Abstract
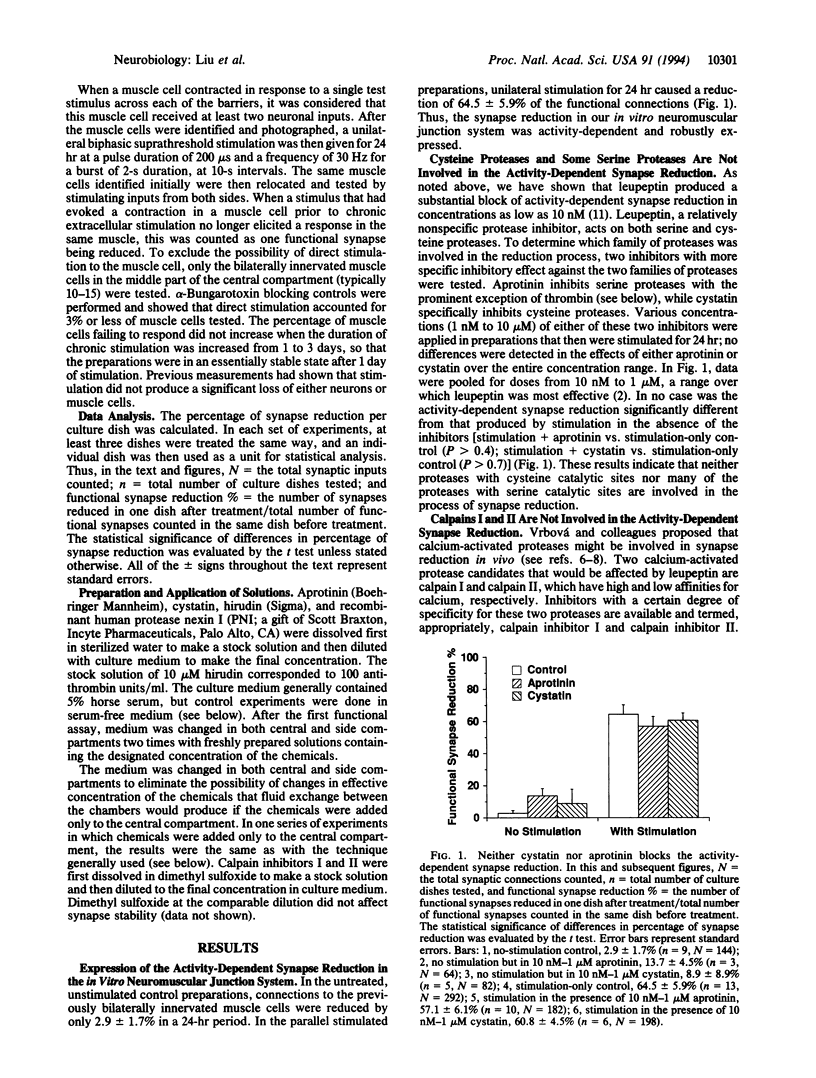
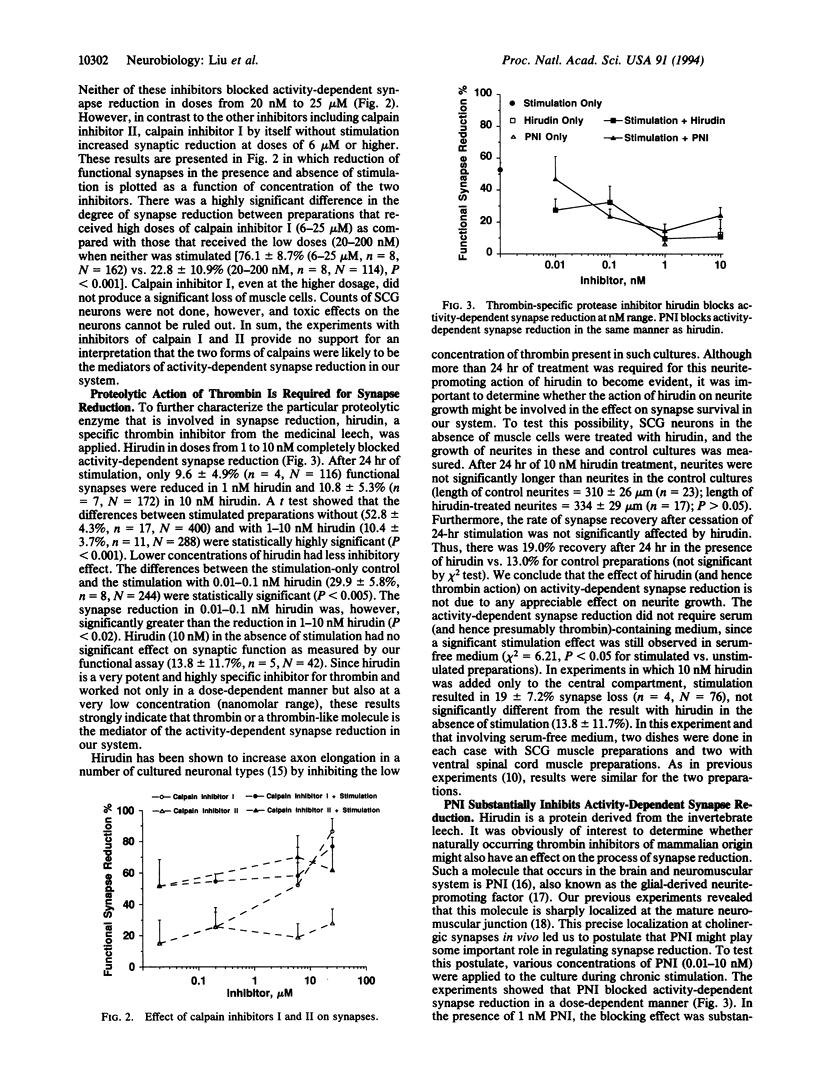
Molecular mechanisms of activity-dependent synapse reduction were studied in an in vitro mammalian neuromuscular preparation. Synapse reduction in this model is activity-dependent and is substantially reduced by the broad-spectrum protease inhibitor, leupeptin, suggesting the role of activity-dependent proteolytic action in the process. Our present experiments show that a potent and specific thrombin inhibitor, hirudin, at nanomolar concentration completely blocked the activity-dependent synapse reduction. Furthermore, a naturally occurring serine protease inhibitor, protease nexin I (PNI), which closely colocalizes with acetylcholine receptors at the neuromuscular junction, inhibited the synapse reduction at the same low concentration. In contrast, neither cystatin, a cysteine protease inhibitor, nor aprotinin, a serine protease inhibitor that does not inhibit thrombin, blocked the synapse reduction. Similarly, neither of the inhibitors of the calcium-activated proteases calpain I and II prevented the reduction of synapses. These results strongly suggest that serine proteolytic action by thrombin or thrombin-like molecules is required for synapse reduction in our in vitro model of the mammalian neuromuscular junction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Balice-Gordon R. J., Lichtman J. W. In vivo observations of pre- and postsynaptic changes during the transition from multiple to single innervation at developing neuromuscular junctions. J Neurosci. 1993 Feb;13(2):834–855. doi: 10.1523/JNEUROSCI.13-02-00834.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher K. L., Andersen T. T., Fenton J. W., 2nd, Festoff B. W. Thrombin receptor peptides induce shape change in neonatal murine astrocytes in culture. J Neurosci Res. 1994 Jan;37(1):108–115. doi: 10.1002/jnr.490370115. [DOI] [PubMed] [Google Scholar]
- Campenot R. B. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh K. P., Gurwitz D., Cunningham D. D., Bradshaw R. A. Reciprocal modulation of astrocyte stellation by thrombin and protease nexin-1. J Neurochem. 1990 May;54(5):1735–1743. doi: 10.1111/j.1471-4159.1990.tb01228.x. [DOI] [PubMed] [Google Scholar]
