memory in humans (Fernandez-Serrano et al., 2011; Ornstein et al., 2000; Tramullas et al., 2007) and correlates with both treatment outcomes and treatment retention rates in drug-addicted individuals (Aharonovich, 2006). Moreover, drug addiction is associated with functional and neuroanatomical changes in brain areas such as dorsolateral prefrontal cortex (Liu et al., 2009; Liu et al., 2005), a region thought to be critical for accurate performance in working memory procedures, such as delayed nonmatching-to-sample (DNMTS) (Levy and Goldman-Rakic, 1999). Overall, pharmacological or behavioral approaches that specifically target executive functions such as working memory may therefore provide novel treatment strategies for the development of medications and behavioral interventions for drug addiction (Sofuoglu, 2010; Sofuoglu et al., 2013).
Most preclinical procedures for examining the behavioral and neurobiological mechanisms of working memory utilize a variant of the delayed matching-to-sample (DMTS) procedure (e.g., Blough, 1959; for review, see White, 2013). For example, a subject is required to choose among two comparison stimuli, one of which is physically identical to the sample stimulus presented previously in the trial. When choice of the comparison that matches the previously presented sample is reinforced, the procedure is called (DMTS); whereas, when choice of the other nonmatching sample is reinforced, the procedure is called DNMTS. In general, preference for the reinforced comparison decreases monotonically as a function of the duration of the retention or delay interval interposed between the offset of the sample stimulus and presentation of the comparison stimuli (Rubin and Wenzel, 1996; White, 2001).
Training of DMTS or DNMTS procedures in nonhuman primates has been previously reported to take greater than 12 months (Weed et al., 1999; Gould et al., 2012; 2013). In addition to these protracted training periods, baseline accuracy has been shown to increase over time necessitating individual subject adjustments of both delays and distractors for the DMTS procedure to maintain a delay-dependent decrease in performance (Weed et al., 1999; Bain et al., 2003; Gould et al., 2013; Uslaner et al., 2013; Kromley et al., 2015). Thus, the aim of the present study was to develop an automated DNMTS procedure in experimentally-naïve rhesus monkeys using a touch-sensitive screen attached to the home cage. DNMTS was used to facilitate comparison to prior studies in rhesus monkeys (Weed et al., 1999) and because this task engages brain regions exhibiting abnormal functioning in opioid addicted humans (Levy and Goldman-Rakic, 1999; Liu et al., 2005; Liu et al., 2009). We hypothesized that the use of two unidimensional stimuli (white and black boxes) would facilitate DNMTS training and reliably maintain a delay-dependent decrement in accuracy. Additionally, the effects of environmental manipulations that consisted of either manipulating the magnitude of the reinforcer (extinction and prefeeding) or manipulating the intertrial interval, and two pharmacological manipulations, acute Δ9-tetrahydrocannabinol and methylphenidate, were determined to validate the procedure. These environmental (Odum et al., 2005; Taffe, 2004) and pharmacological (Aigner, 1988; Schulze et al., 1988) manipulations have been previously examined on DMTS or DNMTS performance in preclinical studies.
2. Method
2.1. Subjects
Four adult male rhesus monkeys (Macaca mulatta) served as subjects. All monkeys were experimentally naïve at the beginning of the study. Monkeys weighed 7–10 kg and were maintained on a diet of fresh fruit and food biscuits (Lab Diet High Protein Monkey Biscuits no. 5045; PMI Nutrition, St. Louis, MO) provided following daily experimental sessions. Water was continuously available in the home cage via an automatic watering system. A 12-h light-dark cycle was in effect (lights on from 0600 to 1800 h). Animal research and maintenance were conducted according to the 8th edition of the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (National Academies Press, 2011). Animal facilities were licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee approved the research protocol. Monkeys had visual, auditory, and olfactory contact with other monkeys throughout the study. Operant procedures and foraging devices were provided for environmental manipulation and enrichment. Videos were played daily in animal housing rooms to provide additional environmental enrichment.
2.2. Apparatus
Monkeys were housed individually in well-ventilated, stainless steel chambers (66×76×94 cm) that also served as experimental chambers. Each chamber was equipped with a custom-made, stainless steel screen enclosure (Lafayette Instrument, Lafayette, USA), which was mounted on the front wall of the chamber to provide access to a 15″ touch-sensitive screen (33.6×26.4 cm Model 1537L; Elo TouchSystems, Menlo Park, CA). Each chamber was also equipped with a pellet dispenser (Model ENV-203-1000; Med Associates, St Albans, VT) mounted on a shelf above the chamber. All experimental events and data were collected using custom programming in ABET II Touch software (Lafayette Instrument, Lafayette, USA) in tandem with a Whisker server (Cambridge University, UK) controlled the touch-sensitive apparatus. Touchscreen stimuli were made in Microsoft PowerPoint for Mac 2011 using the hue-saturation-brightness slider. Sample and comparison stimuli were different shades of gray constructed by adjusting the hue and saturation to 0.0 and varying brightness/intensity. Brightness was set at 1.8% (black) and 100.0% (white) throughout the present study.
