Abstract
c-Abl is a non-receptor protein-tyrosine kinase lacking a clear physiological role. A clue to its normal function is suggested by overexpression of Abl in fibroblasts, which leads to inhibition of cell growth. This effect requires tyrosine kinase activity and the Abl C-terminus. c-Abl is localized to the cell nucleus, where it can bind DNA, and interacts with the retinoblastoma protein, a potential mediator of the growth-inhibitory effect. Nuclear localization of Abl can be directed by a pentalysine nuclear localization signal in the Abl C-terminus. Here, we have identified two additional basic motifs in the Abl C-terminus, either of which can function independently of the pentalysine signal to localize Abl to the nucleus. Using a quantitative transfection assay, we show that both c-Abl and transforming Abl proteins inhibit entry into S phase and this effect is absolutely dependent on nuclear localization. Further, we demonstrate that the Abl cytostatic effect requires both the Rb and p53 tumor suppressor gene products. These results indicate that Abl inhibits cell proliferation by interacting with central elements of the cell cycle control apparatus in the nucleus, and suggest a direct connection between p53 and Rb in this growth-inhibitory pathway.
Full text
PDF

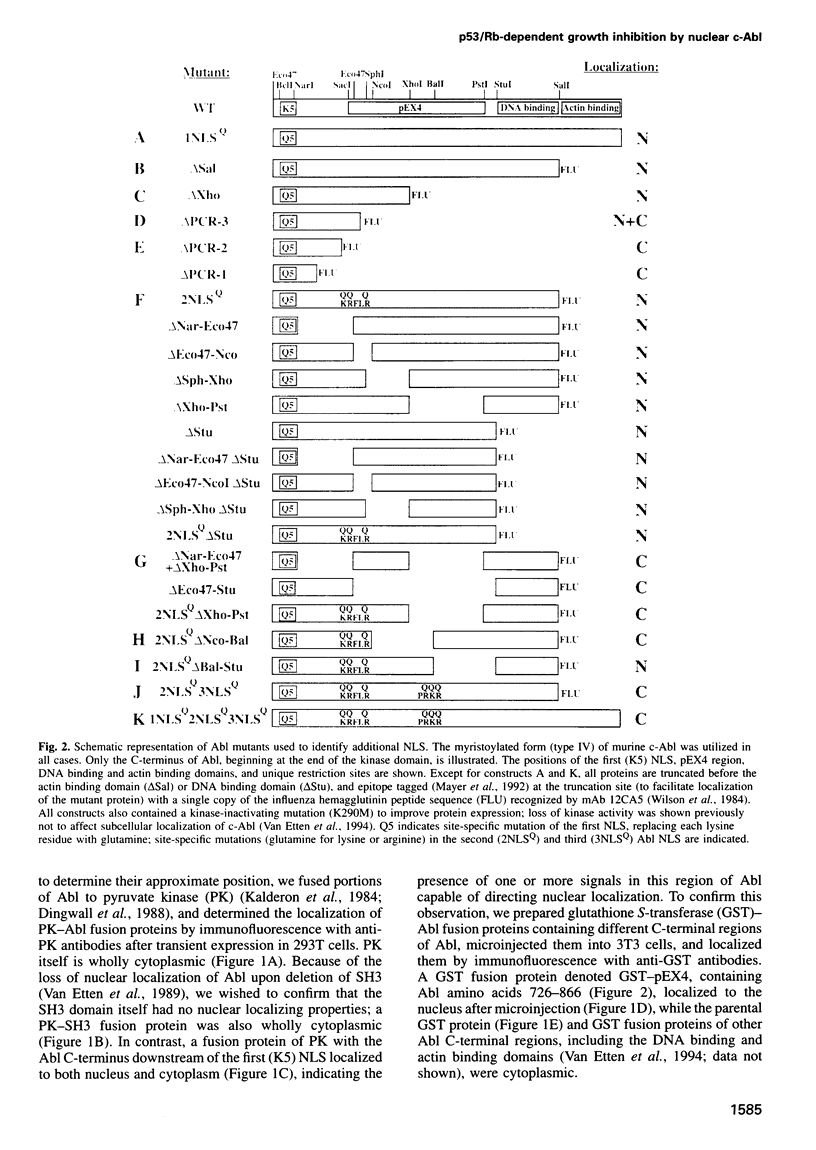
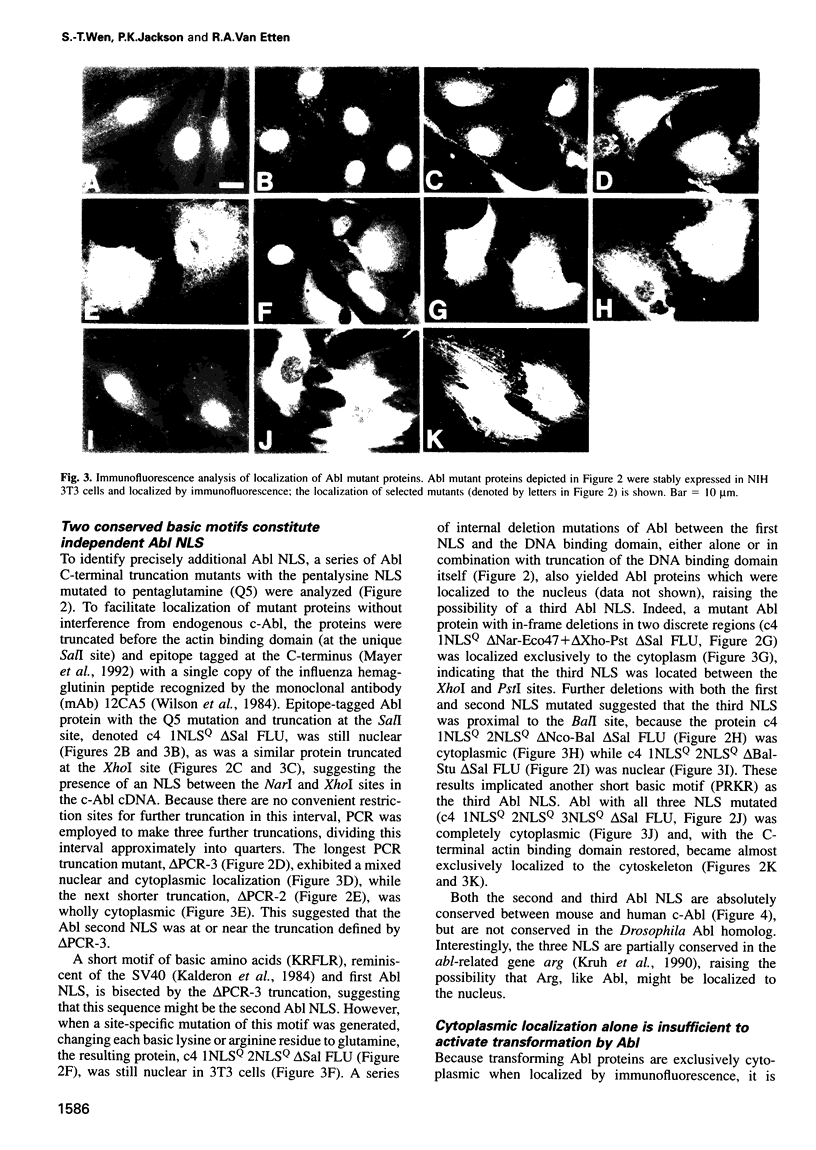
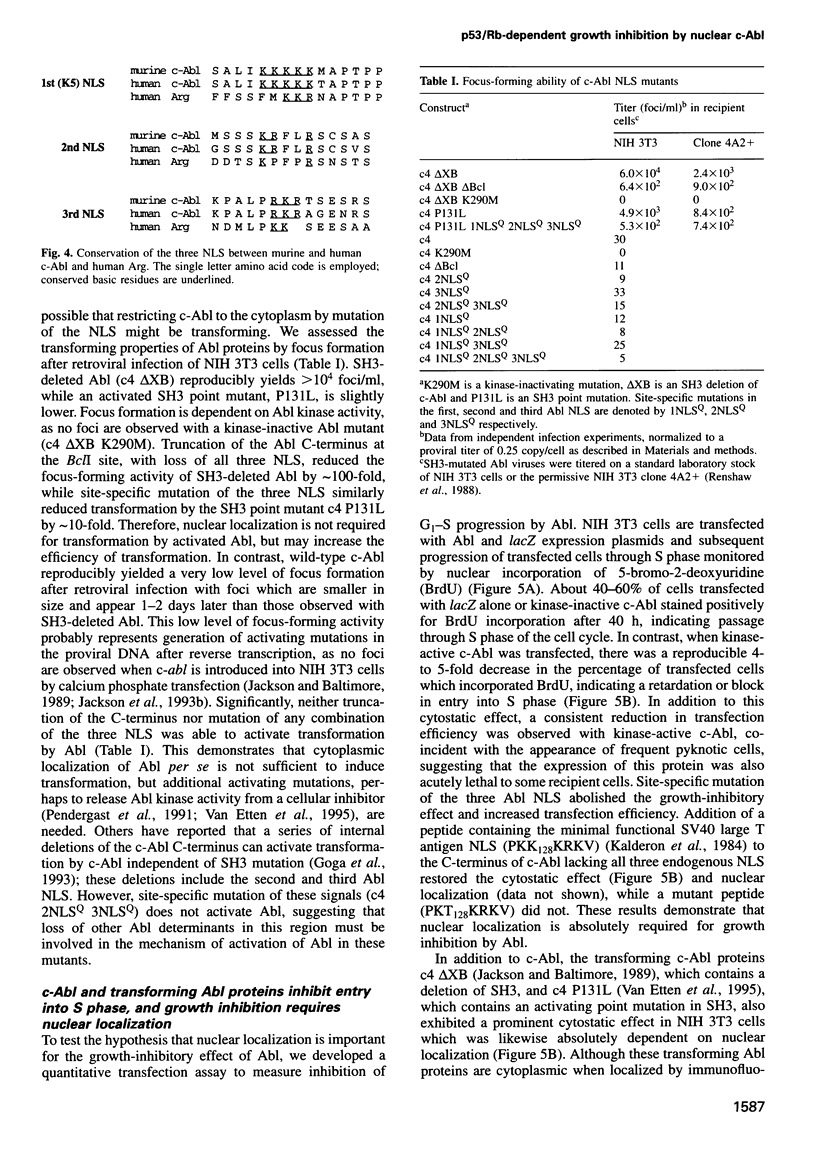
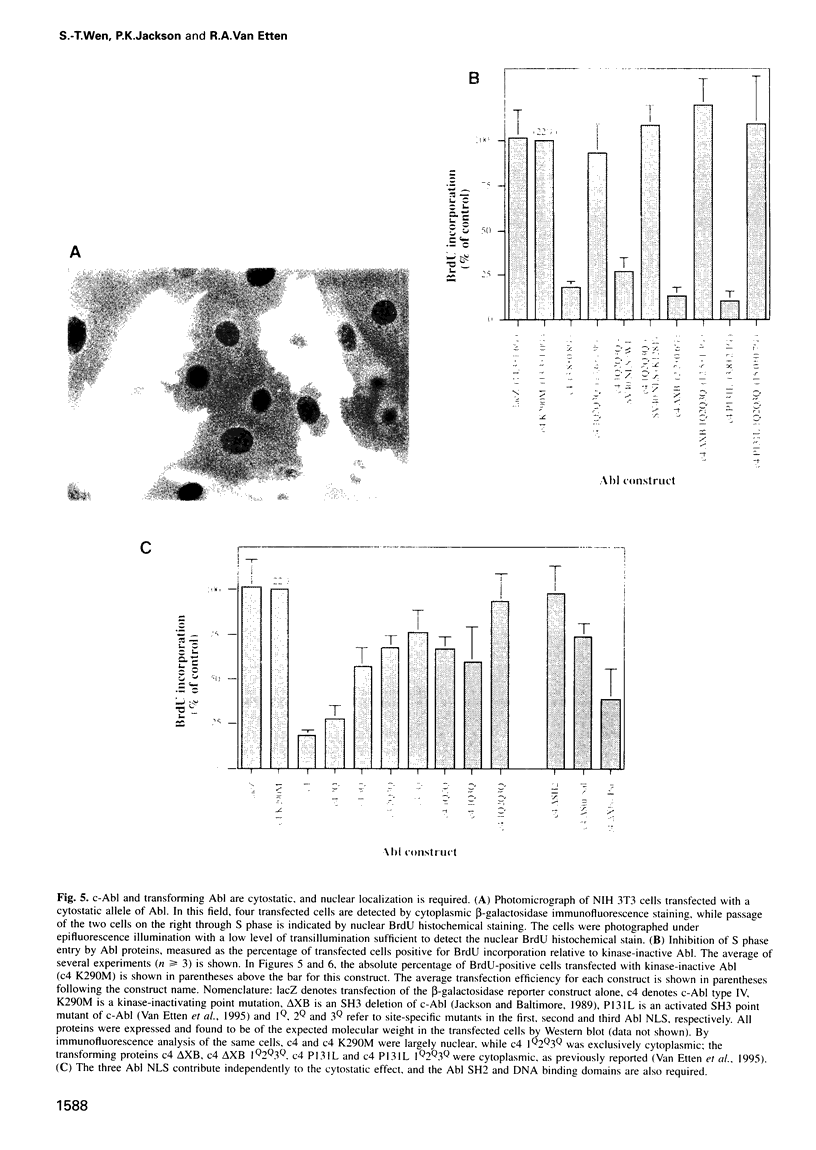

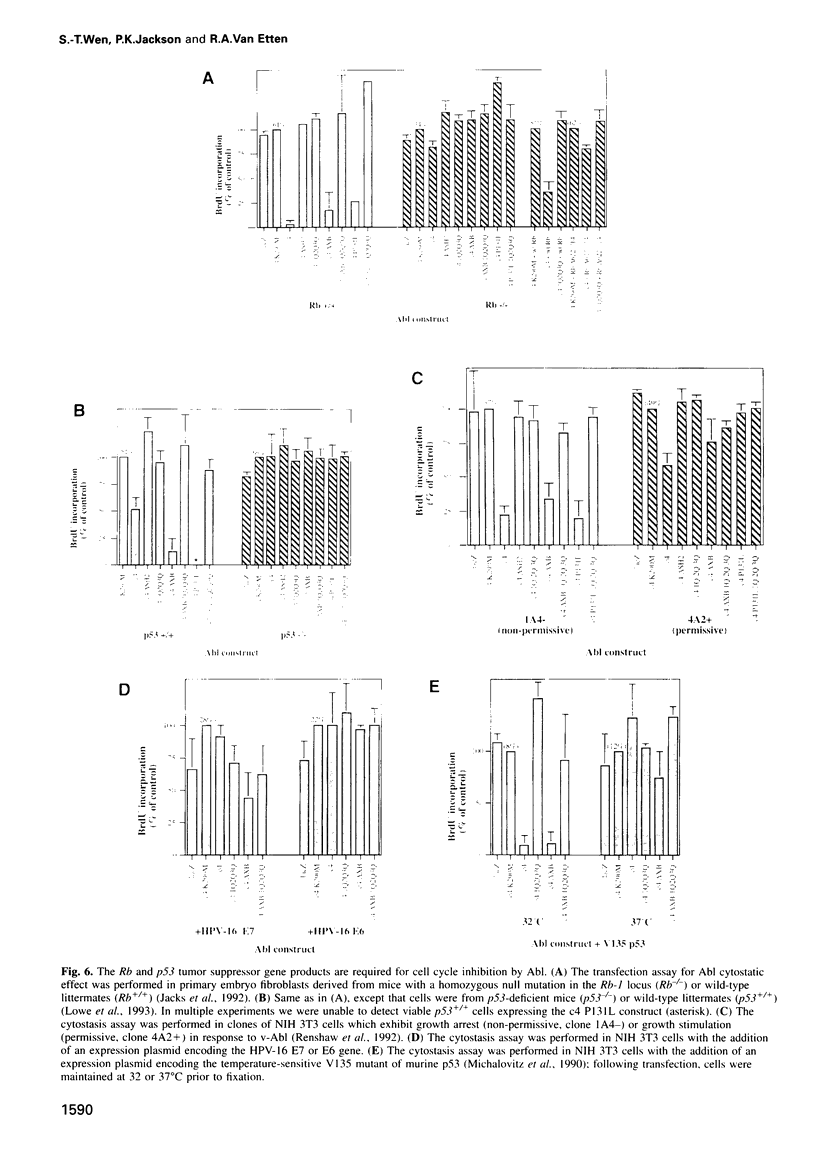





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison C., Jenkins J. R., Stürzbecher H. W. The p53 nuclear localisation signal is structurally linked to a p34cdc2 kinase motif. Oncogene. 1990 Mar;5(3):423–426. [PubMed] [Google Scholar]
- Baskaran R., Dahmus M. E., Wang J. Y. Tyrosine phosphorylation of mammalian RNA polymerase II carboxyl-terminal domain. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11167–11171. doi: 10.1073/pnas.90.23.11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Neriah Y., Bernards A., Paskind M., Daley G. Q., Baltimore D. Alternative 5' exons in c-abl mRNA. Cell. 1986 Feb 28;44(4):577–586. doi: 10.1016/0092-8674(86)90267-9. [DOI] [PubMed] [Google Scholar]
