Abstract
Background
Obstructive sleep apnea (OSA) may worsen asthma, but large studies are lacking and the underlying mechanisms are unknown.
Objective
Determine the prevalence of OSA risk among patients with asthma of different severity compared to normal controls (NC), and among asthmatics, test the relationship of OSA risk with asthma burden and airway inflammation.
Methods
Subjects with severe (SA, n=94) and non-severe asthma (NSA, n=161), and NC (n=146) were recruited in an add-on sub-study, to the observational Severe Asthma Research Program (SARP) II; subjects completed sleep quality, sleepiness and OSA risk (Sleep Apnea scale of the Sleep Disorders Questionnaire [SA-SDQ]) questionnaires and clinical assessments. Sputum was induced in a subset of asthmatics.
Results
Relative to NC, despite similar sleep duration, the SA and NSA subjects had worse sleep quality, were sleepier and had higher SA-SDQ scores. Among asthmatics, higher SA-SDQ was associated with increased asthma symptoms, β-agonist use, health care utilization, and worse asthma quality of life. Significant association of SA-SDQ with sputum polymorphonuclear cells% was noted: each increase in SA-SDQ by its standard deviation (6.85 units) was associated with a rise in % sputum neutrophils of 7.78 (95 % CI 2.33-13.22, p = 0.0006), independent of obesity and other confounders.
Conclusions
OSA symptoms are more prevalent among asthmatics, in whom they are associated with higher disease burden. OSA risk is associated with a neutrophilic airway inflammation in asthma, suggesting that OSA may be an important contributor to the neutrophilic asthma. Further studies are necessary to confirm these findings and better understand the mechanistic underpinnings of this relationship.
Keywords: Asthma; sleep apnea, obstructive; airway inflammation
1. Introduction
Asthma ranks among the most common chronic medical conditions and poses an increased health care burden. In the US, prevalence of asthma has risen from 7.3% (in 2001) to 8.2% (in 2011).1 The health care costs associated with asthma are significant, and they correlate strongly with disease severity.2 Despite significant progress in research and improved therapies, up to 75% of asthmatics do not achieve disease control and continue to be impaired in their quality of life.1, 3 For many people with asthma, no apparent causes are found and optimized therapies remain inadequate.4 These observations highlight the need for new interventions.
Obstructive sleep apnea (OSA) is another frequent breathing disorder5 involving the upper airway, and is often associated with asthma. There is accumulating evidence for etiologic interactions between the two conditions, apart from mere coexistence due to their common occurence.6 On one hand, studies consistently report higher prevalence of OSA symptoms,7-9 or polysomnography (PSG)-diagnosed OSA in asthmatic patients10, 11 in relationship with asthma severity.10 Conversely, OSA appears to worsen asthma. OSA risk is associated with poor asthma control,12 both during the day and at night.13 Moreover, treatment for OSA improves asthma outcomes, such as symptoms,14-16 bronchodilator use,14 peak expiratory flow (PEF) rates,14 and disease-specific quality of life.17 Last, OSA is a risk factor for frequent exacerbations in difficult-to-control asthma.18
Among several plausible pathways put forth to explain the aggravation of asthma by OSA is that of inflammation.6, 13 In OSA, a neutrophil-predominant inflammation starts in the nose,19 and extends to the lower airways,20, 21 where it correlates with disease severity.20 Underlying clinical asthma involves a complex inflammatory milieu that leads to bronchial reactivity, mucus secretion and remodeling of airway walls.22 A variety of cells, including dendritic, eosinophils, mastocytes and neutrophils are involved. Traditionally, eosinophils have been thought to play the most prominent role because of their intense infiltration of asthmatic airways and because they release a variety of potent inflammatory mediators.23 There is emerging evidence, however, that asthma is heterogeneous in its inflammatory cellular profiles.24, 25 Recent studies demonstrate that up to 60% of asthmatics who have persistent symptoms have a non-eosinophilic, neutrophil-rich type of asthma26 that responds poorly to standard therapies.27, 28 Whether neutrophils are attracted to the asthmatic airway as a result of smoking, obesity, or corticosteroid use remains unclear.24, 27, 29 Nonetheless, the similarities discussed above give rise to the question: can OSA be an unrecognized contributor to the airway neutrophilia in asthma?
Although the aforementioned investigations represent important contributions to a nascent field, studies of OSA prevalence in large samples of subjects who have a wide-range of baseline characteristics and different degree of asthma severity, are lacking. In addition, the broad impact of OSA on the burden of asthma disease control and health care use, as well as underlying mechanisms remain to be studied. The aims of the present study were to: 1) determine the prevalence of OSA symptoms among a large sample of asthma patients who were well-phenotyped for disease severity, relative to normal subjects; and 2) among asthmatics, to examine the relationships of OSA symptoms with asthma disease burden and the type of lower airway inflammation. Preliminary results have been published in abstract form.30
2. Methods
This study began in October 2007, as an add-on sub-study to the established and ongoing Severe Asthma Research Program (SARP) II protocol,31 which had been enrolling subjects since January 2007.
2.1. Subjects
Subjects with severe (SA) or non-severe asthma (NSA) and normal controls (NC) were enrolled. Details on subject recruitment and group assignment are described in the Supplemental Material. Of note, as part of the parent SARP study, at some of the sites subjects with a clinical history of OSA or positive airway pressure (PAP) use were not enrolled. Each institutional review board approved the study and all subjects signed informed consent.
Of the 474 SARP subjects who completed the sleep questionnaires, 61 were excluded because they were younger than 18 years of age. Among the remaining 413, 28 subjects had been diagnosed with OSA. Because positive airway pressure (PAP) treatment for OSA could influence OSA symptoms and asthma outcomes,8, 12-17 and confound the results of our analysis, the 12 subjects with OSA who were using PAP (4 in each group, ie 3% of NC, 2% of NSA and 4% of SA, p=0.75) were excluded from further analyses. 8, 12, 13, 32 Thus, our final sample included a total of 401 subjects (146 NC, 161 NSA and 94 SA). Not all assessments were performed in all subjects; the numbers of subjects contributing to the analysis are presented with the respective variable in Table 1. Of 132 NC subjects who underwent methacholline challenge, a PC20 could not be estimated in 125; the data from these subjects was not included when testing for group differences in baseline variables.
Table 1.