2.3. Touch Response Training
Monkeys were first trained to touch the screen at a central location under a fixed-ratio (FR) 1 schedule for 1-g banana-flavored pellets (5TUR, Test Diets, Richmond, IN). Sessions ended after either 80 pellets were earned or two hours, whichever occurred first. A custom grid with dimensions of 800×600 pixels, divided into 48, 100×100 pixel boxes was used throughout the present study. During the first training phase, the entire screen was signaled active by a white display and each touch (FR 1) produced a food pellet and was followed by a 3 s intertrial interval (ITI) in which the screen was blank (black). Subsequent training phases consisted of progressively decreasing dimensions of the active response location until reliable responding (≥ 60 pellets earned per session) within the final 200×200 pixel (9.6×8.8 cm) dimensions was established. This required one intermediate active location size (500×500 pixel) and no more than 10 sessions for any monkey. After the terminal size of the active location was established, the fixed-ratio requirement was increased across sessions to a terminal value of FR 10. Finally, monkeys were given two training sessions in which the active location alternated between left, center, and right.
2.4. Delayed Nonmatching-to-Sample (DNMTS)
Each trial (Fig. 1A) began with a black or white sample stimulus appearing at the center location of the screen on a red background. Once the sample stimulus was touched ten times within 15 s of trial onset (15-s limited hold), the sample stimulus was removed. Following a delay, comparison stimuli were presented in the left and right locations. A single touch of a comparison stimulus (5-s limited hold) removed all stimuli from the screen. Correct responses (touches to the shade that did not match the sample) resulted in the presentation of a 1-g pellet, followed by a 3-s ITI. Incorrect responses (touches to the shade that matched the sample) resulted in a 9-s ITI. Sessions consisted of an equal number of trials in which each sample was presented on 40 trials (80 trials total) and the same sample could not be presented on more than four consecutive trials. In addition, the identity of the sample stimulus and the location of the correct, nonmatching comparison (left or right), were counterbalanced and programmed to occur an equal number of times each session.
Fig. 1.

Schematic of the delayed nonmatching-to-sample (DNMTS) procedure. (A) Sequence of trial events. (B) The 2×2 matrix of events in a DNMTS procedure. Ci is the number of correct or nonmatching comparison choices, Ei is the number of error or matching comparison choices.
Initial training consisted of manipulating the delay between sample offset and comparison onset (i.e., delay interval). DNMTS performance was first established under simultaneous nonmatching conditions where completion of the response requirement on the sample stimulus produced the comparison stimuli, but did not result in the removal of sample stimulus. Next, the delay was increased to 0.1 s and then 1 s. Each training delay remained in effect until a monkey three consecutive sessions at ≥ 0.85 proportion correct. Once a monkey met terminal DNMTS training criteria, delay test sessions were conducted.
Training sessions were conducted every day except for Tuesdays and Fridays, when test sessions were conducted. Both session types consisted of 80 trials, but differed in the delay between sample offset and comparison onset. Training sessions consisted of a single delay presented on each trial and testing sessions consisted of four different delay intervals presented in quasi-random order. DNMTS performance was assessed under sets of long and short delay distributions. The long distribution consisted of a 1 s training delay during training sessions 3.2, 10, 32, or 100 s test delays. The short distribution consisted of a 0.1 s training delay and 1, 3.2, 10, or 32, s test delays.
2.5. Environmental manipulations
Once DNMTS performance was stable, the effects of extinction and prefeeding were examined under long and short delay interval distributions. Each manipulation was in effect for a single test session. The extinction test consisted of removing pellets from the dispenser; in all other respects extinction conditions were identical to a standard test session. The prefeeding manipulation consisted of placing 80 pellets into the food trough 1 hr prior to the test session. Following the final environmental manipulation under the short delay interval distribution, the ITI was increased to 60 s.
2.6. Pharmacological manipulations
The effects of Δ9-THC and methylphenidate on DNMTS performance were determined under the short delay interval distribution and a 3 s ITI. Doses of Δ9-THC (0.032 – 0.32 mg/kg, i.m.) or vehicle were administered 60 min prior to test sessions. Doses of (±)-methylphenidate hydrochloride (0.1 – 0.56 mg/kg, i.m.) or vehicle were administered 15 min prior to test sessions. The order of testing with vehicle and drug doses was varied across subjects using a Latin-square design. Δ9-THC was dissolved in a 1:1:10 mixture of ethanol (Pharmaco-AAPER, Belmont, NC), cremophor (Sigma Aldrich, St. Louis, MO) and sterile water. Methylphenidate was dissolved in sterile saline. Δ9-THC and methylphenidate hydrochloride provided by the National Institute on Drug Abuse (Bethesda, MD) Drug Supply Program.
2.7. Data analysis
The primary dependent measures in preliminary touch training were the numbers of touches per session from the last two sessions of each ratio requirement. Active touches were within-trial touches that occurred anywhere within the centrally located stimulus box, inactive touches were within-trial touches outside the stimulus box, and ITI touches were between-trial touches anywhere on the screen. DNMTS accuracy was displayed as proportion correct (total correct responses)÷(total responses) at each retention interval (i.e., a forgetting function). In addition, a measure of discriminability log d (Fig. 1B) derived from a behavioral model of signal detection (Davison & Tustin, 1978; see also Davison & Nevin, 1999) was calculated as
| (1) |
where C1 and C2 are correct (nonmatching) responses and E1 and E2 are error (matching) responses following each sample stimulus. Discriminability measured as log d is the logarithm (base 10) of the ratio of correct to error responses averaged across each sample presentation. Averaging the response ratios across each sample stimulus produces a measure free of any bias in responding towards one comparison stimulus. In addition, log d provides an equal interval measure of discrimination. Because log d is undefined when one response type is 0, a modified correction procedure (Brown and White, 2005) was used. The constant added to each response count was proportional to the number of trials completed at a given delay and set 2.2 as the maximum log d. A secondary dependent measure was the total number of choices or proportion of trials completed (correct and error choices) made at each delay.