- Birchenall-Roberts M. C., Ruscetti F. W., Kasper J. J., Bertolette D. C., 3rd, Yoo Y. D., Bang O. S., Roberts M. S., Turley J. M., Ferris D. K., Kim S. J. Nuclear localization of v-Abl leads to complex formation with cyclic AMP response element (CRE)-binding protein and transactivation through CRE motifs. Mol Cell Biol. 1995 Nov;15(11):6088–6099. doi: 10.1128/mcb.15.11.6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Jackson P. K., Bernards A., Baltimore D. Nonmyristoylated Abl proteins transform a factor-dependent hematopoietic cell line. Mol Cell Biol. 1992 Apr;12(4):1864–1871. doi: 10.1128/mcb.12.4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R., Cai Y., Wong P. M., Chung S. W. Deregulation of c-abl mediated cell growth after retroviral transfer and expression of antisense sequences. Oncogene. 1995 Apr 20;10(8):1607–1614. [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995 Aug 25;82(4):675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Dikstein R., Heffetz D., Ben-Neriah Y., Shaul Y. c-abl has a sequence-specific enhancer binding activity. Cell. 1992 May 29;69(5):751–757. doi: 10.1016/0092-8674(92)90287-m. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Robbins J., Dilworth S. M., Roberts B., Richardson W. D. The nucleoplasmin nuclear location sequence is larger and more complex than that of SV-40 large T antigen. J Cell Biol. 1988 Sep;107(3):841–849. doi: 10.1083/jcb.107.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Engelman A., Rosenberg N. Isolation of temperature-sensitive Abelson virus mutants by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8021–8025. doi: 10.1073/pnas.84.22.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangioni J. V., Neel B. G. Use of a general purpose mammalian expression vector for studying intracellular protein targeting: identification of critical residues in the nuclear lamin A/C nuclear localization signal. J Cell Sci. 1993 Jun;105(Pt 2):481–488. doi: 10.1242/jcs.105.2.481. [DOI] [PubMed] [Google Scholar]