Baseline characteristics of normal control (NC), non-severe (NSA) and severe asthma (SA) subjects.
| NC (n=146)* | NSA (n=161)* | SA (n=94)* | P-value† | |
|---|---|---|---|---|
| Age (years) | 31 (11) | 34 (13) | 44 (13) | <0.0001‡ |
| Female n, (%) | 91 (62%) | 94 (58%) | 57 (61%) | 0.78 |
| BMI (kg/m2) | 26 (7) | 30 (7) | 32 (10) | <0.0001 |
| Obese n, (%) | 30 (21%) | 59 (37%) | 51 (54%) | <0.0001 |
| Race n, (%): | 0.22 | |||
| Caucasians | 101 (69%) | 98 (61%) | 61 (65%) | |
| African-Americans | 31 (21%) | 45 (28%) | 31(33%) | |
| Others | 14 (10%) | 18 (11%) | 2 (2%) | |
| Hx/o smoking n, (%) | 21 (14%) | 35 (22%) | 27 (29%) | 0.03¥ |
| Asthma duration (years) | - | 21 (12) | 26 (17) | 0.008 |
| Pre-bronchodilator FEV1 % predicted | 97 (10) | 84 (18) | 63 (22) | <0.0001‡ |
| Pre-bronchodilator FVC % predicted | 98 (11) | 92 (16) | 78 (19) | <0.0001‡ |
| Pre-bronchodilator FEV1/FVC % predicted | 99 (7) | 90 (11) | 79 (16) | <0.0001‡ |
| Methacholine PC20 (log) (7/124/39) | 0.62 (1.23) | 0.14 (0.70) | -0.23 (0.90) | <0.0001¥,€ |
| FEV1 (%) reversibility to 8 | 5 (3) | 16 (18) | 29 (35) | <0.007‡ |
| puffs of albuterol (n=143/160/91) | ||||
| Daily symptoms n, (%) | ||||
| Wheezing | 0 | 29 (18%) | 38 (40%) | <0.0001‡ |
| Cough | 0 | 27 (17%) | 38 (40%) | <0.0001‡ |
| Chest tightness | 0 | 18 (11%) | 34 (36%) | <0.0001‡ |
| Shortness of breath | 0 | 29 (18%) | 47 (50%) | <0.0001‡ |
| Any | 0 | 52 (32%) | 63 (67%) | <0.0001‡ |
| Nightly symptoms n, (%) | 1 | 24 (15%) | 39 (41%) | <0.0001‡ |
| Daily rescue β-agonist use n, (%): | ||||
| Inhaled | 0 | 43 (27%) | 44 (47%) | <0.0001‡ |
| Nebulized | 0 | 1 (1%) | 19 (20%) | <0.0001¥,€ |
| Any | 0 | 43 (27%) | 48 (53%) | <0.0001‡ |
| Health care utilization due to asthma in the past year n, (%): (n=143/160/93) | ||||
| hx/o MD visits | 0 | 95 (60%) | 82 (87%) | <0.0001‡ |
| hx/o ER visits | 0 | 23 (14%) | 47 (50%) | <0.0001‡ |
| hx/o hospitalizations | 0 | 4 (3%) | 29 (31%) | <0.0001¥,€ |
| hx/o ER visits or hospitalizations | 0 | 25 (16%) | 49 (52%) | <0.0001‡ |
| hx/o ICU admission | 0 | 2 (1%) | 11 (12%) | <0.0001¥,€ |
| Health care utilization ever due to asthma n, (%): (n=143/159/92) | ||||
| hx/o ER visits | 0 | 96 (60%) | 80 (85%) | <0.0001‡ |
| hx/o hospitalizations | 0 | 56 (35%) | 63 (67%) | <0.0001‡ |
| hx/o ICU admission | 0 | 11 (7%) | 32 (34%) | <0.0001¥,€ |
| hx/o assisted ventilation | 0 | 5 (3%) | 18 (20%) | <0.0001¥,€ |
| AQLQ (n=51/155/89) | ||||
| Total score | 6.95 (0.07) | 4.91 (1.19) | 3.97 (1.22) | <0.0001‡ |
| Symptoms | 6.92 (0.12) | 4.97 (1.22) | 3.87 (1.39) | <0.0001‡ |
| Activities | 6.95 (0.10) | 4.91 (1.27) | 4.13 (1.18) | <0.0001‡ |
| Emotions | 7.00 (0) | 4.93 (1.50) | 3.73 (1.63) | <0.0001‡ |
| Environment | 6.98 (0.08) | 4.66 (1.43) | 4.12 (1.52) | <0.0001‡ |
| Inflammatory markers | ||||
| IgE (log) (n=103/64) | - | 2.20 (0.64) | 2.07 (2.94) | 0.27 |
| Blood Eos% (n=103/64) | - | 3.60 (2.63) | 3.57 (2.97) | 0.97 |
| eNO (n=111/64) | - | 32 (25) | 63 (64) | 0.0004 |
| Sputum Eosinophils % (n=94/45) | - | 4 (10) | 8 (14) | 0.07 |
| Sputum PMNs% (n=94/45) | - | 44 (25) | 49 (24) | 0.26 |
Values are means (standard deviations) or counts (%).
Numbers of subjects except for PC20, FEV1 reversibility, health care utilization, AQLQ, and inflammatory markers (where numbers are shown for NC/NSA/SA).
Denotes overall P-values from general linear models (for 3 groups) or from t-tests (for 2 groups analyses).
Significant pairwise comparisons between the 3 groups from the general linear analysis with post-hoc contrasts are shown as:
all groups differ,
SA vs. NC;
NSA vs. NC;
SA vs. NSA.
Abbreviations: BMI= body mass index; FEV1=Forced expiratory volume in first second of the forced vital capacity maneuver; FVC=Forced vital capacity; PC20=the provocative concentration of methacholine, necessary to produce a 20% fall in FEV1; AQLQ=Asthma Quality of Life Questionnaire; eNO=fraction of exhaled nitric oxide; PMNs=polymorphonuclear neutrophils.
2.2. Clinical Questionnaires
Clinical SARP-specific and validated questionnaires were administered. The SARP-specific instruments,33 administered by study coordinators included general information on demographics, smoking, asthma symptoms, medical history including healthcare utilization (past year and lifelong) due to asthma and comorbidities and medication history. The symptoms questions assessed cough, wheezing, chest tightness, shortness of breath, and nighttime asthma (waking from sleep, use of albuterol, early morning chest tightness) symptoms in the prior 3 months, on a scale 1-6 ranging from never to at least twice a day. Inhaled (via metered dose inhaler [MDI] or nebulized) rescue β-agonist use in the prior 3 months was ascertained on an identical scale.
The validated, self-administered questionnaires included: 1) the Sleep Apnea scale of the Sleep Disorders Questionnaire (SA-SDQ), which assesses OSA risk;34 2) a question on perceived excessive daytime sleepiness (EDS) (“Do you think you are overly (too) sleepy during the day?”) and the Epworth Sleepiness Scale (ESS); 3) the Pittsburgh Sleep Quality Index (PSQI) which measures sleep quality. A PSQI score > 5 distinguishes poor from good sleepers, with good sensitivity and specificity; and 4) Asthma Quality of Life Questionnaire (AQLQ). These questionnaires are described in further detail in the Supplemental Material.
2.3. Pulmonary function and inflammatory markers
Subjects completed pulmonary function testing including spirometry, maximal bronchodilator reversibility (8 puffs of β-agonist) and methacholine challenge (if baseline FEV1 ≥50% predicted, adopted from the Asthma Clinical Research Network31, 35 and approved by the SARP Data and Safety Monitoring Board31). In addition, subjects completed online exhaled nitric oxide (eNO) by chemiluminescence, at a constant expiratory flow (50 mL/s) according to published guidelines,36 and sputum induction in those without contraindication. The induction method (adopted from the Asthma Clinical Research Network37) and sample processing have been previously described in detail by the SARP group.25 Percent WBC, bronchial and squamous epithelial cells were calculated for total cell counts. Samples with >80% squamous were considered inadequate. Percent neutrophils, eosinophils, macrophages and lymphocytes were calculated for WBC count, with the former two populations being the focus of this analysis. Subjects had a venous blood sample drawn for measurement of total serum IgE, and a complete WBC count with differential for absolute count and % eosinophils.