Effects of environmental and pharmacological variables were analyzed in two ways. First, log d, total choices, and the proportion of total trials completed were analyzed with delay and experimental manipulation as a fixed effect and subject as a random effect in a linear mixed effect (LME; Pinheiro and Bates, 2000; Young et al., 2009) model conducted in JMP Pro 11 for Mac (SAS Institute, Cary, NC). All continuous variables (e.g. time and drug dose) were treated as such in mixed model analyses and vehicle was assigned a numeric value of −1 log unit less than the lowest dose tested. Second, model-comparison was used to determine the effects of delay distribution, ITI, and dose of Δ9-THC or methylphenidate on the slope and y-intercept of the forgetting function. A negative exponential function was fit to obtained forgetting functions with the delay scaled as
| (2) |
where log d0 is the y-intercept or the estimated log d at a 0 s retention interval t and k is the slope of the function which provides an estimate of the rate of forgetting. Therefore, log d0 provides an estimate of delay-independent changes in discriminability, while k estimates delay-dependent changes in discriminability. The exponential function with delay interval scaled as has previously been shown to provide an excellent fit to functions obtained with different species (Ruben and Wenzel, 1996; White, 2001). The free parameters of Eq. 2 were estimated by least-squares regression (GraphPad Prism version 6.0 for Mac OS X, San Diego California USA). Model comparison was used to determine whether manipulations had an effect on y-intercept (log d0) or slope (k) values by an extra sum-of-squares F-test (Motulsky and Christopoulos, 2003). This test computes an F statistic from a simpler Null hypothesis model and a more complex Alternative hypothesis model that assumes some parameters differ across treatments. The Null model fixes parameter estimates across each level of an independent variable, while the Alternative model does not constrain the parameter estimates to be equal, and, consequently, contains greater degrees of freedom. The criterion for statistical significance was set a priori at the 95% confidence level (P<0.05).
3. Results
3.1. Touch Response and Discrimination Training
Fig. 2 shows acquisition of touchscreen responding for contingent food presentation as the FR schedule requirement increased from 1–10. Fig. 2A shows that active responses increased as the FR requirement increased (F(1,7)=12.26, P=0.01). The number of inactive and ITI responses did not systematically change as the FR requirement increased. Fig. 2B shows the number of sessions required for each monkey to meet the ≥0.85 proportion correct criterion. There was no statistically significant effect of training delay on the speed with each monkey met the performance criterion.
Fig. 2.
Touch location training and the effects of increasing FR schedule requirements on active touch responses in rhesus monkeys (n=4). Ordinates: (A) Total responses per session. (B) Number of sessions required to achieve ≥ 0.85-proportion correct criterion. Abscissae: (A) Fixed-ratio schedule requirement (N). (B) delay to comparison presentation. Each symbol represents performance of a different monkey.
3.2. Baseline DNMTS performance
Fig. 3 shows mean forgetting functions averaged over the final two testing sessions under each delay distribution (A; long and B; short). Fig. 3 shows that accurate nonmatching decreased rapidly as the delay increased and approached 0 discriminability (proportion correct=0.5) at the 32-s delay. Fits of Eq. 2 accounted for a large percent of the variance in the delay functions (VAF=86.4) under both delay distributions. The model comparison analysis using Eq. 2 found that neither the y-intercepts nor the slopes of the functions were different (Table 1). Fig. 4 shows delay functions (Fig. 4A–B) and proportion of trials completed (Fig. 4C–D) within the experimental test session. There was no statistically significant effect of trial block on any dependent measure examined during the delay function.
Fig. 3.
Baseline forgetting functions and proportion trials completed in a DNMTS procedure with a long or short delay interval distribution in rhesus monkeys (n=4). Ordinates: (A Left) log d (A Right) proportion correct (B) proportion of trials completed. Abscissa: delay interval in seconds.
Table 1.
Effects of environmental and pharmacological manipulations on the parameters of Eq. 2 based on multi-model comparison. Estimates of log d0 (y-intercept) and k (slope) and variance accounted for (VAF) for each drug dose. Cells with -- indicate that a parameter estimate did not differ across levels of a manipulation. See text for further explanation.
| log d0 | k | VAF | ||
|---|---|---|---|---|
| Distribution | ||||
| Long | 2.50 | −0.65 | 86.3 | |
| Short | -- | -- | 86.3 | |
| ITI | ||||
| 3 s | 2.50 | −0.63 | 75.0 | |
| 60 s | -- | −0.31 | 76.5 | |
| Δ9-THC | ||||
| Vehicle | 2.50 | −0.48 | 72.7 | |
| 0.032 | -- | −0.56 | 52.0 | |
| 0.1 | -- | −0.76 | 45.6 | |
| 0.32 | -- | −1.23 | 43.7 | |
| Methylphenidate | ||||
| Vehicle | 2.38 | −0.52 | 72.3 | |
| 0.1 | -- | -- | 37.5 | |
| 0.32 | -- | -- | 43.6 | |
| 0.56 | -- | -- | 41.9 | |
Fig. 4.
