Abstract
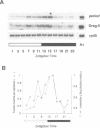
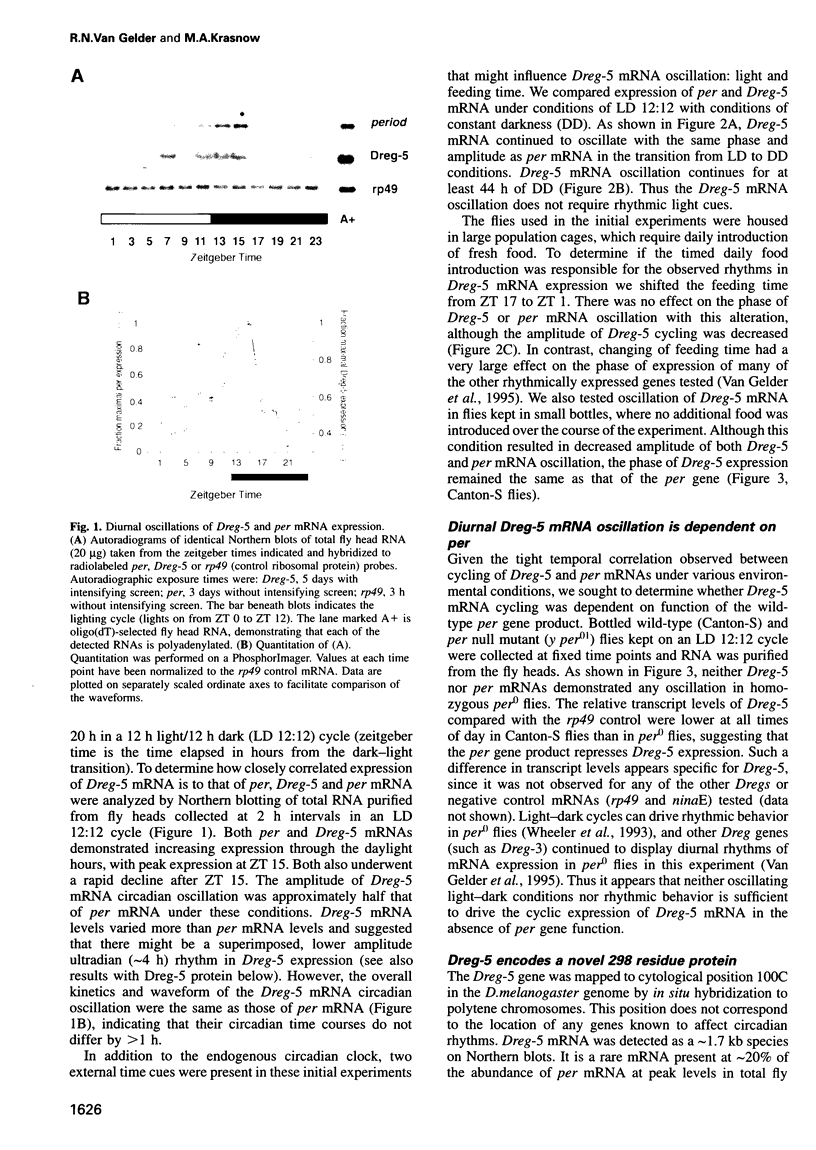
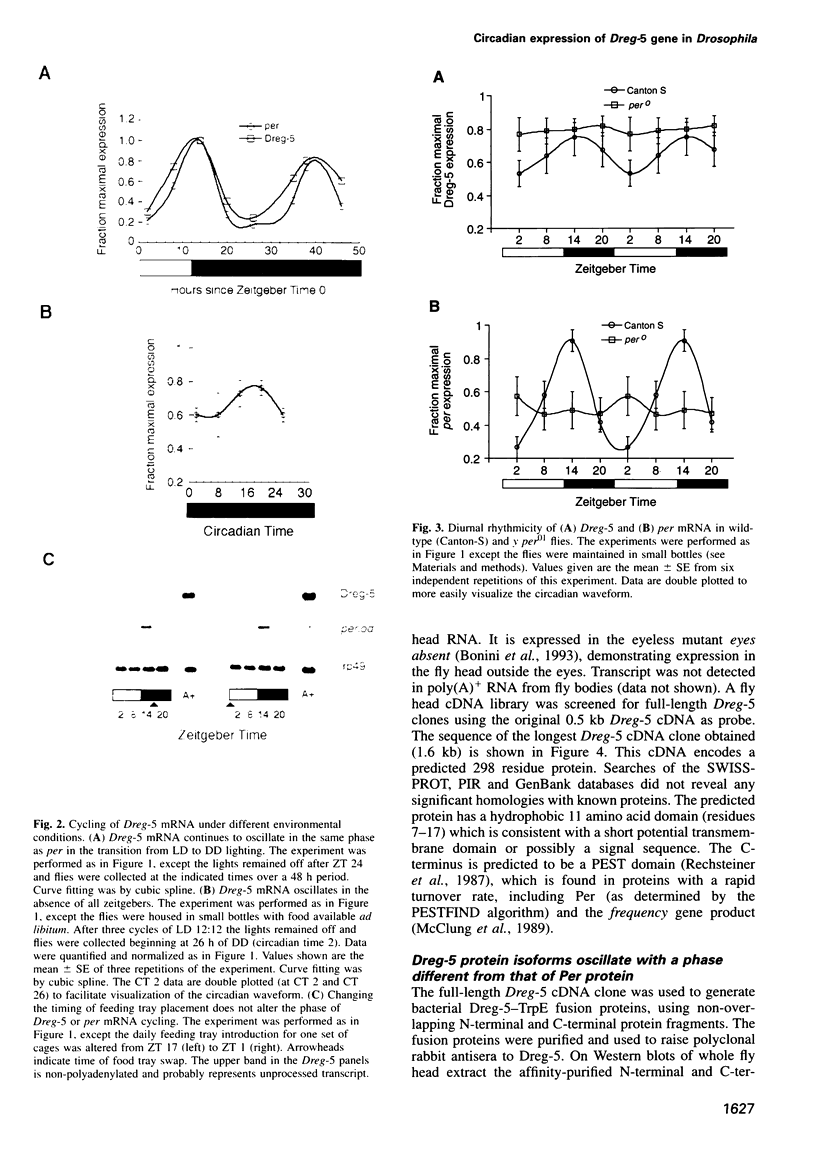
The Drosophila melanogaster period (per) gene is required for expression of endogenous circadian rhythms of locomotion and eclosion. per mRNA is expressed with a circadian rhythm that is dependent on Per protein; this feedback loop has been proposed to be essential to the central circadian pacemaker. This model would suggest the Per protein also controls the circadian expression of other genetic loci to generate circadian behavior and physiology. In this paper we describe Dreg-5, a gene whose mRNA is expressed in fly heads with a circadian rhythm nearly identical to that of the per gene. Dreg-5 mRNA continues to cycle in phase with that of per mRNA in conditions of total darkness and also when the daily feeding time is altered. Like per mRNA, Dreg-5 mRNA is not expressed rhythmically in per null mutant flies. Dreg-5 encodes a novel 298 residue protein and Dreg-5 protein isoforms also oscillate in abundance with a circadian rhythm. The phase of Dreg-5 protein oscillation, however, is different from that of Per protein expression, suggesting that Dreg-5 and per have common translational but different post-translational control mechanisms. These results demonstrate that the per gene is capable of modulating the rhythmic expression of other genes; this activity may form the basis of the output of circadian rhythmicity in Drosophila.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson B. D., Johnson K. A., Loros J. J., Dunlap J. C. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science. 