2.4. Data Analysis
Obesity was defined by BMI≥30 kg/m2, following the Centers for Disease Control's guidelines. African-Americans — a group with particular susceptibility for worse asthma1 — were compared to Caucasians and all other races (American Indian or Alaskan Native, Asian, Native Hawaiian, others) combined. From the SA-SDQ, habitual snoring was identified by scores of 4 or 5 (“usually” or “always”). The validated SA-SDQ scores≥36 for men and ≥32 for women defined high OSA risk.34 Due to the small numbers of asthma participants meeting these cutoffs, the SDQ was normalized (ie, each individual score divided by the sample standard deviation). This approach was also taken to facilitate the interpretation of results in a clinical context. Asthma symptoms and bronchodilator use were categorized as occurring at least daily (scores≥5). Global variables of “any asthma symptoms” (wheezing, cough, chest tightness or shortness of breath) or “any (via MDI or nebulized) bronchodilator use” were constructed, and similarly categorized by their daily occurrence (scores≥5). PC20 methacholine and total IgE data were log(10)-transformed before analysis, to assure a normal distribution. Asthma sputum inflammatory phenotypes were defined using the cut-offs previously published by the SARP group: eosinophilic (eosinophils≥2% and neutrophils<40%), neutrophilic (eosinophils<2% and neutrophils≥40%), mixed granulocytic neutrophilic (eosinophils≥2% and neutrophils≥40%) and paucigranulocytic phenotypes (eosinophils<2% and neutrophils<40%).25
SAS statistical software Version 9.3 (SAS Institute, Cary, NC) was used for analyses. Categorical variables are summarized as counts and percentages, and continuous variables as means and standard deviations (SD) unless otherwise specified. Chi-squared or Fisher's exact tests were used, as appropriate, to analyze categorical variables.
To test for differences between the 3 groups in baseline and sleep measures (Table 1 and Table 2—both univariate and multivariate models with adjustment for age, gender, race, obesity and smoking history), we used general linear models with post-hoc contrasts. Because this was an exploratory ancillary study, we did not control for multiple comparisons.
Table 2.
Sleep measures, OSA symptoms and OSA risk in normal control (NC), non-severe (NSA) and severe asthma (SA) subjects, in univariate and multivariate (adjusted for age, gender, obesity, race and smoking history) analyses.
| NC (n=146) | NSA (n=161) | SA (n=94) | Unadjusted P-value (3 groups)† | Adjusted P-values* | |||
|---|---|---|---|---|---|---|---|
| SA vs NC | NSA vs NC | SA vs NSA | |||||
| Sleep duration (hours) | 7 (1) | 7 (2) | 7 (3) | 0.75 | 0.97 | 0.97 | 0.99 |
| Daytime sleepiness n, (%) | 18 (12%) | 64 (40%) | 38 (40%) | <0.0001¥,§ | <0.0001 | <0.0001 | 0.44 |
| ESS | 5 (4) | 7 (4) | 8 (5) | <0.0001¥,§ | <0.0001 | <0.0001 | 0.33 |
| Abnormal ESS (score >10) n, (%) | 11 (8%) | 31 (19%) | 29 (31%) | <0.0001‡ | <0.0001 | 0.004 | 0.01 |
| PSQI | 7 (3) | 9 (3) | 11 (3) | <0.0001‡ | <0.0001 | <0.0001 | 0.0003 |
| Abnormal PSQI (score > 5) n, (%) | 126 (86%) | (158) 98% | 94 (100%) | <0.0001¥,§ | 0.0003 | <0.0001 | 0.74 |
| Any snoring n, (%) | 68 (47%) | 114 (71%) | 68 (72%) | <0.0001 | 0.03 | 0.0004 | 0.46 |
| Habitual snoring n, (%) | 10 (7%) | 29 (18%) | 19 (20%) | 0.004 ¥,§ | 0.15 | 0.06 | 0.93 |
| Witnessed apnea n, (%) | 10 (7%) | 31 (19%) | 24 (26%) | 0.0003¥,§ | 0.04 | 0.03 | 0.74 |
| SA-SDQ | 19 (6) | 26 (7) | 29 (6) | <0.0001‡ | <0.0001 | <0.0001 | 0.25 |
| High OSA risk£ n, (%) | 4 (3%) | 26 (16%) | 24 (26%) | <0.0001‡ | 0.04 | 0.02 | 0.85 |
Values are means (standard deviations) or numbers (%).
Denotes overall P-values from general linear models. Significant group pairwise comparisons from the general linear analysis post-hoc contrasts are shown as:
all groups differ (p<0.0001 for each comparison),
SA vs. NC p<0.0001,
NSA vs. NC p<0.0001.
P-values adjusted for age, gender, obesity, race, hx/o smoking.
defined as SA-SDQ scores ≥36 for men and ≥32 for women.
Abbreviations: ESS=Epworth Sleepiness Scale; PSQI=Pittsburgh Sleep Quality Index; SA-SDQ=Sleep Apnea scale of the Sleep Disorders Questionnaire.
Among asthma subjects, we used Student t-tests to compare continuous baseline variables between the two groups (Table 1). General linear models were used to test for associations of normalized SDQ with continuous asthma outcomes (Tables 3-4) and inflammatory markers (Table 5), in both, univariate and multivariate analyses. For dichotomous dependent variables (Tables 3-4), logistic regression was employed.
Table 3.
Associations of OSA risk* with asthma control indices.
| Univariate Analyses† | Multivariate Analyses†‡ | |||
|---|---|---|---|---|
| Parameter estimate or OR (95% CI) | P-value | Parameter estimate or OR (95% CI) | P-value | |
| Daily symptoms: | ||||
| Wheezing | 2.22 (1.60, 3.07) | <0.0001 | 1.75 (1.15, 2.66) | 0.009 |
| Cough | 1.86 (1.36, 2.53) | <0.0001 | 1.25 (0.82, 1.91) | 0.29 |
| Chest tightness | 1.68 (1.21, 2.34) | 0.002 | 1.38 (0.88, 2.14) | 0.16 |
| Shortness of breath | 1.92 (1.41, 2.59) | <0.0001 | 1.37 (0.92, 2.05) | 0.13 |
| Any | 2.44 (1.79, 3.33) | <0.0001 | 1.56 (1.05, 2.31) | 0.03 |
| Nightly symptoms | 1.89 (1.38, 2.58) | <0.0001 | 1.76 (1.16, 2.67) | 0.008 |
| Daily β-agonist rescue use: | ||||
| Inhaled | 1.66 (1.25, 2.19) | 0.0004 | 1.46 (1.00, 2.14) | 0.05 |
| Nebulized | 1.59 (0.99, 2.53) | 0.05 | 1.61 (0.85, 3.07) | 0.14 |
| Any | 1.70 (1.28, 2.24) | 0.0002 | 1.52 (1.04, 2.22) | 0.03 |
| Lung function: | ||||
| FEV % predicted 1 | -6.46 (-9.19, -3.72) | <0.0001 | -0.01 (-3.62, 3.59) | 0.99 |
| FVC % predicted | -5.91 (-8.11, -3.70) | <0.0001 | 0.82 (-1.94, 3.58) | 0.56 |
| FEV/FVC % predicted 1 | -1.83 (-3.67, 0.01) | 0.05 | -1.08 (-3.65, 1.48) | 0.41 |
| Log PC20 | -0.14 (-0.26, -0.02) | 0.03 | -0.06 (0.23, 0.12) | 0.54 |
| FEV1 reversibility to 8 puffs of inhaled albuterol | 2.44 (-0.83, 5.71) | 0.14 | -0.41 (-5.00, 4.18) | 0.86 |
Sleep Apnea scale of the Sleep Disorders Questionnaire score normalized by its standard deviation.
