Abstract
Background
Inhaled corticosteroids (ICSs) are recommended as first-line therapy for children with mild persistent asthma; however, specific patient characteristics may modify the treatment response.
Objective
Identify demographic, clinical, and atopic characteristics that may modify the ICS treatment response among children enrolled in the Treating Children to Prevent Exacerbations of Asthma (TREXA) trial.
Methods
Children age 6-18 years with mild persistent asthma were randomized to 44 weeks of combined, daily, rescue, or placebo treatment. Daily treatment consisted of 40 mcg of beclomethasone twice daily. Rescue treatment consisted of 40 mcg beclomethasone accompanying each symptom-driven albuterol actuation. Combined treatment consisted of both. Outcomes included time to first exacerbation and proportion of asthma control days. Fourteen baseline characteristics were selected for interaction testing based on their clinical relevance.
Results
Two-hundred-eighty-eight children were randomized. Seventy-five percent were white and 55% were male. As measured by time to first exacerbation, 4 characteristics identified children who received greater benefit from treatment: non-Hispanic ethnicity, positive aeroallergen skin test, serum immunoglobulin E level ≥350 K/μL, and history of oral corticosteroid use in the year prior to enrollment. As measured by asthma control days, 4 characteristics identified children who received greater benefit from treatment: male sex, positive aeroallergen skin test, serum immunoglobulin E level ≥350 K/μL, and incomplete run-in asthma control.
Conclusions
Children with mild persistent asthma who have markers of atopic asthma or who have greater asthma burden may obtain greater benefit from beclomethasone therapy. Additional study is needed to confirm whether these markers can guide individualized therapy.
Keywords: asthma, pediatric, inhaled corticosteroid, stratified analysis, intermittent treatment
Introduction
Asthma is no longer thought to define a single disease, but rather to encompass a number of phenotypes characterized by clustering of specific clinical and physiologic features that predict the natural course of disease and its response to treatment.(1) This latter feature is most relevant to clinicians because not all children may respond equally to the current mainstay of treatment, inhaled corticosteroids.(2) In the future, advances in pharmacogenomics may lead to individually tailored treatment based on the presence of unique biologic markers.(3) Until this new paradigm is realized, clinicians need better information on how to best utilize existing treatments for persistent asthma.
Inhaled corticosteroids are the most efficacious treatment for children with mild-to-moderate asthma.(4) However, clinical trials comparing inhaled corticosteroid treatment with placebo or montelukast have suggested that some subgroups of children may experience greater benefits from inhaled corticosteroids than others.(5-7) These trials suggest that children with markers of allergic asthma or markers of more severe asthma may experience greater benefits from inhaled corticosteroid treatment than children without such markers.(2) Responders may have asthma that is preferentially mediated by abnormalities in the T helper (TH2) immune pathway.(1) This allergic asthma phenotype is associated with atopy, type 1 hypersensitivity reactions, and eosinophilic inflammation; it is also more prevalent among children than adults. Cluster analyses of children with more severe asthma have demonstrated that only a portion of children express this phenotype which is considered to be steroid responsive.(8)
The Treating Children to Prevent Exacerbations of Asthma (TREXA) trial, a randomized, double-blind, placebo controlled trial of 3 inhaled corticosteroid dosing strategies among children with mild persistent asthma provided an opportunity to investigate whether specific demographic, asthma burden, and atopic characteristics modified the clinical response to treatment with inhaled corticosteroids as compared to placebo.
Methods
TREXA's study design, statistical approach, and results have been detailed elsewhere.(9) Briefly, children 6-18 years of age from 5 clinical centers in the United States were randomized to 1 of 4 treatment groups: combined, daily, rescue, or placebo. Daily treatment consisted of 40 mcg of beclomethasone dipropionate (hereafter called beclomethasone) twice daily. Rescue treatment consisted of one 40 mcg beclomethasone actuation to accompany each symptom-driven albuterol actuation. Combined treatment consisted of concurrent daily and rescue use. Children were provided with placebo inhalers for daily and rescue use to ensure blinding.
Eligible children had mild persistent asthma and demonstrated good asthma control and high protocol adherence during the study's 4-week run-in period. Children also had to demonstrate airway reversibility and methacholine responsiveness; however, these requirements were later relaxed to aid recruitment. Children were excluded if they demonstrated any of the following markers of severe asthma: baseline forced expiratory volume in one second (FEV1) <60% of predicted, asthma-related hospitalization within the past year, an asthma exacerbation in the past 3 months or more than 2 in the previous year, or a prior life-threatening exacerbation. The Institutional Review Boards of each participating institution approved the clinical trial protocol; children gave assent and parents gave consent.