- Franz W. M., Berger P., Wang J. Y. Deletion of an N-terminal regulatory domain of the c-abl tyrosine kinase activates its oncogenic potential. EMBO J. 1989 Jan;8(1):137–147. doi: 10.1002/j.1460-2075.1989.tb03358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Tabin C. J., Wang J. Y., Weinberg R., Baltimore D. Transfection of fibroblasts by cloned Abelson murine leukemia virus DNA and recovery of transmissible virus by recombination with helper virus. J Virol. 1982 Jan;41(1):271–285. doi: 10.1128/jvi.41.1.271-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goga A., Liu X., Hambuch T. M., Senechal K., Major E., Berk A. J., Witte O. N., Sawyers C. L. p53 dependent growth suppression by the c-Abl nuclear tyrosine kinase. Oncogene. 1995 Aug 17;11(4):791–799. [PubMed] [Google Scholar]
- Goga A., McLaughlin J., Pendergast A. M., Parmar K., Muller A., Rosenberg N., Witte O. N. Oncogenic activation of c-ABL by mutation within its last exon. Mol Cell Biol. 1993 Aug;13(8):4967–4975. doi: 10.1128/mcb.13.8.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Craik C., Hiraoka Y. Homeodomain of yeast repressor alpha 2 contains a nuclear localization signal. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6954–6958. doi: 10.1073/pnas.87.18.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y., Yamato K., Tsuchida N. A temperature sensitive mutant of the human p53, Val138, arrests rat cell growth without induced expression of cip1/waf1/sdi1 after temperature shift-down. Oncogene. 1995 May 18;10(10):1879–1885. [PubMed] [Google Scholar]
- Hu Q. J., Dyson N., Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990 Apr;9(4):1147–1155. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. S., Duyster J., Wang J. Y. Biological response to phorbol ester determined by alternative G1 pathways. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4793–4797. doi: 10.1073/pnas.92.11.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Jackson P. K., Paskind M., Baltimore D. Mutation of a phenylalanine conserved in SH3-containing tyrosine kinases activates the transforming ability of c-Abl. Oncogene. 1993 Jul;8(7):1943–1956. [PubMed] [Google Scholar]