Analyses used general linear models (for continuous dependent variables) or logistic regression (for dichotomous dependent variables).
Adjusted for age, gender, race and obesity.
Abbreviations: OR=odds ratios; CI= confidence interval.
Table 4.
Associations of OSA risk* with asthma-related health care utilization and quality of life.
| Univariate Analyses† | Multivariate Analyses†‡ | |||
|---|---|---|---|---|
| Parameter estimate or OR (95% CI) | P-value | Parameter estimate or OR (95% CI) | P-value | |
| Health care utilization in the past year: | ||||
| hx/o MD visits | 1.50 (1.13, 2.01) | 0.006 | 1.15 (0.76, 1.73) | 0.52 |
| hx/o ER visits | 1.48 (1.11, 1.97) | 0.008 | 1.35 (0.91, 2.00) | 0.14 |
| hx/o hospitalizations | 1.96 (1.33, 2.89) | 0.0007 | 1.50 (0.90, 2.52) | 0.12 |
| hx/o ER visits or hospitalizations | 1.54 (1.16, 2.05) | 0.003 | 1.30 (0.88, 1.93) | 0.18 |
| hx/o ICU admission | 1.61 (0.92, 2.81) | 0.09 | 1.41 (0.68, 2.95) | 0.36 |
| Health care utilization ever: | ||||
| hx/o ER visits | 1.62 (1.21, 2.18) | 0.001 | 1.45 (0.96, 2.20) | 0.08 |
| hx/o hospitalizations | 1.46 (1.13, 1.90) | 0.005 | 1.10 (0.76, 1.59) | 0.60 |
| hx/o ICU admission | 1.59 (1.11, 2.29) | 0.01 | 1.35 (0.83, 2.20) | 0.23 |
| hx/o assisted ventilation | 2.06 (1.32, 3.22) | 0.002 | 1.87 (1.05, 3.35) | 0.03 |
| AQLQ | ||||
| total score | -0.53 (-0.67, -0.38) | <0.0001 | -0.40 (-0.61, -0.20) | 0.0002 |
| symptoms | -0.53 (-0.70, -0.37) | <0.0001 | -0.42 (-0.65, -0.19) | 0.0004 |
| activities | -0.49 (-0.65, -0.34) | <0.0001 | -0.34 (-0.55, -0.13) | 0.002 |
| emotions | -0.59 (-0.79, -0.39) | <0.0001 | -0.48 (-0.76, -0.21) | 0.0007 |
| environment | -0.51 (-0.69, -0.33) | <0.0001 | -0.41 (-0.66, -0.10) | 0.001 |
Sleep Apnea scale of the Sleep Disorders Questionnaire score normalized by its standard deviation.
Analyses used general linear models (for continuous dependent variables) or logistic regression (for dichotomous dependent variables).
Adjusted for age, gender, race and obesity.
Abbreviations: OR=odds ratios; CI= confidence interval; AQLQ=Asthma Quality of Life Questionnaire.
Table 5.
Associations of OSA risk* with blood and airway inflammatory markers in subjects with asthma.
| Univariate Analyses† | Multivariate Analyses†‡ | |||
|---|---|---|---|---|
| Parameter estimate (95% CI) | p-value | Parameter estimate (95% CI) | p-value | |
| Log IgE | -0.04 (-0.16, 0.07) | 0.42 | -0.12 (-0.28, 0.03) | 0.11 |
| Blood eos count | -0.001 (-0.04, 0.03) | 0.94 | -0.004 (-0.05, 0.04) | 0.87 |
| Blood Eosinophils% | -0.12 (-0.55, 0.32) | 0.60 | 0.01 (-0.57, 0.59) | 0.97 |
| eNO | -3.43 (-10.21, 3.35) | 0.32 | -0.30 (-9.14, 8.54) | 0.94 |
| Sputum Eosinophils % | 0.61 (-1.24, 2.45) | 0.52 | 0.28 (-2.27, 2.82) | 0.83 |
| Sputum PMNs% | 5.68 (1.76, 9.60) | 0.005 | 7.78 (2.33, 13.22) | 0.006 |
Sleep Apnea scale of the Sleep Disorders Questionnaire score normalized by its standard deviation.
Analyses used general linear models.
Adjusted for obesity, smoking history (yes/no) and any (inhaled or systemic) current corticosteroid use.
Abbreviations: CI= confidence interval; eNO=fraction of exhaled nitric oxide; PMNs=polymorphonuclear neutrophils.
When we assessed the associations of normalized SA-SDQ score with asthma outcomes, we included age, gender, BMI and race, as covariates.38 For associations of normalized SA- SDQ score with inflammatory markers, we included covariates that have been proposed to impact non-eosinophilic pathways in the lower airway (smoking history, obesity, and current inhaled or systemic corticosteroid use).24, 27, 29
All tests were two-tailed, with significance set at p<0.05 and trends noted for p=0.05-0.10.
3. Results
3.1. Baseline characteristics of study subjects
Table 1 presents the baseline characteristics of subjects. Both asthma groups were significantly older than NC, had higher prevalence of obesity and increased African-American representation. In seven control subjects, the PC20 could be estimated, but after corroborating with their lack of symptoms and with other clinical data available, these subjects were found appropriate for enrollment by their Center PIs. Only one control subject reported nightly symptoms, but had a FEV1% reversibility=4.95 from baseline, a negative methacholine challenge (PC20 could not be estimated) and interestingly endorsed frequent snoring.
Among asthmatics, SA had a longer duration of disease, worse lung physiology and methacholine PC20, and increased bronchial reversibility compared to NSA. As expected, both asthma groups showed worse lung physiology measures, health care use and quality of life than NC; among individuals with asthma, SA uniformly had worse such outcomes than NSA. With the exception of higher eNO levels and a trend for higher sputum eosinophil % among SA subjects, the other markers of airway inflammation were similar between the two groups.
3.2. Sleep measures and sleep apnea symptoms among all study subjects
As shown in Table 2, despite similar duration of sleep, NSA and SA subjects reported excessive daytime sleepiness, scored higher on ESS and had abnormal ESS scores more often than NC. In addition, NSA and SA, compared to NC subjects, had higher PSQI global scores; these scores were uniformly abnormal.
Other OSA symptoms, such as snoring and witnessed apneas, were also more prevalent among asthmatics. SA-SDQ scores and prevalence of high OSA risk (Table 2) were higher among asthmatics. The average SA-SDQ score among all asthmatics was 26.9 (SD 6.9).
3.3. Associations of SA-SDQ with disease control measures among participants with asthma
In univariate analyses (Table 3), higher SA-SDQ scores were significantly associated with daily (wheezing, cough, chest tightness, shortness of breath, or any) and nightly asthma symptoms, daily (via MDI or nebulization) β-agonist rescue use, measures of airways obstruction and methacholine PC20. After adjusting for covariates such as age, gender, BMI and race (Table 3), SA-SDQ scores remained significantly associated with daily wheezing or any asthma symptom, nightly asthma symptoms and use of any β-agonist rescue; no relationships with pulmonary physiologic measures (FEV1% and FVC% predicted, and logPC20) were found.