Outcomes included time to first exacerbation (TFE) requiring oral corticosteroids and asthma control days (ACDs) reported on daily diary cards during the 44-week intervention. ACDs were defined as days without symptom-driven albuterol use, daytime or nighttime symptoms, unscheduled asthma-related health care visits, or asthma-related school absences.
Fourteen baseline characteristics based on clinical relevance and availability were investigated for their potential to modify the treatment response to inhaled corticosteroids: gender (male or female), age group (6-11 or 12-18 years), race (white or non-white), ethnicity (Hispanic or non-Hispanic), age of diagnosis (≤ 3 years or > 3 years), history of parental asthma (yes or no), history of eczema (yes or no), positive aeroallergen skin test (yes or no), serum IgE concentration (<350 K/μL or ≥350 K/μL), FEV1 reversibility (<12% or ≥12%), dose of methacholine at which a 20% decline in FEV1 was observed (PC20, ≤12.5 mg/mL or >12.5 mg/mL), fractional exhaled nitric oxide (FENO) concentration (<25 ppb or ≥25 ppb), complete asthma control during run-in (yes or no), and use of oral corticosteroids in the 12 months prior to baseline (yes or no). The FENO and IgE cut-points were based on suggestions outlined in the National Institutes of Health and Agency for Healthcare Research and Quality's Asthma Outcomes Workshop report on biomarkers.(10)
Analyses and reporting follow established recommendations for stratified analyses.(11-13) All analyses were conducted post-hoc. Descriptive analyses of categorical variables are presented as proportions or percentages. Descriptive analyses of continuous variables are presented as means and standard deviations (SD) or medians with 95% confidence intervals.
To identify characteristics that predicted a differential response to inhaled corticosteroid treatment, each characteristic was individually evaluated using a test of formal interaction. This approach provides the strongest evidence to support a treatment response modification. Two types of interaction are possible: interactions where the direction of effect is different (qualitative) and interactions where the direction is similar but the magnitude is different (quantitative).(12) Interaction testing is preferred to simple pairwise comparisons within strata because it minimizes Type I error; however, interaction testing typically requires a larger sample and/or effect size to identify significant relationships.(14)
An interaction was deemed present if the interaction term was significant at the p<0.10 level (3 treatment groups x 14 characteristics x 2 outcomes = 84 total comparisons). If the interaction term was significant, pairwise comparisons (stratified analyses) were conducted with comparisons being made between each treatment group and placebo. All stratified analyses controlled for clinical center and the original eligibility criteria (met versus not met). Additional covariates were only included if a significant univariate relationship existed between the covariate and the specified outcome. For example, analyses using TFE also controlled for ethnicity and analyses using ACDs controlled for age group, ethnicity, skin test status, IgE level, history of eczema, and run-in asthma control. Stratified analyses were considered significant at p<0.05.
Analyses of TFE were conducted using Cox proportional hazards models and analyses of ACDs were conducted using multivariate linear regression. Analyses of TFE were reported as hazard ratios (HR) and analyses of asthma control days were reported as mean change in days per year. All analyses were conducted using STATA 12 (College Station, TX).
Results
Two hundred and eighty-eight children with mild persistent asthma were randomized. Children were similar across all measured characteristics. (Table 1) Seventy-five percent of children were white and 55% were male. Children tended to be younger (age 6-11 years) rather than older (age 12-18 years). Seventy-eight percent of children were skin test positive and 50% reported eczema, yet only 31% had IgE levels ≥350 K/μL. While 21% of children demonstrated ≥12% reversibility following bronchodilator administration, 66% were responsive to methacholine at a dose of ≤12.5 mg/dL. Few children had markers of poorly controlled asthma: only 17% had an FENO level ≥25 ppb, only 36% had less than complete asthma control during the run-in, and only 29% reported oral corticosteroid use in the year prior to enrollment.
Table 1.