- Jackson P., Baltimore D. N-terminal mutations activate the leukemogenic potential of the myristoylated form of c-abl. EMBO J. 1989 Feb;8(2):449–456. doi: 10.1002/j.1460-2075.1989.tb03397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P., Baltimore D., Picard D. Hormone-conditional transformation by fusion proteins of c-Abl and its transforming variants. EMBO J. 1993 Jul;12(7):2809–2819. doi: 10.1002/j.1460-2075.1993.tb05942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kharbanda S., Ren R., Pandey P., Shafman T. D., Feller S. M., Weichselbaum R. R., Kufe D. W. Activation of the c-Abl tyrosine kinase in the stress response to DNA-damaging agents. Nature. 1995 Aug 31;376(6543):785–788. doi: 10.1038/376785a0. [DOI] [PubMed] [Google Scholar]
- Kipreos E. T., Lee G. J., Wang J. Y. Isolation of temperature-sensitive tyrosine kinase mutants of v-abl oncogene by screening with antibodies for phosphotyrosine. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1345–1349. doi: 10.1073/pnas.84.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipreos E. T., Wang J. Y. Cell cycle-regulated binding of c-Abl tyrosine kinase to DNA. Science. 1992 Apr 17;256(5055):382–385. doi: 10.1126/science.256.5055.382. [DOI] [PubMed] [Google Scholar]
- Kipreos E. T., Wang J. Y. Differential phosphorylation of c-Abl in cell cycle determined by cdc2 kinase and phosphatase activity. Science. 1990 Apr 13;248(4952):217–220. doi: 10.1126/science.2183353. [DOI] [PubMed] [Google Scholar]
- Kruh G. D., Perego R., Miki T., Aaronson S. A. The complete coding sequence of arg defines the Abelson subfamily of cytoplasmic tyrosine kinases. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5802–5806. doi: 10.1073/pnas.87.15.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Lyons R. H., Ferguson B. Q., Rosenberg M. Pentapeptide nuclear localization signal in adenovirus E1a. Mol Cell Biol. 1987 Jul;7(7):2451–2456. doi: 10.1128/mcb.7.7.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioni T., Jackson P. K., Bchini-Hooft van Huijsduijnen O., Picard D. Cell cycle arrest by tyrosine kinase Abl involves altered early mitogenic response. Oncogene. 1995 Apr 6;10(7):1325–1333. [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Van Etten R. A., Baltimore D. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol Cell Biol. 1992 Feb;12(2):609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter J. R., Wang J. Y. An actin-binding function contributes to transformation by the Bcr-Abl oncoprotein of Philadelphia chromosome-positive human leukemias. EMBO J. 1993 Apr;12(4):1533–1546. doi: 10.1002/j.1460-2075.1993.tb05797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Muller A. J., Young J. C., Pendergast A. M., Pondel M., Landau N. R., Littman D. R., Witte O. N. BCR first exon sequences specifically activate the BCR/ABL tyrosine kinase oncogene of Philadelphia chromosome-positive human leukemias. Mol Cell Biol. 1991 Apr;11(4):1785–1792. doi: 10.1128/mcb.11.4.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992 Oct 16;258(5081):424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- Oppi C., Shore S. K., Reddy E. P. Nucleotide sequence of testis-derived c-abl cDNAs: implications for testis-specific transcription and abl oncogene activation. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8200–8204. doi: 10.1073/pnas.84.23.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos P., Ridge S. A., Boucher C. A., Stocking C., Wiedemann L. M. The novel activation of ABL by fusion to an ets-related gene, TEL. Cancer Res. 1995 Jan 1;55(1):34–38. [PubMed] [Google Scholar]
- Pawson T., Schlessingert J. SH2 and SH3 domains. Curr Biol. 1993 Jul 1;3(7):434–442. doi: 10.1016/0960-9822(93)90350-w. [DOI] [PubMed] [Google Scholar]
- Pear W. S., Nolan G. P., Scott M. L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast A. M., Muller A. J., Havlik M. H., Clark R., McCormick F., Witte O. N. Evidence for regulation of the human ABL tyrosine kinase by a cellular inhibitor. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5927–5931. doi: 10.1073/pnas.88.13.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast A. M., Traugh J. A., Witte O. N. Normal cellular and transformation-associated abl proteins share common sites for protein kinase C phosphorylation. Mol Cell Biol. 1987 Dec;7(12):4280–4289. doi: 10.1128/mcb.7.12.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Srinivasan A. Nucleotide sequence of Abelson murine leukemia virus genome: structural similarity of its transforming gene product to other onc gene products with tyrosine-specific kinase activity. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3623–3627. doi: 10.1073/pnas.80.12.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R., Ye Z. S., Baltimore D. Abl protein-tyrosine kinase selects the Crk adapter as a substrate using SH3-binding sites. Genes Dev. 1994 Apr 1;8(7):783–795. doi: 10.1101/gad.8.7.783. [DOI] [PubMed] [Google Scholar]