Baseline within-session forgetting functions and total choices in a DNMTS procedure with a long or short delay interval distribution in rhesus monkeys (n=4). Ordinates: (A–B Left) log d (A–B Right) proportion correct and (C–D) number of choices. Abscissa: delay interval in seconds.
3.3. Environmental and pharmacological manipulations
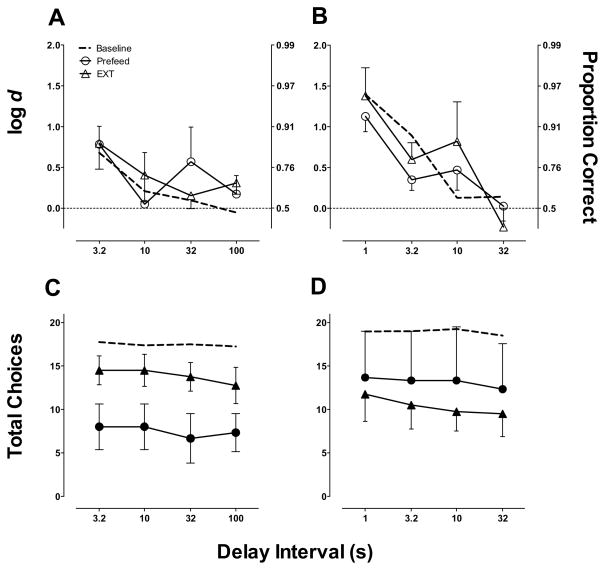
During the training days that preceded long or short delay distribution test days, DNMTS performance was accurate with long (log d=1.45 ± 0.11; proportion correct=0.94 ± 0.02) and short (log d=1.93 ± 0.01; proportion correct=0.98 ± 0.002) delay distributions and monkeys completed the majority of trials (long: 75.6 ± 1.0; short: 78.8 ± 0.6). Fig. 5 shows the effects of extinction and prefeeding manipulations on DNMTS performance. Discriminability decreased as a function of increasing delay interval (Fig 5, A: F(1,35.4)=4.38, P=0.0436 and B: F(1,39)=15.98, P=0.0003). Extinction and prefeeding manipulations did not significantly alter discriminability, but did decrease choices at all delay intervals (Fig 5, C: F(2,35.1)=59.57, P<0.0001 and D: F(2,35.1)=59.95, P<0.0001). Figure 6 shows that increasing the ITI from 3 s to 60 s increased discriminability (delay interval: F(1, 25)=14.5, P=0.0008; ITI: F(1, 25)=10.07, P=0.004). Model comparison indicated that the slopes of the forgetting functions were significantly different (F(2,36)=10.08, P=0.0003), suggesting the 60-s ITI produced less rapid forgetting compared to the 3-s ITI (Table 1).
Fig. 5.
Effects of environmental manipulations on DNMTS forgetting delay functions and total choices with either a long (A, C) or short (B, D) delay interval distribution in rhesus monkeys (n=4). Ordinates: A–B Left) log d (A–B Right) proportion correct and (C–D) number of choices completed per delay. Abscissa: delay in seconds. Filled points in C–D represent delay intervals at which total choices were statistically different from baseline (p<0.05).
Fig. 6.
Effects of ITI duration on DNMTS performance in rhesus monkeys (n=4). Ordinates: (A Left) log d (A Right) proportion correct (B) proportion of trials completed. Abscissa: delay interval in seconds.
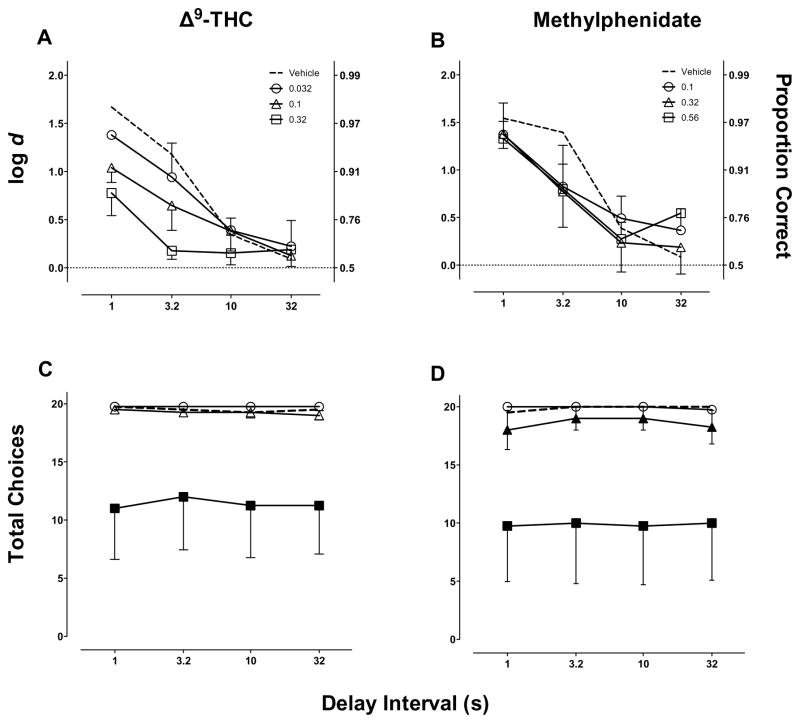
During the training days that preceded pharmacological test days, DNMTS performance was accurate (log d=1.98 ± 0.13; proportion correct=0.99 ± 0.005) and monkeys completed the majority of trials (78.6 ± 0.82). Figure 7(A, C) shows the effects of Δ9-THC on DNMTS performance. The LME analysis of the forgetting functions demonstrated a significant effect of delay interval (F(1,51.5)=41.19, P<0.0001), Δ9-THC dose (F(1,53.5)=14.11, P=0.0004), and the interaction (F(1,51.5)=6.00, P=0.0177). Δ9-THC dose also produced a significant decrease in total choices (F(1,57)=28.2, P<0.0001). Model comparison indicated that the forgetting functions (Fig. 7A) were significantly affected by Δ9-THC doses compared to vehicle (F(3,55)=7.39, P=0.0003). The best fitting model indicated that Δ9-THC produced a dose-dependent increase in the slope of the forgetting function (Table 1). Figure 7(B, D) shows the effects of methylphenidate on the forgetting function and number of choices. The LME analysis of the forgetting functions demonstrated a significant effect of delay interval (F(1,49.3)=21.93, P<0.0001), but no effect of methylphenidate dose nor the interaction. Methylphenidate significantly decreased total choices (F(1,57)=25.8, P<0.0001). Consistent with the LME analysis, model comparison also indicated that methylphenidate did not systematically alter the forgetting funcitons (Table 1).