Baseline Demographic and Clinical Characteristics by Treatment Assignment
| Baseline Characteristic | OVERALL (n, %) | PLACEBO (n, %) | RESCUE (n, %) | DAILY (n, %) | COMBINED (n, %) |
|---|---|---|---|---|---|
| All Subjects | 288 (100) | 74 (100) | 71 (100) | 72 (100) | 71 (100) |
| Male | 159 (55.2) | 41 (55.4) | 37 (52.1) | 42 (58.3) | 39 (54.9) |
| Age 6-11 years | 188 (65.3) | 50 (67.6) | 47 (66.2) | 47 (65.3) | 44 (62.0) |
| White | 217 (75.3) | 59 (79.7) | 57 (80.3) | 51 (70.8) | 50 (70.4) |
| Hispanic or Latino | 103 (35.8) | 26 (35.1) | 27 (38.0) | 26 (36.1) | 24 (33.8) |
| Diagnosis ≤3 years | 139 (48.9) | 37 (50.7) | 34 (47.9) | 33 (46.5) | 35 (50.7) |
| Parental Asthma | 131 (48.5) | 36 (51.4) | 33 (46.5) | 30 (46.2) | 32 (50.0) |
| Eczema | 143 (49.7) | 36 (48.6) | 37 (52.1) | 34 (47.2) | 36 (50.7) |
| Skin test positive* | 220 (78.0) | 54 (73.0) | 57 (85.1) | 52 (74.3) | 57 (80.3) |
| IgE level* >350 K/μL | 85 (30.5) | 18 (24.7) | 25 (36.8) | 25 (36.2) | 17 (24.6) |
| FEV1 reversibility ≥12% | 59 (20.5) | 14 (18.9) | 16 (22.5) | 15 (20.8) | 14 (19.7) |
| PC20 ≤12.5 mg/dL | 191 (66.3) | 52 (70.3) | 49 (69.0) | 44 (61.1) | 46 (64.8) |
| FENO level* < 25 ppb | 228 (83.2) | 57 (80.3) | 60 (87.0) | 62 (87.3) | 49 (77.8) |
| Complete run-in control | 183 (63.5) | 51 (68.9) | 47 (66.2) | 39 (54.2) | 46 (64.8) |
| ≥1 OCS past year | 83 (28.8) | 21 (28.4) | 24 (33.8) | 19 (26.4) | 19 (26.8) |
Indicates missing data: skin test, 6 missing; IgE, 9 missing; eNO, 14 missing. Abbreviations: FENO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second ; IgE, serum immunoglobulin E; OCS, oral corticosteroid requiring exacerbation; PC20, concentration of methacholine causing a 20% decline in FEV1.
Several baseline characteristics were associated with a more severe natural history of asthma among untreated children (placebo); however, differences were only observed using ACDs and not TFE. Children who were skin test positive (p<0.05), had bronchodilator reversible asthma (p<0.05), or who had less than complete run-in asthma control (p<0.001) reported fewer asthma control days than their respective counterparts. Summary data of TFE and ACDs by baseline characteristic are presented in the Appendix.
Time to First Exacerbation
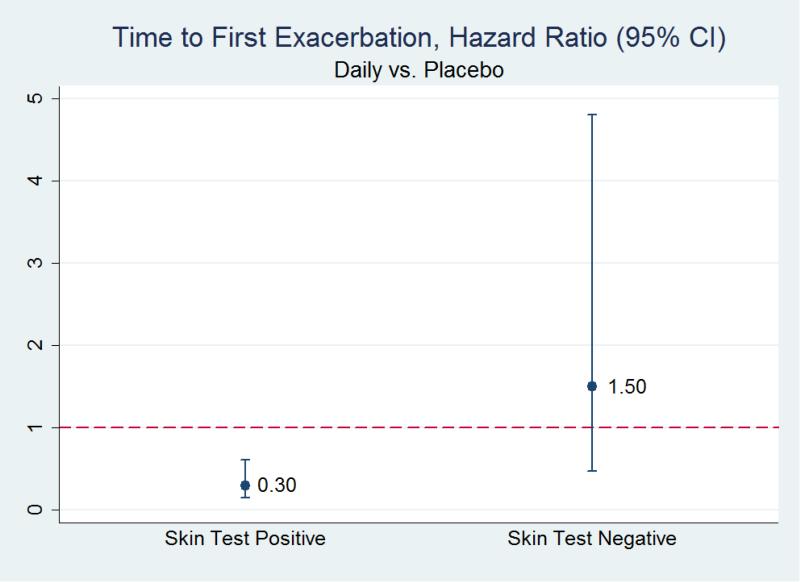
4 characteristics were associated with at least one significant interaction term among the combined and daily groups compared to placebo: skin test status, ethnicity, IgE level, and history of oral corticosteroid use. (Table 2) No significant interaction terms were identified among children receiving rescue treatment as compared to placebo. Among children receiving daily treatment (as compared to placebo), less frequent exacerbations were observed among children with a positive skin test, HR 0.30 (CI95% 0.15, 0.61; p<0.01), but not among those with a negative skin test. (Figure 1) Among those receiving combined treatment (as compared to placebo), less frequent exacerbations were observed among non-Hispanic children, HR 0.37 (CI95% 0.18, 0.75; p<0.01), but not among Hispanic children; less frequent exacerbations were observed among those with IgE levels ≥350 K/μL, HR 0.16 (CI95% 0.03, 0.92; p<0.05), but not among those with IgE levels <350 K/μL; and less frequent exacerbations were observed among children who did not report oral corticosteroid use in the year prior to enrollment, HR 0.35 (CI95% 0.16, 0.76; p<0.01), but not among those who did.