3.4. Associations of SA-SDQ with disease-related health care use and quality of life amongsubjects with asthma
In univariate analyses, higher SA-SDQ scores were associated with more frequent visits to physicians and emergency room, and hospitalizations in the prior year; additionally, higher SA-SDQ scores were associated with increased odds for lifetime emergency room visits, hospitalizations, ICU admissions and need for assisted ventilation (Table 4). In multivariate analyses, a significant association of SA-SDQ was maintained with a lifetime history of assisted ventilation.
Higher SA-SDQ was related to worse AQLQ global and individual domain scores (Table 4), with estimates of the relationships almost always surpassing the established minimal clinically difference of 0.5.39 With adjustment for covariates, the significance of all these relationships was maintained (Table 4); although the magnitude of the estimates was reduced slightly, they generally remained within the reported range (0.42-0.58) for a minimal clinically important difference.39
3.5. Associations of SA-SDQ with asthma-related inflammatory markers
Analyses of sputum cells showed higher % PMNs in subjects with high OSA risk compared to those without high OSA risk (p=0.001), whereas % sputum eosinophils were similar (p=0.66) (Figure 1). Overall, subjects with high OSA risk tended to have higher representation of any inflammatory (neutrophilic, eosinophilic or mixed) vs. paucigranulocytic than individuals without high OSA risk (80% vs. 63%, p=0.07). In analyses of specific inflammatory phenotypes (Figure 2), individuals with high OSA risk trended toward higher proportions of neutrophilic and mixed granulocytic, and lower proportions of eosinophilic and paucigranulocytic phenotypes (43% vs. 30%, 31% vs. 18%, 6% vs. 15% and 20% vs. 37%, respectively, Fisher's exact p=0.06 for overall relationship).
Figure 1.
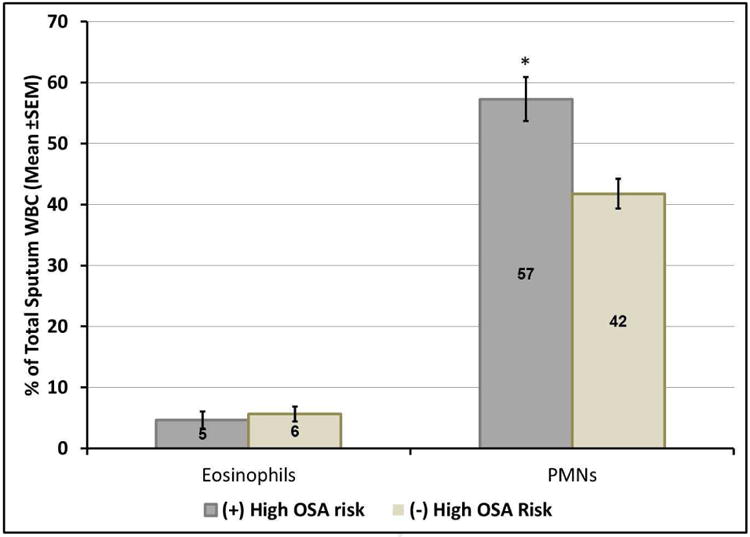
Sputum eosinophils and PMNs (expressed as percentage of sputum total white blood cell counts) in asthmatic subjects with and without high OSA risk (defined as scores on sleep apnea scale of the Sleep Disorders Questionnaire [SA-SDQ] ≥36 for men and ≥32 for women).
In subjects with high OSA risk compared to those without high OSA, there were higher % PMNs, whereas % sputum eosinophils were similar between the two groups.
*P-value=0.001 (two-sample Student t-test).
Abbreviations: OSA=obstructive sleep apnea.
Figure 2.
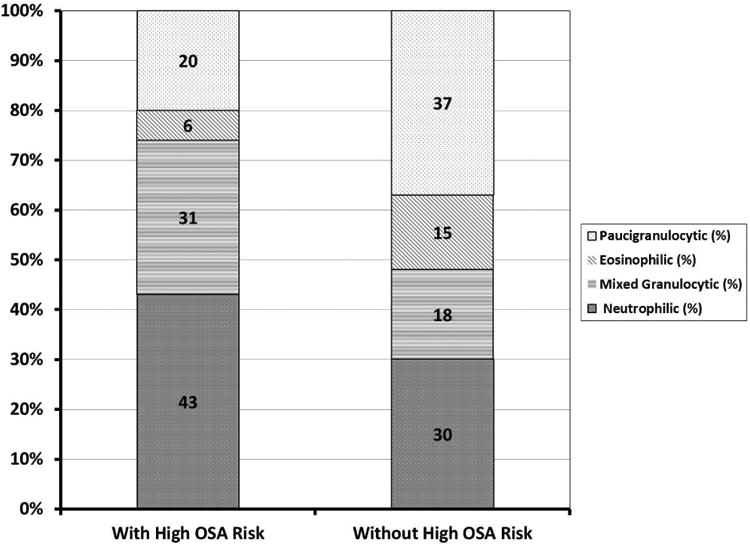
Proportion of sputum inflammatory phenotypes in asthmatic subjects with and without high OSA risk (defined as scores on sleep apnea scale of the Sleep Disorders Questionnaire [SA-SDQ] ≥36 for men and ≥32 for women).
Asthmatic subjects with high OSA risk, compared to those without high OSA risk, tended to have higher proportions of neutrophilic and mixed granulocytic, and lower proportions of eosinophilic and paucigranulocytic phenotypes (p=0.06, Fisher's exact test).
Abbreviations: OSA=obstructive sleep apnea.
In univariate analyses, SA-SDQ was not associated with markers of eosinophilic inflammation from blood and airway samples (Table 5). In contrast, there was a strong positive association of SA-SDQ with sputum neutrophils; the strength of this association was further magnified when it was adjusted for the covariates (obesity, prior smoking history and current inhaled or systemic corticosteroid use): each increase in SD-SDQ by its standard deviation (6.85 units) was associated with a rise in % sputum neutrophils of 7.78 (95% confidence interval = [2.33, 13.22], p=0.006).
4. Discussion
In this large sample of well-characterized subjects, OSA symptoms were more prevalent among subjects with asthma, apart from traditional risks, and were associated with worse asthma control indices, more frequent disease-related health care use and lesser quality of life. In subjects with asthma, a greater risk for OSA was significantly associated with airway neutrophilia, independent of obesity and other confounders. This suggests that OSA may be an important, previously unrecognized contributor to neutrophilic asthma. Further studies with objective measures of OSA are necessary to confirm these findings and better understand the underlying mechanisms.