- Renshaw M. W., Capozza M. A., Wang J. Y. Differential expression of type-specific c-abl mRNAs in mouse tissues and cell lines. Mol Cell Biol. 1988 Oct;8(10):4547–4551. doi: 10.1128/mcb.8.10.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw M. W., Kipreos E. T., Albrecht M. R., Wang J. Y. Oncogenic v-Abl tyrosine kinase can inhibit or stimulate growth, depending on the cell context. EMBO J. 1992 Nov;11(11):3941–3951. doi: 10.1002/j.1460-2075.1992.tb05488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W. D., Roberts B. L., Smith A. E. Nuclear location signals in polyoma virus large-T. Cell. 1986 Jan 17;44(1):77–85. doi: 10.1016/0092-8674(86)90486-1. [DOI] [PubMed] [Google Scholar]
- Robbins J., Dilworth S. M., Laskey R. A., Dingwall C. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: identification of a class of bipartite nuclear targeting sequence. Cell. 1991 Feb 8;64(3):615–623. doi: 10.1016/0092-8674(91)90245-t. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., Callahan W., Witte O. N. Dominant negative MYC blocks transformation by ABL oncogenes. Cell. 1992 Sep 18;70(6):901–910. doi: 10.1016/0092-8674(92)90241-4. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., McLaughlin J., Goga A., Havlik M., Witte O. The nuclear tyrosine kinase c-Abl negatively regulates cell growth. Cell. 1994 Apr 8;77(1):121–131. doi: 10.1016/0092-8674(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Sawyers C. L., McLaughlin J., Witte O. N. Genetic requirement for Ras in the transformation of fibroblasts and hematopoietic cells by the Bcr-Abl oncogene. J Exp Med. 1995 Jan 1;181(1):307–313. doi: 10.1084/jem.181.