Table 2.
Stratified Analyses of Baseline Characteristics Associated with a Significant (p<0.10) Univariate Interaction Term (Treatment Assignment × Baseline Characteristic)
| Rescue | Daily | Combined | ||
|---|---|---|---|---|
| Hazard Ratio (CI95%) of Time to First Exacerbation as compared to Placebo | ||||
| Ethnicity | Hispanic | 1.16 (0.47, 2.86) | ||
| Non-Hisp. | 0.37 (0.18, 0.75)** | |||
| Skin test status | Positive | 0.30 (0.15, 0.61)** | ||
| Negative | 1.50 (0.47, 4.80) | |||
| IgE level | <350 K/μL | 0.56 (0.30, 1.07) | ||
| ≥350 K/μL | 0.16 (0.03, 0.92)* | |||
| OCS past year | Yes | 1.12 (0.48, 2.65) | ||
| No | 0.35 (0.16, 0.76)** | |||
|
Change (CI95%) in Asthma Control Days (per year) as compared to Placebo |
||||
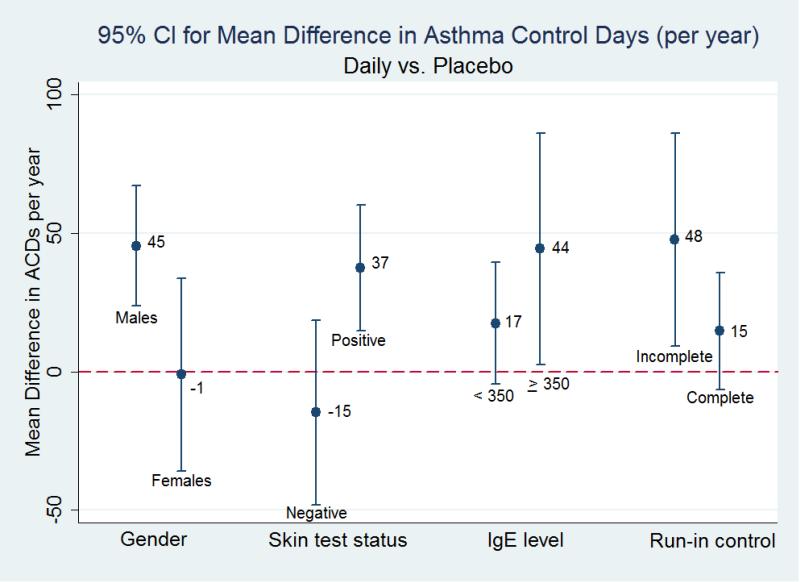
| Gender | Male | 45 ( 23, 68)** | ||
| Female | −1 (−37, 35) | |||
| Skin test status | Positive | 37 ( 14, 60)** | ||
| Negative | −15 (−50, 20) | |||
| IgE level | <350 K/μL | 17 (−5, 40) | 26 (6, 47)* | |
| ≥350 K/μL | 44 ( 1, 88)* | 49 (1, 98)* | ||
| Run-in control | Complete | −12 (−36, 11) | 15 (−7, 36) | |
| Incomplete | 18 (−29, 64) | 48 (8, 87)* | ||
IgE, serum immunoglobulin E; OCS, oral corticosteroid requiring exacerbation.
p<0.05
p<0.01
Empty cells denote the absence of a significant (p<0.10) univariate interaction between treatment assignment and placebo. Analyses of time to first exacerbation and asthma control days control for clinical center and eligibility criteria. Analyses of time to first exacerbation additionally control for ethnicity. Analyses of asthma control days additionally control for age group, ethnicity, skin test status, IgE level, and run-in control.
Figure 1.