Fig. 7.
Effects of Δ9-THC (A, C) and methylphenidate (B, D) on DNMTS performance in rhesus monkeys (n=4). Ordinates: (A–B Left) log d (A–B Right) proportion correct and (C–D) number of choices completed per delay. Abscissa: delay interval. Filled points in C–D represent delay intervals at which total choices were statistically different from vehicle rates (p<0.05).
4. Discussion
The aims of the present study were to develop and validate a novel delayed nonmatching-to-sample (DNMTS) procedure to determine the effects of environmental and pharmacological manipulations on working memory in unrestrained rhesus monkeys. There were three main findings. First, the procedure required 2.46 ± 0.26 months from initial touch-apparatus training to the first delay test session. Second, the forgetting functions for individual monkeys were stable over the course of the entire experimental period (~6 months; cf. Table 1). Lastly, the results demonstrate the sensitivity of this novel working memory procedure in unrestrained rhesus monkeys to environmental and pharmacological manipulations. Moreover, unique aspects of the procedure may provide increased experimental flexibility for interrogating the neurobiological mechanisms of working memory as well as changes in these process produced by drugs of abuse.
The methods in this study are derived from two-alternative forced-choice and DMTS procedures extensively used human and nonhuman laboratory studies of recognition memory (Jan, Wixted, and Huber, 2009; see also White and Wixted, 2010). The rapid decline in log d over delay intervals lasting several seconds in the present study is consistent with numerous DMTS studies in pigeons (for a recent review see White, 2013), rats (Harper et al., 2005), nonhuman primates (Wright, Urcuioli, and Sands, 1986), and humans (Williams, Johnston, and Saunders, 2006). Furthermore, log d showed a consistent delay interval-dependent decrease across repeated tests and typically decreased to near chance levels within 10 seconds. Taken together, these results suggest that the DNMTS procedure represents a valid assessment of short-term remembering and forgetting that will permit future examinations of behavioral and neurobiological mechanisms specific to this dimension of memorial function.
The impact of acutely eliminating food delivery and prefeeding on DNMTS performance were evaluated 1) to determine the impact of reinforcer magnitude on DNMTS performance and 2) to provide a comparison to acute pharmacological treatments. Removing food delivery and prefeeding with 80 pellets generally decreased number of choices, but did not systematically alter forgetting functions suggesting that these measures of DNMTS performance are independent. The effects on log d appear to be consistent with Taffe (2004) where providing rhesus monkeys with at least 400 grams of chow/day was necessary to decrease DMTS performance. Previous studies have also demonstrated that the sensitivity and time-course of the disruptive effects of environmental and pharmacological manipulations differ between rate and accuracy measures of discrimination performance (Katz, 1982, 1983; Nevin, Milo, Odum, and Shahan, 2003). Following disruptive manipulations, rate-based measures are generally more sensitive to disruption and are evident sooner compared to accuracy measures (Nevin, 1967). Finally, in agreement with previous DMTS studies, the disruptive effects of the delay interval on log d were consistently greater than extinction and prefeeding (Odum, Shahan, and Nevin, 2005; Berry and Odum, 2014).
Consistent with previous studies in rhesus monkeys, acute administration of Δ9-THC produced a dose-dependent disruption of DNMTS performance (Ainger, 1988; Schulze et al, 1989). A negative exponential function was fit to the data to confirm that Δ9-THC produced delay-dependent effects (i.e., working memory). Previous research has shown the negative exponential parameters can be altered independently by a variety of manipulations and that the slope parameter represents memorial features of the task (White 1985; 2001). Model comparison showed that Δ9-THC produced a dose-dependent increase in the slope of the forgetting function. This effect is consistent with an increase in the rate of forgetting reported in DMTS with humans (Lane et al., 2005), DMTS/DNMTS with rodents (Hampson and Deadwyler, 1999) and other working memory procedures with rhesus monkeys (Taffe 2012). Acute administration of methylphenidate failed to systematically alter the forgetting functions over a range of behaviorally active doses. The lack of effect of methylphenidate on working memory in the DNMTS procedure in present study is also consistent with a recent study in rhesus monkeys using the CANTAB DMTS procedure (Soto et al., 2013).