- Connold A. L., Evers J. V., Vrbová G. Effect of low calcium and protease inhibitors on synapse elimination during postnatal development in the rat soleus muscle. Brain Res. 1986 Jul;393(1):99–107. doi: 10.1016/0165-3806(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D., Gurwitz D. Proteolytic regulation of neurite outgrowth from neuroblastoma cells by thrombin and protease nexin-1. J Cell Biochem. 1989 Jan;39(1):55–64. doi: 10.1002/jcb.240390107. [DOI] [PubMed] [Google Scholar]
- Dihanich M., Kaser M., Reinhard E., Cunningham D., Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991 Apr;6(4):575–581. doi: 10.1016/0896-6273(91)90060-d. [DOI] [PubMed] [Google Scholar]
- Farmer L., Sommer J., Monard D. Glia-derived nexin potentiates neurite extension in hippocampal pyramidal cells in vitro. Dev Neurosci. 1990;12(2):73–80. doi: 10.1159/000111836. [DOI] [PubMed] [Google Scholar]
- Festoff B. W., Rao J. S., Chen M. Protease nexin I, thrombin- and urokinase-inhibiting serpin, concentrated in normal human cerebrospinal fluid. Neurology. 1992 Jul;42(7):1361–1366. doi: 10.1212/wnl.42.7.1361. [DOI] [PubMed] [Google Scholar]
- Festoff B. W., Rao J. S., Hantaï D. Plasminogen activators and inhibitors in the neuromuscular system: III. The serpin protease nexin I is synthesized by muscle and localized at neuromuscular synapses. J Cell Physiol. 1991 Apr;147(1):76–86. doi: 10.1002/jcp.1041470111. [DOI] [PubMed] [Google Scholar]
- Fields R. D., Nelson P. G. Activity-dependent development of the vertebrate nervous system. Int Rev Neurobiol. 1992;34:133–214. doi: 10.1016/s0074-7742(08)60098-7. [DOI] [PubMed] [Google Scholar]
- Liu Y., Fields R. D., Fitzgerald S., Festoff B. W., Nelson P. G. Proteolytic activity, synapse elimination, and the Hebb synapse. J Neurobiol. 1994 Mar;25(3):325–335. doi: 10.1002/neu.480250312. [DOI] [PubMed] [Google Scholar]
- Low D. A., Baker J. B., Koonce W. C., Cunningham D. D. Released protease-nexin regulates cellular binding, internalization, and degradation of serine proteases. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2340–2344. doi: 10.1073/pnas.78.4.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki K., Hasegawa M., Funahashi K., Umeda M. A metalloproteinase inhibitor domain in Alzheimer amyloid protein precursor. Nature. 1993 Apr 29;362(6423):839–841. doi: 10.1038/362839a0. [DOI] [PubMed] [Google Scholar]