Hazard Ratio of Time to First Exacerbation among Participants Treated with a Daily Inhaled Corticosteroid as compared to Placebo Stratified by Skin Test Status.
Asthma Control Days
4 characteristics were associated with at least one significant interaction term among the 3 treatment groups: gender, skin test status, IgE level, and run-in control. (Table 2) Among children receiving rescue treatment (as compared to placebo), the interaction term for level of run-in asthma control was significant, but neither complete nor less than complete control was associated with a significant increase in ACDs in the stratified analyses. Among children receiving daily treatment (as compared to placebo), a significant improvement in ACDs was observed among males, 45 (CI95% 23, 68; p<0.01) more days a year, but not among females; a significant improvement was seen among skin test positive children, 37 (CI95% 14, 60; p<0.01) more days a year, but not among those who were skin test negative; a significant improvement was seen among children with an IgE level ≥350 K/μL, 44 (CI95% 1, 88; p<0.05) more days a year, but not among those with a level <350 K/μL; and, a significant improvement was seen among children with less than complete run-in asthma control, 48 (CI95% 8, 87; p<0.05) more days a year, but not among those with complete control. (Figure 2) Among children receiving combined treatment (as compared to placebo), a significant improvement was seen among both those with IgE levels ≥350 K/μL and <350 K/μL, 49 (CI95% 1, 98; p<0.05) and 26 (CI95% 6, 47; p<0.01) more days a year, respectively.
Figure 2.
Mean Change in Asthma Control Days among Participants Treated with a Daily Inhaled Corticosteroid as compared to Placebo Stratified by Gender, Skin Test Status, Immunoglobulin E (IgE) Level, and Run-In Asthma Control.
Discussion
Children who have markers of allergic asthma (e.g., positive skin test) or more burdensome asthma (e.g., less than complete run-in control) may obtain greater benefit from beclomethasone treatment than children without such markers. Because differences between the 3 active groups (rescue, daily, and combined) were small, it is not possible to draw any conclusions regarding differential responsiveness between the inhaled corticosteroid dosing strategies. These markers could reflect differential physiologic responsiveness to corticosteroid treatment; alternatively, they could distinguish between asthma phenotypes of greater or lesser severity in which one phenotype has more to gain from treatment than the other. Because we did not characterize physiologic and/or immunologic profiles of individual children, we can only speculate as to the link between underlying physiologic differences and differential corticosteroid responsiveness.
Our findings suggest that certain demographic and clinical characteristics could be used to identify children who may obtain the greatest benefit from inhaled corticosteroid treatment. However, the converse is not equally true. Our data do not support withholding or delaying treatment among children without such characteristics. First, our results did not identify any characteristics that predict worse outcomes when treated (qualitative interactions). Rather, our analyses identified characteristics that were associated with larger, relative improvements (quantitative interactions). Second, because TREXA was not designed to answer this question it was underpowered to detect meaningful, but small improvements within subgroups. It is quite possible that a number of subgroups experienced benefits that we could not detect; therefore, the absence of observed treatment effect is not sufficient to conclude the absence of effect. More definitive answers will only come from larger trials specifically designed to examine subgroup responses. These trials should also characterize individual immunologic and physiologic factors that might account for differential treatment responsiveness, should include children with more heterogeneous asthma severity, and should account for prior inhaled corticosteroid exposure.
Our estimates likely represent the upper boundary of difference. By design, TREXA preferentially studied children who were most likely to be steroid responders because it excluded children who were unable to maintain asthma control during the 4-week run-in using daily low-dose beclomethasone. Therefore, the predictors we identified may not be as informative in general practice where there is greater heterogeneity of potential treatment response. This concept is illustrated by the Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial which attempted to identify predictors of short- and long-term response to inhaled corticosteroids among adults.(15) Short-term response to treatment, as measured by change in FEV1, was predicted by markers of pulmonary function like bronchodilator reversibility. Short-term responders who were maintained on treatment maintained control; short-term responders whose treatment was withdrawn subsequently lost control. Non-responders did equally well whether their treatment was maintained or withdrawn. TREXA's results are most generalizable to children who have already demonstrated a favorable response to an initial trial of daily inhaled corticosteroid treatment.