Increasing the ITI to 60 s produced a marked increase in discriminability at all but the 32-s retention interval. This result is in agreement with several prior studies demonstrating that increases in the ITI produce more accurate matching in DMTS in humans (Mackay and Gould, 1992; Williams, Johnston, and Saunders 2006) and pigeons (Edhouse & White, 1988; Roberts & Kraemer, 1982). Moreover, White (1985) reported that increasing the ITI increased log d0 and decreased k parameters of Eq. 2. Model comparison results in the present study indicated that increasing the ITI did not alter log d0 but increased discriminability in a delay-interval dependent manner by decreasing the rate of forgetting (k). The lack of effect on log d0 in the present study may have been due to a ceiling effect on discriminability at the training delay. Intertrial interval effects on the forgetting function are understood to result from proactive interference and the effects of increasing the ITI on the forgetting function in the present study are consistent with decreased interference (Wright Urcuioli, Sands, 1986).
The DNMTS procedure developed in this study required less than 3 months of training to produce high levels of nonmatching accuracy during training sessions and stable, delay interval-dependent forgetting functions during test sessions. The relatively brief training period and consistent delay interval-dependent decreases in discriminability are in contrast to a prior report on DNMTS acquisition in rhesus monkeys using the Cambridge Neuropsychological Test Automated Battery (CANTAB; Weed et al., 1999). Weed et al., (1999) reported that accuracy continued to increase over 26 months of training and that the effects of the delay interval on accuracy changed over time. The pattern of delay interval effects reported in that study suggests a decrease in the rate of forgetting with continued training. There are differences in the implementation of DNMTS between Weed et al., (1999) and the present study. For example, the CANTAB DNMTS typically employs daily testing and a minimal (FR1) sample requirement. There is little reason to suspect that these variables would dramatically prolong acquisition, however, as daily testing would be predicted to facilitate acquisition and sample response requirements are known to produce delay-independent effects on accuracy (White, 1985).
Perhaps the most significant differences between the CANTAB and present versions of the DNMTS procedure are the number of discriminative stimuli that serve as sample and comparison stimuli. Throughout the present study only two discriminative stimuli were used, while CANTAB typically includes thousands of unique stimuli. Previous studies have evaluated the effects of stimulus set size on DMTS performance. Although most studies have not reported acquisition data, one previous study with pigeons directly assessed the effects of stimulus set size on MTS acquisition (Wright et al., 1988). Wright et al., (1988) comparing MTS acquisition using either 2 or 156 trial-unique stimuli demonstrated a greater than 20-fold increase in the number of sessions required to achieve greater than 75% accuracy with the larger stimulus set. Therefore, the much shorter acquisition period in this study were likely due to the number of discriminative stimuli.
Previous studies in rhesus monkeys using touch screen technology have reported that much longer delay intervals than used in this study are required to produce large decrements in accuracy (e.g., Gould, Gage, and Nader, 2012). As discussed above, previous studies with humans, nonhuman primates, and pigeons have evaluated the effects of stimulus set size on working memory. These studies have found that the forgetting function steepens as the number of discriminative stimuli decreases (Williams et al., 1998; 2006). Notably, Overman and Doty (1980) reported that in DMTS with trial-unique stimuli, accuracy remains above chance following a 24 hr retention interval. The effects of stimulus set size on rate of forgetting have been attributed to the presence of greater proactive interference with smaller stimulus sets (Wright Urcuioli, and Sands, 1986). Taken together, these studies suggest that larger sets of discriminative stimuli require more training time and are less susceptible to the degrading effects of the retention interval on discrimination.
Because log d declined so rapidly in the DNMTS procedure we sought to determine whether a shorter distribution of delay intervals would yield comparable forgetting functions. The form of the forgetting functions did not depend on the distribution of delay intervals. We are aware of no DNMTS studies that have directly assessed the effects of the range of delay intervals. Sargisson and White (2003), however, studied arithmetic and logarithmic delay interval distribution progressions by sampling five delays each session from a set of 20 delays drawn from an arithmetic or logarithmic progression. They reported that delays drawn from a progression fell on the same forgetting function. Taken together, this suggests that under the present DNMTS procedure different delay intervals were sampling the same underlying forgetting function. Thus, the delay intervals used in test sessions may be chosen on the basis of factors such as overall session time with no loss of information. For example, shorter delay-interval distributions have the advantage of minimizing differences in pharmacological time-course effects on working memory.
Conclusions
The present study established performance of a novel touchscreen-based DNMTS procedure in unrestrained rhesus monkeys to assess working memory. Employing only two discriminative stimuli appears to be an efficient means of facilitating acquisition and producing stable, retention interval-dependent forgetting functions. The acute environmental and pharmacological manipulations examined here suggest that rate-based measures can be altered independent of memorial function.
Highlights.