By studying a large asthma population that has been well-phenotyped for disease severity, our study adds key strengths to existing evidence that suggests a bidirectional relationship of OSA with asthma. On one hand, we find that –despite similar sleep duration— asthmatics, compared to normal controls, reported increased sleepiness and worse sleep quality (Table 2). At the same time, they more often reported snoring and witnessed apneas, scored higher on SA-SDQ and more often met the definition of high OSA risk (Table 2). Several clinical studies have shown a high prevalence of OSA symptoms or PSG-diagnosed OSA in asthma patients;7-11 this prevalence is higher than in normal controls7, 10 or in other types of patients.9 Underlying this relationship may be factors more unique to this population (including the disease itself and inhaled corticosteroid usage). 38, 40 Conversely, our study finds that the risk of OSA is related to an increased asthma burden. Higher OSA risk was significantly associated with several indices of poor disease control, such as increased asthma symptoms and rescue inhaler use (Table 3). We also show that high OSA risk was associated with increased healthcare utilization and lower quality of life (Table 4). Thus, our study strengthens previous findings from patients with asthma where higher risk for OSA was associated with worse overall asthma control,12 daytime and nighttime symptoms,13 and with increased exacerbations.18 Likewise, these data align well with studies of CPAP therapy for OSA in small sets of patients with asthma. in which this treatment improved asthma outcomes, including daytime14 and nighttime14-16 symptoms, rescue bronchodilator use,14 PEF rates,14 and disease-specific quality of life.17
Our study provides novel evidence for a potential mechanism whereby OSA aggravates asthma through non-eosinophillic inflammatory pathways. We found that higher OSA risk was associated with enhanced presence of PMNs in the sputum (Figure 1). In subjects with high OSA risk relative to those without high OSA risk, we also observed a 2.5 fold decreased prevalence of eosinophilic asthma and a 1.4 fold increased risk of neutrophilic asthma (Figure 2). Furthermore, after adjusting for covariates, higher SA-SDQ was significantly associated with sputum PMNs%, whereas no association was found with sputum % or peripheral blood markers of eosinophilic inflammation (Table 5). This suggests that comorbid OSA may influence the pathogenesis of a neutrophil – rich rather than an eosinophilic type of asthma. There is increased recognition of the heterogeneity of asthma, encompassing phenotypes with potentially different underlying inflammatory pathologies. For example, a neutrophilic phenotype of asthma has been observed more often in patients with chronic persistent, severe, and even fatal asthma.26, 27 PMNs can contribute to tissue damage and inflammation, mucus hypersecretion and airway remodeling, all of which are characteristic of asthma. PMNs secrete a salvo of powerful cytotoxic and extracellular matrix – disrupting enzymes such as elastase, myeloperoxidase and matrix metalloproteases. The neutrophil elastase, for example, is a potent secretagogue and enhancer of vascular permeability, leading to excess mucus production. PMNs produce mediators, such as leukotrienes, with pleiotropic activity on eosinophils and T cells, which release Th2 cytokines and promote bronchospasm. PMNs are an important source of TGF-β, implicated in angiogenesis, smooth muscle proliferation, and trafficking of cells involved ultimately in airway remodeling.41 At the same time, there is an increasing body of evidence that OSA induces neutrophilic inflammation of the airways. OSA patients, compared to normal subjects, have increased neutrophil numbers in the induced sputum20, 21 and these correlate with the apnea-hypopnea index (AHI).20 In OSA, neutrophil activation was documented via increased ROS formation and NF-κB upregulation.42 Additionally, neutrophil apoptosis, unlike that of other cells, is inhibited, rather than induced, by hypoxia.43 In this context, our novel results in asthma patients suggest that OSA—a common occurrence in these patients—may contribute to the non-eosinophilic/neutrophilic inflammation, and more severe asthma. These alterations in the lower airway cellular profile could relate to OSA's key features—intermittent hypoxia, mechanical stress from increased work of breathing and sleep fragmentation. Indeed, a recent experimental study found that exposure to chronic intermittent hypoxia during allergen-induced inflammation in rats shifted the pattern of airway inflammation from its traditional Th-2 eosinophilic to more Th-1-phenotype, with airway infiltration by monocytes.44 More importantly, these immune alterations were associated with airflow limitation. This physiologic deficit resulted from heterogeneous structural changes consisting of proximal airway collagen deposition, and co-occurred with distal matrix degradation in the small airways and lung parenchyma.44 Whether the other OSA features have different effects, and how all these often coexistent features interact in an individual patient with asthma to modulate the lower airway inflammatory milieu, needs to be studied.
There are several limitations to our study. First, at some of the centers, in order to be enrolled in the parent SARP study, subjects had to lack a clinical history of OSA or PAP use. As a consequence, we likely studied a narrower range, ie milder sleep-disordered breathing and lacked more severe cases. Furthermore, in difficult-to-control asthma, OSA is highly prevalent (88-95.5%),10, 11 but because of exclusions in the parent study, we likely missed some OSA cases. Thus, our data likely represents an underestimation of the true relationships between OSA and asthma. Future studies will need to incorporate a wide range of severity of OSA. Another study limitation is its cross-sectional design which precludes causal inferences. For example, sleepiness in asthma could be due to asthma-related sleep disturbance,45 OSA8, 46 or a combination thereof. Furthermore, although the interaction of OSA with asthma is likely to be bidirectional,6, 46 interventional studies suggest an influential role of coexistent OSA on asthma. Altogether, these studies call for further investigations of the bidirectional links between OSA and asthma. Last, a questionnaire-based assessment, rather than an objective diagnosis of OSA, was employed. This approach has been common in the OSA and OSA/asthma literature,9 where the expense of technology and personnel time would have made the conduct of such large study cost-prohibitive. We note the SA-SDQ is a well-established assessment instrument for OSA, with good performance in sleep-based and other clinic populations, when compared to PSG.34, 47
We want to acknowledge that obesity is a frequent confounder in studies of OSA/asthma. In our study, SA subjects were more obese than NSA and NC subjects, similar to previous reports.48 Some of our findings were attenuated by adjustment for covariates, including obesity (Tables 3, 4, 5). It is possible that obese subjects in our study were at high risk for both OSA and asthma simply because of their obesity,49 or it is possible that OSA mediates the relationship between obesity and asthma. Obesity has been suggested to have an important role in promoting the development/severity of asthma.50 However, obesity is also a well-established risk factor for OSA.5 Our analyses on associations of OSA symptoms with asthma control measures are adjusted for obesity and suggest a role of OSA in asthma control that is independent of obesity. Furthermore, when accounting for SA-SDQ (Table 5), obesity was not associated with sputum PMNs% or eosinophils% (-7.13 [-18.39, 4.13], p=0.21; and -0.46 [-5.72, 4.79], p=0.86, respectively), or with other markers of eosinophilic inflammation shown in Table 5 (data not shown). This agrees with recent literature that suggests adipose tissue can directly affect the airway, rather than through enhanced airway inflammation.50 This phenomenon may explain why studies that addressed the relationship of obesity with lower airway inflammation in asthma have provided negative results,49, 51 prompting calls to consider OSA in this type of investigations.29, 49 In addition, interventional studies of CPAP treatment for OSA report improvement in asthma outcomes.14, 15, 17 Although weight changes are not reported, it is unlikely that substantial loss could have occurred this quickly (2 weeks-2 months follow-up) to yield these improvements in asthma; in contrast, a meta-analysis of several experimental CPAP studies showed trivial, non-significant rather weight gain, following OSA treatment.52 Last, in SARP, neutrophils were highest in Cluster 5 which had also the highest proportion of obesity and possibly OSA.53 Whether the neutrophilia in this cluster is driven by OSA, or something intrinsic to this sub-phenotype, remains to be further studied.