Our results are consistent with other trials investigating differential responses to inhaled corticosteroids. (Table 3) The Prevention of Early Asthma in Kids (PEAK) trial reported stratified results following treatment with fluticasone or placebo among children 2-3 years of age at high risk of asthma.(5) Daily fluticasone provided greater benefit to children who were male, white, skin test positive, who had prior asthma-related emergency department visits and/or hospitalizations, or who had fewer episode free days during the study run-in period. Differences in treatment response were observed using multiple outcomes including episode free days, oral corticosteroid use, and healthcare utilization. As in our study, these characteristics seemed to identify children with inherently unequal exacerbation risk and symptom control when untreated. Therefore, it is reasonable to conclude that children who exhibited these markers had more to gain than their counterparts. Once treated, all subgroups appeared to achieve similar levels of exacerbation risk and symptom control.
Table 3.
Summary of Reported Characteristics that May Modify the Response to Daily Treatment with an Inhaled Corticosteroid: TREXA, PEAK, CLIC, and PACT Trials
| TREXA | PEAK | CLIC | PACT | |
|---|---|---|---|---|
| Population | 6-18 years | 2-3 years | 6-17 years | 6-14 years |
| Sample Size | 288 | 285 | 126 | 191 |
| Severity | mild | positive API | mild-to-moderate | mild-to-moderate |
| Duration | 1 year | 2 years | 16 weeks | 1 year |
| ICS | beclomethasone (40 μg bid) | fluticasone (88 μg bid) | fluticasone (100 μg bid) | fluticasone (100 μg bid) |
| Comparison | placebo | placebo | montelukast (5-10 mg qd) | montelukast (5 mg qd) |
| Risk Dimension | Time to First Exacerbation | Oral Corticosteroids | % Δ FEV1 | Time to First Exacerbation |
| Ethnicity3 | Gender | Age | Parental Asthma History | |
| IgE Level3 | Prior Healthcare Use | Asthma Control Days | PC20 <2mg/mL Prior ICS Use | |
| Prior OCS use3 | Race | Body Mass Index | ||
| Skin Test2 | Skin Test | Eosinophil Cationic Protein | ||
| FEV1 / FVC | ||||
| ED and Urgent Care Visits | ||||
| Prior Healthcare Use | ||||
| Race | ||||
| Skin Test | ||||
| Control Dimension | Asthma Control Days | Episode Free Days | Asthma Control Days | Asthma Control Days |
| Gender2 | Gender | FENO | FENO >25 ppb Parental | |
| IgE Level2,3 | Prior Healthcare Use | Asthma History | ||
| Run-In Control1,2 | Race | |||
| Skin Test2 |
API, asthma predictive index; ED, emergency department; FENO, fractional exhaled nitric oxide; FEV1 forced expiratory volume in 1 second; FVC, forced vital capacity; PC20, methacholine concentration associated with 20% decline in FEV1; ICS, inhaled corticosteroid; IgE, immunoglobulin E.
Symptom-driven ICS treatment (rescue)
Daily ICS treatment (daily)
Both symptom-driving and daily ICS treatment (combined).
CLIC, Characterizing the Response to a Leukotriene Receptor Antagonist and an Inhaled Corticosteroid trial; PACT, Pediatric Asthma Controller trial; PEAK, Prevention of Early Asthma in Kids trial; TREXA, Treating Children to Prevent Exacerbations trial.
The Characterizing the Response to a Leukotriene Receptor Antagonist and an Inhaled Corticosteroid (CLIC) trial reported stratified results of children 6-17 years of age with mild-to-moderate asthma who were treated with fluticasone and montelukast in a cross-over design.(7, 16) The Pediatric Asthma Controller Trial (PACT) reported stratified results from children 6-14 years of age with mild-to-moderate asthma who were treated with either fluticasone or montelukast.(6) Both trials investigated characteristics that predicted a differential response to fluticasone treatment. While the absence of a placebo group makes these analyses difficult to reconcile with ours, both found that markers of inflammation tended to predict a greater response to fluticasone as compared to montelukast. PACT and CLIC data were reanalyzed using the 4 phenotype (cluster) classification derived from the Severe Asthma Research Program (SARP): late onset symptomatic asthma with normal lung function, early onset atopic asthma with mild airflow limitation, earliest onset atopic asthma with mild airflow limitation and comorbidity, or early onset atopic asthma with advanced airflow limitation and greatest medication use.(8, 17) While treatment response was similar across the 4 clusters, children in the early onset atopic asthma with comorbidity cluster were least responsive to montelukast or fluticasone treatment; however, this cluster was also the smallest comprising only 7% of characterized children.