Experimentally naïve monkeys learned DNMTS within 60 training days
DNMTS forgetting functions were stable over the 6 month experimental period
Increasing the intertrial interval enhanced DNMTS performance
Δ9-THC significantly disrupted DNMTS performance, whereas methylphenidate did not alter DNMTS performance
Acknowledgments
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers R21DA036383 and T32 DA007027.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and alcohol dependence. 2006;81:313–322. doi: 10.1016/j.drugalcdep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Aigner TG. Delta-9-tetrahydrocannabinol impairs visual recognition memory but not discrimination learning in rhesus monkeys. Psychopharmacology. 1988;95:507–511. doi: 10.1007/BF00172964. [DOI] [PubMed] [Google Scholar]
- Bain JN, Prendergast MA, Terry AV, Jr, Arneric SP, Smith MA, Buccafusco JJ. Enhanced attention in rhesus monkeys as a common factor for the cognitive effects of drugs with abuse potential. Psychopharmacology. 2003;169:150–160. doi: 10.1007/s00213-003-1483-1. [DOI] [PubMed] [Google Scholar]
- Berry MS, Odum AL. Reinforcer magnitude and resistance to disruption of forgetting functions and response rates. Journal of the experimental analysis of behavior. 2014;101:373–384. doi: 10.1002/jeab.86. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology. 2012;221:361–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough DS. Delayed matching in the pigeon. Journal of the Experimental Analysis of Behavior. 1959;2:151–160. doi: 10.1901/jeab.1959.2-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GS, White KG. The optimal correction for estimating extreme discriminability. Behavior Research Methods. 2005;37:436–449. doi: 10.3758/bf03192712. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ. Estimation of working memory in macaques for studying drugs for the treatment of cognitive disorders. Journal of Alzheimer’s Disease. 2008;15:709–720. doi: 10.3233/jad-2008-15414. [DOI] [PubMed] [Google Scholar]
- Davison MC, Tustin RD. The relation between the generalized matching law and signal-detection theory. Journal of the Experimental Analysis of Behavior. 1978;29:331–336. doi: 10.1901/jeab.1978.29-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison M, Nevin JA. Stimuli, reinforcers, and behavior: An integration. Journal of the Experimental Analysis of behavior. 1999;71:439–482. doi: 10.1901/jeab.1999.71-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edhouse WV, White KG. Sources of proactive interference in animal memory. Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:56. [Google Scholar]
- Fernández-Serrano MJ, Pérez-García M, Verdejo-García A. What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neuroscience & Biobehavioral Reviews. 2011;35:377–406. doi: 10.1016/j.neubiorev.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Gould RW, Gage HD, Nader MA. Effects of chronic cocaine self-administration on cognition and cerebral glucose utilization in rhesus monkeys. Biological psychiatry. 2012;72:856–863. doi: 10.1016/j.biopsych.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Garg PK, Garg S, Nader MA. Effects of nicotinic acetylcholine receptor agonists on cognition in rhesus monkeys with a chronic cocaine self-administration history. Neuropharmacology. 2013;64:479–488. doi: 10.1016/j.neuropharm.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life sciences. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Harper DN, Wisnewski R, Hunt M, Schenk S. ($\pm$) 3, 4-Methylenedioxymethamphetamine, d-Amphetamine, and Cocaine Impair Delayed Matching-to-Sample Performance by an Increase in Susceptibility to Proactive Interference. Behavioral neuroscience. 2005;119:455. doi: 10.1037/0735-7044.119.2.455. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Characterization and localization of cannabinoid receptors in brain: an in vitro technique using slide-mounted tissue sections. NIDA Res Monogr. 1991;112:129–145. [PubMed] [Google Scholar]
- Jahn H. Memory loss in Alzheimer’s disease. Dialogues in clinical neuroscience. 2013;15:445. doi: 10.31887/DCNS.2013.15.4/hjahn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang Y, Wixted JT, Huber DE. Testing signal-detection models of yes/no and two-alternative forced-choice recognition memory. Journal of Experimental Psychology: General. 2009;138:291. doi: 10.1037/a0015525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL. Effects of drugs on stimulus control of behavior: I. Independent assessment of effects on response rates and stimulus control. The Journal of Pharmacology and Experimental Therapeutics. 1982 [PubMed] [Google Scholar]
- Katz JL. Effects of drugs on stimulus control of behavior. II. Degree of stimulus control as a determinant of effect. The Journal of Pharmacology and Experimental Therapeutics. 1983 Sep;226:756–763. [PubMed] [Google Scholar]
- Kraemer PJ, Roberts WA. Short-term memory for visual and auditory stimuli in pigeons. Animal Learning & Behavior. 1984;12:275–284. [Google Scholar]
- Kromrey SA, Gould RW, Nader MA, Czoty PW. Effects of prior cocaine self-administration on cognitive performance in female cynomolgus monkeys. Psychopharmacology. 2015:1–10. doi: 10.1007/s00213-015-3865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Lieving LM, Tcheremissine OV. Marijuana effects on human forgetting functions. Journal of the experimental analysis of behavior. 2005;83:67–83. doi: 10.1901/jeab.2005.22-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett TA, Voineskos AN, Kennedy JL, Levine B, Daskalakis ZJ. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biological psychiatry. 2014;75:361–370. doi: 10.1016/j.biopsych.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Association of storage and processing functions in the dorsolateral prefrontal cortex of the nonhuman primate. The Journal of Neuroscience. 1999;19:5149–5158. doi: 10.1523/JNEUROSCI.19-12-05149.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Hao Y, Kaneko Y, Ouyang X, Zhang Y, Xu L, et al. Frontal and cingulate gray matter volume reduction in heroin dependence: Optimized voxel-based morphometry. Psychiatry and Clinical Neurosciences. 2009;63:563–568. doi: 10.1111/j.1440-1819.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- Liu N, Liu Y, Fan Y, Yu H, Wilson FA, Ma Y, et al. EEG activities in the orbitofrontal cortex and dorsolateral prefrontal cortex during the development of morphine dependence, tolerance and withdrawal in rhesus monkeys. Brain research. 2005;1053:137–145. doi: 10.1016/j.brainres.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Mackay HA, Gould DD. Relative sample recency and proactive interference in adults with mental retardation. Experimental Analysis of Human Behavior Bulletin. 1992;10:1. [Google Scholar]