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schwartzberg P. L., Stall A. M., Hardin J. D., Bowdish K. S., Humaran T., Boast S., Harbison M. L., Robertson E. J., Goff S. P. Mice homozygous for the ablm1 mutation show poor viability and depletion of selected B and T cell populations. Cell. 1991 Jun 28;65(7):1165–1175. doi: 10.1016/0092-8674(91)90012-n. [DOI] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Slebos R. J., Lee M. H., Plunkett B. S., Kessis T. D., Williams B. O., Jacks T., Hedrick L., Kastan M. B., Cho K. R. p53-dependent G1 arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5320–5324. doi: 10.1073/pnas.91.12.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Roudebush M., Day R., Mosser S. D., Gibbs J. B., Feig L. A. Dominant inhibitory Ras mutants demonstrate the requirement for Ras activity in the action of tyrosine kinase oncogenes. Oncogene. 1991 Dec;6(12):2297–2304. [PubMed] [Google Scholar]
- Stam K., Heisterkamp N., Grosveld G., de Klein A., Verma R. S., Coleman M., Dosik H., Groffen J. Evidence of a new chimeric bcr/c-abl mRNA in patients with chronic myelocytic leukemia and the Philadelphia chromosome. N Engl J Med. 1985 Dec 5;313(23):1429–1433. doi: 10.1056/NEJM198512053132301. [DOI] [PubMed] [Google Scholar]
- Sánchez I., Hughes R. T., Mayer B. J., Yee K., Woodgett J. R., Avruch J., Kyriakis J. M., Zon L. I. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994 Dec 22;372(6508):794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Underwood M. R., Fried H. M. Characterization of nuclear localizing sequences derived from yeast ribosomal protein L29. EMBO J. 1990 Jan;9(1):91–99. doi: 10.1002/j.1460-2075.1990.tb08084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Debnath J., Zhou H., Casasnovas J. M. Introduction of a loss-of-function point mutation from the SH3 region of the Caenorhabditis elegans sem-5 gene activates the transforming ability of c-abl in vivo and abolishes binding of proline-rich ligands in vitro. Oncogene. 1995 May 18;10(10):1977–1988. [PubMed] [Google Scholar]
- Van Etten R. A., Jackson P. K., Baltimore D., Sanders M. C., Matsudaira P. T., Janmey P. A. The COOH terminus of the c-Abl tyrosine kinase contains distinct F- and G-actin binding domains with bundling activity. J Cell Biol. 1994 Feb;124(3):325–340. doi: 10.1083/jcb.124.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten R. A., Jackson P., Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989 Aug 25;58(4):669–678. doi: 10.1016/0092-8674(89)90102-5. [DOI] [PubMed] [Google Scholar]