There are a number of limitations that should be considered. Most importantly, TREXA was underpowered to identify differential responses to treatment, especially given 3 active treatment groups. We undertook 84 tests of formal interaction at significance level of p<0.10; therefore, 8 significant results would be expected by chance alone. For this reason, our analysis is hypothesis generating and should not be used to guide clinical care. Instead, we have identified the characteristics most likely to yield a differential treatment response and ones that should be of highest priority in future investigations. We had also planned to investigate characteristics associated with a differential response to the 3 dosing inhaled corticosteroid dosing strategies; however, all 3 groups obtained similar benefit making these comparisons impractical. Furthermore, TREXA investigated children who had mild asthma and who were most likely to be steroid responsive; therefore, generalizability to a broader clinical population is limited. If future studies are conducted, they will require substantially more children to have any reasonable expectation of identifying characteristics associated with a differential treatment response.
Conclusion
Our findings, in conjunction with others, indicate that children who exhibit markers of allergic inflammation and/or greater asthma burden may obtain a greater benefit from treatment with inhaled corticosteroids than children without such markers. Because TREXA was not powered to detect small improvements within subgroups, we cannot conclude that subgroups exist in which no benefit accrues. While the markers examined in this study are clinically relevant, they may be of limited utility in guiding treatment in individual children. Even if the markers were more specific, additional treatment alternatives are needed for potential corticosteroid non-responders before individualized treatment for children with asthma will be readily viable. Nonetheless, these current markers help identify children who would be expected to obtain the greatest benefit from daily inhaled corticosteroid treatment.
Supplementary Material
1. What is already known about this topic?
Specific markers of allergic inflammation and/or greater disease burden tend to identify groups of children with mild asthma who exhibit greater benefit from inhaled corticosteroid treatment than children without such markers.
2. What does this article add to our knowledge?
This article reaffirms that markers of allergic inflammation (serum IgE and skin test status) and disease burden (prior oral corticosteroid use and asthma control) appear to identify children who obtain greatest benefit from inhaled corticosteroid treatment.
3. How does this study impact current management guidelines?
When children with mild persistent asthma exhibit markers of allergic inflammation and/or greater disease burden, clinicians can expect inhaled corticosteroid treatment to yield meaningful exacerbation risk reduction and greater symptom control.
Acknowledgments
Funding support: Supported by grants from the National Heart, Lung, and Blood Institute (HL064307, HL064288, HL064295, HL064287, HL064305, and HL064313), the National Institute of Allergy and Infectious Diseases (T32AI007635), and the Clinical Translational Science Award program of the National Center for Research Resources (UL1-RR025011 [Wisconsin], UL1-RR025780 [Colorado], and UL1-RR024992 [St. Louis]). This study was performed in part by the General Clinical Research Centers at Washington University School of Medicine (M01-RR00036), National Jewish Health (M01-RR00051), and the University of Wisconsin (M01-RR03186)
Abbreviations
- ACD
asthma control day
- FENO
fractional exhaled nitric oxide
- CI95%
95% confidence interval
- CLIC
Characterizing the Response to a Leukotriene Receptor Antagonist and an Inhaled Corticosteroid trial
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- IgE
serum immunoglobulin E
- OCS
oral corticosteroid
- PACT
Pediatric Asthma Controller Trial
- PEAK
Prevention of Early Asthma in Kids trial
- PC20
methacholine concentration causing a 20% reduction in FEV1
- PTF
proportion treatment failure
- TFE
time to first exacerbation
- TREXA
Treating Children to Prevent Exacerbations of Asthma trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joe K. Gerald, Mel and Enid Zuckerman College of Public Health The University of Arizona 1295 North Martin Avenue P.O. Box 245210 Tucson, AZ 85724-5210.
Lynn B. Gerald, Mel and Enid Zuckerman College of Public Health Arizona Respiratory Center Tucson, Arizona.
Monica M. Vasquez, Mel and Enid Zuckerman College of Public Health Arizona Respiratory Center Tucson, Arizona.
Wayne J Morgan, Arizona Respiratory Center College of Medicine University of Arizona Tucson, Arizona.
Susan J Boehmer, Department of Public Health Sciences, Penn State Hershey College of Medicine Hershey, Pennsylvania.
Robert F Lemanske, Jr, University of Wisconsin School of Medicine and Public Health Madison, Wisconsin.
David T. Mauger, Department of Public Health Sciences Penn State Hershey College of Medicine Hershey, Pennsylvania.
Robert C. Strunk, Department of Pediatrics Washington University School of Medicine St. Louis, Missouri.