- Motulsky H, Christopoulos A. Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. Oxford University Press; 2003. [Google Scholar]
- Nevin JA. Effects of reinforcement scheduling on simultaneous discrimination performance. Journal of the Experimental Analysis of Behavior. 1967;10:251–260. doi: 10.1901/jeab.1967.10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA, Milo J, Odum AL, Shahan TA. Accuracy of discrimination, rate of responding, and resistance to change. Journal of the Experimental Analysis of Behavior. 2003;79:307–321. doi: 10.1901/jeab.2003.79-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Odum AL, Shahan TA, Nevin JA. Resistance to change of forgetting functions and response rates. Journal of the Experimental Analysis of Behavior. 2005;84:65–75. doi: 10.1901/jeab.2005.112-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, et al. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Overman WH, Doty RW. Prolonged visual memory in macaques and man. Neuroscience. 1980;5:1825–1831. doi: 10.1016/0306-4522(80)90032-9. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. Springer Science & Business Media; New York: 2000. [Google Scholar]
- Robbins TW. Dissociating executive functions of the prefrontal cortex. Philosophical Transactions of the Royal Society B: Biological Sciences. 1996;351:1463–1471. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Wenzel AE. One hundred years of forgetting: A quantitative description of retention. Psychological review. 1996;103:734. [Google Scholar]
- Sargisson RJ, White KG. On the form of the forgetting function: The effects of arithmetic and logarithmic distributions of delays. Journal of the experimental analysis of behavior. 2003;80:295–309. doi: 10.1901/jeab.2003.80-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet A, Ali SF, Slikker W, et al. Acute effects of delta-9-tetrahydrocannabinol in rhesus monkeys as measured by performance in a battery of complex operant tests. Journal of Pharmacology and Experimental Therapeutics. 1988;245:178–186. [PubMed] [Google Scholar]
- Sofuoglu M. Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction. 2010;105:38–48. doi: 10.1111/j.1360-0443.2009.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013;64:452–463. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto PL, Dallery J, Ator NA, Katz BR. A critical examination of best dose analysis for determining cognitive-enhancing potential of drugs: studies with rhesus monkeys and computer simulations. Psychopharmacology. 2013;228:611–622. doi: 10.1007/s00213-013-3070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA. Effects of parametric feeding manipulations on behavioral performance in macaques. Physiology & behavior. 2004;81:59–70. doi: 10.1016/j.physbeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Δ9tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. Journal of Psychopharmacology. 2012;26:1299–1306. doi: 10.1177/0269881112443743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramullas M, Martínez-Cué C, Hurlé MA. Chronic methadone treatment and repeated withdrawal impair cognition and increase the expression of apoptosis-related proteins in mouse brain. Psychopharmacology. 2007;193:107–120. doi: 10.1007/s00213-007-0751-x. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Tye SJ, Eddins DM, Wang X, Fox SV, Savitz AT, et al. Orexin receptor antagonists differ from standard sleep drugs by promoting sleep at doses that do not disrupt cognition. Science translational medicine. 2013;5:179ra44–179ra44. doi: 10.1126/scitranslmed.3005213. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, et al. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Cognitive Brain Research. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- White KG. Characteristics of forgetting functions in delayed matching to sample. Journal of the Experimental Analysis of Behavior. 1985;44:15–34. doi: 10.1901/jeab.1985.44-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White KG. Forgetting functions. Animal Learning & Behavior. 2001;29:193–207. [Google Scholar]
- White KG. Remembering and forgetting. In: Madden GJ, Dube WV, Hackenberg TD, Hanley GP, Lattal KA, editors. APA handbook of behavior analysis, Vol. 1: Methods and principles. Washington, DC, US: American Psychological Association; 2013. pp. 411–437. [Google Scholar]
- White KG, Wixted JT. Psychophysics of remembering: To bias or not to bias? Journal of the Experimental Analysis of Behavior. 2010;94:83–94. doi: 10.1901/jeab.2010.94-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DC, Johnston MD, Saunders KJ. Intertrial sources of stimulus control and delayed matching-to-sample performance in humans. Journal of the Experimental Analysis of Behavior. 2006;86:253–267. doi: 10.1901/jeab.2006.67-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AA, Cook RG, Rivera JJ, Sands SF, Delius JD. Concept learning by pigeons: Matching-to-sample with trial-unique video picture stimuli. Animal learning & behavior. 1988;16:436–444. [Google Scholar]
- Wright AA, Urcuioli PJ, Sands SF. Proactive interference in animal memory research. In: Kendrick DF, Rilling M, Denny R, editors. Theories of animal memory. Englewood Cliffs, NJ: Erlbaum; pp. 101–125. [Google Scholar]
- Young ME, Clark MH, Goffus A, Hoane MR. Mixed effects modeling of Morris water maze data: Advantages and cautionary notes. Learning and Motivation. 2009;40:160–177. [Google Scholar]