- Monard D. Cell-derived proteases and protease inhibitors as regulators of neurite outgrowth. Trends Neurosci. 1988 Dec;11(12):541–544. doi: 10.1016/0166-2236(88)90182-8. [DOI] [PubMed] [Google Scholar]
- Monard D., Niday E., Limat A., Solomon F. Inhibition of protease activity can lead to neurite extension in neuroblastoma cells. Prog Brain Res. 1983;58:359–364. doi: 10.1016/S0079-6123(08)60037-0. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Fields R. D., Yu C., Liu Y. Synapse elimination from the mouse neuromuscular junction in vitro: a non-Hebbian activity-dependent process. J Neurobiol. 1993 Nov;24(11):1517–1530. doi: 10.1002/neu.480241106. [DOI] [PubMed] [Google Scholar]
- Suidan H. S., Stone S. R., Hemmings B. A., Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron. 1992 Feb;8(2):363–375. doi: 10.1016/0896-6273(92)90302-t. [DOI] [PubMed] [Google Scholar]
- Talbot M. Biology of recombinant hirudin (CGP 39393): a new prospect in the treatment of thrombosis. Semin Thromb Hemost. 1989 Jul;15(3):293–301. doi: 10.1055/s-2007-1002722. [DOI] [PubMed] [Google Scholar]
- Van Essen D. C., Gordon H., Soha J. M., Fraser S. E. Synaptic dynamics at the neuromuscular junction: mechanisms and models. J Neurobiol. 1990 Jan;21(1):223–249. doi: 10.1002/neu.480210115. [DOI] [PubMed] [Google Scholar]
- Vrbová G., Fisher T. J. The Effect of Inhibiting the Calcium Activated Neutral Protease, on Motor Unit Size after Partial Denervation of the Rat Soleus Muscle. Eur J Neurosci. 1989 Jan;1(6):616–625. doi: 10.1111/j.1460-9568.1989.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Zhu P. H., Vrbová G. The role of Ca2+ in the elimination of polyneuronal innervation of rat soleus muscle fibres. Eur J Neurosci. 1992;4(5):433–437. doi: 10.1111/j.1460-9568.1992.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Zumbrunn A., Stone S., Shaw E. Synthesis and properties of Cbz-Phe-Arg-CHN2 (benzyloxycarbonylphenylalanylarginyldiazomethane) as a proteinase inhibitor. Biochem J. 1988 Mar 1;250(2):621–623. doi: 10.1042/bj2500621. [DOI] [PMC free article] [PubMed] [Google Scholar]