- Vandromme M., Cavadore J. C., Bonnieu A., Froeschlé A., Lamb N., Fernandez A. Two nuclear localization signals present in the basic-helix 1 domains of MyoD promote its active nuclear translocation and can function independently. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4646–4650. doi: 10.1073/pnas.92.10.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden K. H., Vojtesek B., Fisher C., Lane D. HPV-16 E7 or adenovirus E1A can overcome the growth arrest of cells immortalized with a temperature-sensitive p53. Oncogene. 1993 Jun;8(6):1697–1702. [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Watanabe S. M., Witte O. N. Site-directed deletions of Abelson murine leukemia virus define 3' sequences essential for transformation and lethality. J Virol. 1983 Mar;45(3):1028–1036. doi: 10.1128/jvi.45.3.1028-1036.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch P. J., Wang J. Y. A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell. 1993 Nov 19;75(4):779–790. doi: 10.1016/0092-8674(93)90497-e. [DOI] [PubMed] [Google Scholar]
- Wetzler M., Talpaz M., Van Etten R. A., Hirsh-Ginsberg C., Beran M., Kurzrock R. Subcellular localization of Bcr, Abl, and Bcr-Abl proteins in normal and leukemic cells and correlation of expression with myeloid differentiation. J Clin Invest. 1993 Oct;92(4):1925–1939. doi: 10.1172/JCI116786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. A., Niman H. L., Houghten R. A., Cherenson A. R., Connolly M. L., Lerner R. A. The structure of an antigenic determinant in a protein. Cell. 1984 Jul;37(3):767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Zacksenhaus E., Bremner R., Phillips R. A., Gallie B. L. A bipartite nuclear localization signal in the retinoblastoma gene product and its importance for biological activity. Mol Cell Biol. 1993 Aug;13(8):4588–4599. doi: 10.1128/mcb.13.8.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Whitlock C. A., Goff S. P., Gifford A., Witte O. N. Lethal effect of the Abelson murine leukemia virus transforming gene product. Cell. 1981 Dec;27(3 Pt 2):477–486. doi: 10.1016/0092-8674(81)90389-5. [DOI] [PubMed] [Google Scholar]