In summary, our study of a large and well-characterized sample of asthma individuals, shows associations of OSA symptoms with worse asthma control indices, health care utilization and disease-specific quality of life; these associations were independent of other factors, such as obesity, that can affect asthma, and suggest that the underlying mechanisms may act through non-eosinophillic, neutrophilic inflammatory pathways. This study represents an important step forward in our understanding of the interaction of asthma and OSA, beyond common risk factors such as obesity. Further studies that incorporate objective assessments of OSA and that differentiate its pathogenic features may deepen our understanding of these mechanisms, and may offer new interventions for a phenotype that responds poorly to current standard therapies.
Supplementary Material
Highlights.
What is already known about this topic?
Obstructive sleep apnea (OSA) and asthma may be associated, but large studies in well-phenotyped subjects are lacking and mechanisms are unknown. We tested this association and mechanisms in a subset of the Severe Asthma Research Program.
What does this article add to our knowledge?
Our study shows that OSA risk was associated with worse indices of asthma control, increased health care utilization and worse quality of life. Furthermore, OSA risk was associated with neutrophilic airway inflammation.
How does this study impact current management guidelines?
Evaluating and treating for OSA independent of obesity, could be one new approach to include in clinical asthma programs.
Acknowledgments
The authors are grateful to the study subjects for their participation and to Dr. William Busse for his support.
Funding support: National Institutes of Health HL069170, HL069174, HL069167, HL069116, UL1RR025011; and additional resources from the William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin.
Dr. Teodorescu received funding from the Department of Medicine and University of Wisconsin School of Medicine and Public Health, Medical Education and Research Committee-New Investigator Award, University of Wisconsin, and Department of Veterans Affairs for asthma-sleep apnea research.
Abbreviations
- AQLQ
Asthma Quality of Life Questionnaire
- BMI
Body Mass Index (in kilograms per meter squared)
- CI
Confidence Interval (95% reported)
- CPAP
Continuous Positive Airway Pressure
- ESS
Epworth Sleepiness Scale
- FEV1
Forced Expiratory Volume in first second of the Forced Vital Capacity maneuver
- FVC
Forced Vital Capacity
- MDI
Metered Dose Inhaler
- NAEPP
National Asthma Education and Prevention Program
- OR
Odds Ratio
- OSA
Obstructive Sleep Apnea
- PC20
Provocative Concentration of methacholine, necessary to produce a 20% fall in FEV1
- PEF
Peak Expiratory Flow Rate
- PMN
Polymorphonuclear Neutrophils
- PSG
Polysomnography (sleep study)
- PSQI
Pittsburgh Sleep Quality Index
- SAS
Statistical Analysis Software
- SA-SDQ
Sleep Apnea scale of the Sleep Disorders Questionnaire
- SD
Standard Deviation
Footnotes
Author Contributions: Conception: M Teodorescu
Study design and implementation: M Teodorescu, RL Sorkness, G Crisafi, ER Bleecker, S, Erzurum, BM Gaston, SE Wenzel, NN Jarjour
Statistical analysis: M Teodorescu, D Curran-Everett
Interpretation of the data: M Teodorescu, O Broytman, RL Sorkness, NN Jarjour
Drafting of the article: M Teodorescu, O Broytman
Critical revision of the article for important intellectual content: All authors
Mrs. Crisafi, Drs. Broytman, Curran-Everett, Sorkness, Bleecker, Erzurum, Gaston, Wenzel and Jarjour report no conflicts of interest related to this work.
The content of this article is solely the responsibility of the authors and does not represent the views of the Department of Veterans Affairs, NIH or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabe KF, Vermeire PA, Soriano JB, Maier WC. Clinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) study. Eur Respir J. 2000;16:802–7. doi: 10.1183/09031936.00.16580200. [DOI] [PubMed] [Google Scholar]
- 2.Godard P, Chanez P, Siraudin L, Nicoloyannis N, Duru G. Costs of asthma are correlated with severity: a 1-yr prospective study. Eur Respir J. 2002;19:61–7. doi: 10.1183/09031936.02.00232001. [DOI] [PubMed] [Google Scholar]
- 3.Stempel DA, McLaughin TP, Stanford RH, Fuhlbrigge AL. Patterns of asthma control: a 3-year analysis of patient claims. J Allergy Clin Immunol. 2005;115:935–9. doi: 10.1016/j.jaci.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 4.Irwin RS, Curley FJ, French CL. Difficult-to-control asthma. Contributing factors and outcome of a systematic management protocol. Chest. 1993;103:1662–9. doi: 10.1378/chest.103.6.1662. [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am J Epidemiol. 2013 doi: 10.1093/aje/kws342. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. J Investig Med. 2014;62:665–75. doi: 10.2310/JIM.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 7.Janson C, Gislason T, Boman G, Hetta J, Roos BE. Sleep disturbances in patients with asthma. Respir Med. 1990;84:37–42. doi: 10.1016/s0954-6111(08)80092-3. [DOI] [PubMed] [Google Scholar]
- 8.Teodorescu M, Consens FB, Bria WF, Coffey MJ, McMorris MS, Weatherwax KJ, et al. Correlates of daytime sleepiness in patients with asthma. Sleep Med. 2006;7:607–13. doi: 10.1016/j.sleep.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Auckley D, Moallem M, Shaman Z, Mustafa M. Findings of a Berlin Questionnaire survey: comparison between patients seen in an asthma clinic versus internal medicine clinic. Sleep Med. 2008;9:494–9. doi: 10.1016/j.sleep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Julien JY, Martin JG, Ernst P, Olivenstein R, Hamid Q, Lemiere C, et al. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J Allergy Clin Immunol. 2009;124:371–6. doi: 10.1016/j.jaci.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Yigla M, Tov N, Solomonov A, Rubin AH, Harlev D. Difficult-to-control asthma and obstructive sleep apnea. J Asthma. 2003;40:865–71. doi: 10.1081/jas-120023577. [DOI] [PubMed] [Google Scholar]
- 12.Teodorescu M, Polomis DA, Hall SV, Teodorescu MC, Gangnon RE, Peterson AG, et al. Association of obstructive sleep apnea risk with asthma control in adults. Chest. 2010;138:543–50. doi: 10.1378/chest.09-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teodorescu M, Polomis DA, Teodorescu MC, Gangnon RE, Peterson AG, Consens FB, et al. Association of obstructive sleep apnea risk or diagnosis with daytime asthma in adults. J Asthma. 2012;49:620–8. doi: 10.3109/02770903.2012.689408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan CS, Woolcock AJ, Sullivan CE. Nocturnal asthma: role of snoring and obstructive sleep apnea. Am Rev Respir Dis. 1988;137:1502–4. doi: 10.1164/ajrccm/137.6.1502. [DOI] [PubMed] [Google Scholar]
- 15.Ciftci TU, Ciftci B, Guven SF, Kokturk O, Turktas H. Effect of nasal continuous positive airway pressure in uncontrolled nocturnal asthmatic patients with obstructive sleep apnea syndrome. Respir Med. 2005;99:529–34. doi: 10.1016/j.rmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Guilleminault C, Quera-Salva MA, Powell N, Riley R, Romaker A, Partinen M, et al. Nocturnal asthma: snoring, small pharynx and nasal CPAP. Eur Respir J. 1988;1:902–7. [PubMed] [Google Scholar]