Stanley J Szefler, Department of Pediatrics, Pulmonary Section, The Breathing Institute Children's Hospital Colorado University of Colorado School of Medicine Aurora, Colorado.
Robert S Zeiger, Department of Allergy, Kaiser Permanente Southern California and Department of Pediatrics, University of California, San Diego San Diego, and La Jolla, California.
Leonard B. Bacharier, Department of Pediatrics Washington University School of Medicine St. Louis, Missouri.
Elizabeth Bade, University of Wisconsin, Aurora UW Medical Group Milwaukee, Wisconsin.
Ronina A. Covar, Department of Pediatrics National Jewish Health Denver, Colorado.
Theresa W Guilbert, University of Wisconsin School of Medicine and Public Health Madison, Wisconsin.
Hengameh Heidarian-Raissy, Pediatrics Pulmonary University of New Mexico Albuquerque, New Mexico.
H William Kelly, Pediatrics Pulmonary University of New Mexico Albuquerque, New Mexico.
Jonathan Malka-Rais, National Jewish Health Center Denver, Colorado.
Christine A. Sorkness, University of Wisconsin School of Medicine and Public Health Madison, Wisconsin.
Lynn Taussig, Denver University Denver, Colorado.
Vernon M. Chinchilli, Department of Public Health Sciences Penn State Hershey College of Medicine Hershey, Pennsylvania.
Fernando D. Martinez, Arizona Respiratory Center College of Medicine The University of Arizona Tucson, Arizona.
References
- 1.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012 May;18(5):716–25. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 2.Szefler SJ, Martin RJ. Lessons learned from variation in response to therapy in clinical trials. J Allergy Clin Immunol. 2010 Feb;125(2):285–92. doi: 10.1016/j.jaci.2009.10.026. quiz 93-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadsworth SJ, Sandford AJ. Personalised medicine and asthma diagnostics/management. Curr Allergy Asthma Rep. Feb;13(1):118–29. doi: 10.1007/s11882-012-0325-9. [DOI] [PubMed] [Google Scholar]
- 4.National Asthma Education and Prevention Program . Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute; Bethesda, MD: 2007. Contract No.: NIH publication 07-4051. [Google Scholar]
- 5.Bacharier LB, Guilbert TW, Zeiger RS, Strunk RC, Morgan WJ, Lemanske RF, Jr., et al. Patient characteristics associated with improved outcomes with use of an inhaled corticosteroid in preschool children at risk for asthma. J Allergy Clin Immunol. 2009 May;123(5):1077–82. 82, e1–5. doi: 10.1016/j.jaci.2008.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knuffman JE, Sorkness CA, Lemanske RF, Jr., Mauger DT, Boehmer SJ, Martinez FD, et al. Phenotypic predictors of long-term response to inhaled corticosteroid and leukotriene modifier therapies in pediatric asthma. J Allergy Clin Immunol. 2009 Feb;123(2):411–6. doi: 10.1016/j.jaci.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005 Feb;115(2):233–42. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. Feb;127(2):382–9. e1–13. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF, Jr., Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011 Feb 19;377(9766):650–7. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szefler SJ, Wenzel S, Brown R, Erzurum SC, Fahy JV, Hamilton RG, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol. Mar;129(3 Suppl):S9–23. doi: 10.1016/j.jaci.2011.12.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oxman AD, Guyatt GH. A consumer's guide to subgroup analyses. Ann Intern Med. 1992 Jan 1;116(1):78–84. doi: 10.7326/0003-4819-116-1-78. [DOI] [PubMed] [Google Scholar]
- 12.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007 Nov 22;357(21):2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 13.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 340:c117. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- 14.Brookes ST, Whitely E, Egger M, Smith GD, Mulheran PA, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J Clin Epidemiol. 2004 Mar;57(3):229–36. doi: 10.1016/j.jclinepi.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Martin RJ, Szefler SJ, King TS, Kraft M, Boushey HA, Chinchilli VM, et al. The Predicting Response to Inhaled Corticosteroid Efficacy (PRICE) trial. J Allergy Clin Immunol. 2007 Jan;119(1):73–80. doi: 10.1016/j.jaci.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006 Jan;117(1):45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 17.Chang TS, Lemanske RF, Jr., Mauger DT, Fitzpatrick AM, Sorkness CA, Szefler SJ, et al. Childhood asthma clusters and response to therapy in clinical trials. J Allergy Clin Immunol. Feb;133(2):363–9. doi: 10.1016/j.jaci.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.