- 17.Lafond C, Series F, Lemiere C. Impact of CPAP on asthmatic patients with obstructive sleep apnoea. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2007;29:307–11. doi: 10.1183/09031936.00059706. [DOI] [PubMed] [Google Scholar]
- 18.ten Brinke A, Sterk PJ, Masclee AA, Spinhoven P, Schmidt JT, Zwinderman AH, et al. Risk factors of frequent exacerbations in difficult-to-treat asthma. Eur Respir J. 2005;26:812–8. doi: 10.1183/09031936.05.00037905. [DOI] [PubMed] [Google Scholar]
- 19.Bergeron C, Kimoff J, Hamid Q. Obstructive sleep apnea syndrome and inflammation. J Allergy Clin Immunol. 2005;116:1393–6. doi: 10.1016/j.jaci.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Li AM, Hung E, Tsang T, Yin J, So HK, Wong E, et al. Induced sputum inflammatory measures correlate with disease severity in children with obstructive sleep apnoea. Thorax. 2007;62:75–9. doi: 10.1136/thx.2006.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salerno FG, Carpagnano E, Guido P, Bonsignore MR, Roberti A, Aliani M, et al. Airway inflammation in patients affected by obstructive sleep apnea syndrome. Respir Med. 2004;98:25–8. doi: 10.1016/j.rmed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–97. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 23.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–63. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 24.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–13. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 25.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–36 e13. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma : evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–36. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 27.Foley SC, Hamid Q. Images in allergy and immunology: neutrophils in asthma. J Allergy Clin Immunol. 2007;119:1282–6. doi: 10.1016/j.jaci.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Chanez P, Wenzel SE, Anderson GP, Anto JM, Bel EH, Boulet LP, et al. Severe asthma in adults: what are the important questions? J Allergy Clin Immunol. 2007;119:1337–48. doi: 10.1016/j.jaci.2006.11.702. [DOI] [PubMed] [Google Scholar]
- 29.Carpagnano GE, Foschino-Barbaro MP, Carratu P, Lacedonia D, Resta O. Could neutrophilic airway inflammation in obese people be more due to obstructive sleep apnoea syndrome than to asthma? Eur Respir J. 2012;39:1547–9. doi: 10.1183/09031936.00184111. [DOI] [PubMed] [Google Scholar]
- 30.Teodorescu M, Busse WW, Curran-Everett D, Crisafi G Jarjour NNatN-NSARPSI. Obstructive sleep apnea (OSA) symptoms in patients with asthma. Am J Respir Crit Care Med. 2010;181:A2551. [Google Scholar]
- 31.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teodorescu M, Consens FB, Bria WF, Coffey MJ, McMorris MS, Weatherwax KJ, et al. Predictors of habitual snoring and obstructive sleep apnea risk in patients with asthma. Chest. 2009;135:1125–32. doi: 10.1378/chest.08-1273. [DOI] [PubMed] [Google Scholar]
- 33.Luyster FS, Teodorescu M, Bleecker E, Busse W, Calhoun W, Castro M, et al. Sleep quality and asthma control and quality of life in non-severe and severe asthma. Sleep Breath. 2012;16:1129–37. doi: 10.1007/s11325-011-0616-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Douglass AB, Bornstein R, Nino-Murcia G, Keenan S, Miles L, Zarcone VP, Jr, et al. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994;17:160–7. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 35.Martin RJ, Wanger JS, Irvin CG, Bucher Bartelson B, Cherniack RM. Methacholine challenge testing: safety of low starting FEV1. Asthma Clinical Research Network (ACRN) Chest. 1997;112:53–6. doi: 10.1378/chest.112.1.53. [DOI] [PubMed] [Google Scholar]
- 36.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 37.Fahy JV, Boushey HA, Lazarus SC, Mauger EA, Cherniack RM, Chinchilli VM, et al. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am J Respir Crit Care Med. 2001;163:1470–5. doi: 10.1164/ajrccm.163.6.9901105. [DOI] [PubMed] [Google Scholar]
- 38.Sutherland ER, Lehman EB, Teodorescu M, Wechsler ME. Body mass index and phenotype in subjects with mild-to-moderate persistent asthma. J Allergy Clin Immunol. 2009;123:1328–34 e1. doi: 10.1016/j.jaci.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol. 1994;47:81–7. doi: 10.1016/0895-4356(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 40.Teodorescu M, Xie A, Sorkness CA, Robbins J, Reeder S, Gong Y, et al. Effects of Inhaled Fluticasone on Upper Airway during Sleep and Wakefulness in Asthma: A Pilot Study. J Clin Sleep Med. doi: 10.5664/jcsm.3450. In Press http://www.aasmnet.org/jcsm/AcceptedPapers/JC-295-12.pdf. [DOI] [PMC free article] [PubMed]
- 41.Lee KY, Ho SC, Lin HC, Lin SM, Liu CY, Huang CD, et al. Neutrophil-derived elastase induces TGF-beta1 secretion in human airway smooth muscle via NF-kappaB pathway. Am J Respir Cell Mol Biol. 2006;35:407–14. doi: 10.1165/rcmb.2006-0012OC. [DOI] [PubMed] [Google Scholar]
- 42.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–9. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 43.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–15. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broytman O, Braun RK, Morgan BJ, Pegelow DF, Hsu PN, Mei LS, et al. Effects of Chronic Intermittent Hypoxia on Allergen-Induced Airway Inflammation in Rats. Am J Respir Cell Mol Biol. 2015;52:162–70. doi: 10.1165/rcmb.2014-0213OC. [DOI] [PubMed] [Google Scholar]
- 45.Mastronarde JG, Wise RA, Shade DM, Olopade CO, Scharf SM American Lung Association Asthma Clinical Research C. Sleep quality in asthma: results of a large prospective clinical trial. J Asthma. 2008;45:183–9. doi: 10.1080/02770900801890224. [DOI] [PubMed] [Google Scholar]
- 46.Teodorescu M, Barnet JH, Hagen EW, Palta M, Young TB, Peppard PE. Association between asthma and risk of developing obstructive sleep apnea. JAMA. 2015;313:156–64. doi: 10.1001/jama.2014.17822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassetti C, Aldrich MS, Quint D. Sleep-disordered breathing in patients with acute supra-and infratentorial strokes. A prospective study of 39 patients. Stroke. 1997;28:1765–72. doi: 10.1161/01.str.28.9.1765. [DOI] [PubMed] [Google Scholar]
- 48.The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22:470–7. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 49.van Veen IH, Ten Brinke A, Sterk PJ, Rabe KF, Bel EH. Airway inflammation in obese and nonobese patients with difficult-to-treat asthma. Allergy. 2008;63:570–4. doi: 10.1111/j.1398-9995.2007.01597.x. [DOI] [PubMed] [Google Scholar]
- 50.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, et al. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Todd DC, Armstrong S, D'Silva L, Allen CJ, Hargreave FE, Parameswaran K. Effect of obesity on airway inflammation: a cross-sectional analysis of body mass index and sputum cell counts. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2007;37:1049–54. doi: 10.1111/j.1365-2222.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 52.Yang D, Liu Z, Yang H. The impact of effective continuous positive airway pressure on homeostasis model assessment insulin resistance in non-diabetic patients with moderate to severe obstructive sleep apnea. Diabetes Metab Res Rev. 2012;28:499–504. doi: 10.1002/dmrr.2301. [DOI] [PubMed] [Google Scholar]
- 53.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–23. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