1994 Mar 18;263(5153):1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- Arpaia G., Loros J. J., Dunlap J. C., Morelli G., Macino G. The interplay of light and the circadian clock. Independent dual regulation of clock-controlled gene ccg-2(eas). Plant Physiol. 1993 Aug;102(4):1299–1305. doi: 10.1104/pp.102.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini N. M., Leiserson W. M., Benzer S. The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell. 1993 Feb 12;72(3):379–395. doi: 10.1016/0092-8674(93)90115-7. [DOI] [PubMed] [Google Scholar]
- Burbach K. M., Poland A., Bradfield C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri Y., Colot H. V., Jacquier A. C., Yu Q., Hall J. C., Baltimore D., Rosbash M. A family of unusually spliced biologically active transcripts encoded by a Drosophila clock gene. Nature. 1987 Mar 5;326(6108):42–47. doi: 10.1038/326042a0. [DOI] [PubMed] [Google Scholar]
- Crews S. T., Thomas J. B., Goodman C. S. The Drosophila single-minded gene encodes a nuclear protein with sequence similarity to the per gene product. Cell. 1988 Jan 15;52(1):143–151. doi: 10.1016/0092-8674(88)90538-7. [DOI] [PubMed] [Google Scholar]
- Edery I., Zwiebel L. J., Dembinska M. E., Rosbash M. Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. F., Hoyle M. N. Isolation of circadian clock mutants of Neurospora crassa. Genetics. 1973 Dec;75(4):605–613. doi: 10.1093/genetics/75.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. C., Rosbash M. Oscillating molecules and how they move circadian clocks across evolutionary boundaries. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5382–5383. doi: 10.1073/pnas.90.12.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B. A., Palazzolo M. J., Meyerowitz E. M. Rapid isolation of long cDNA clones from existing libraries. Nucleic Acids Res. 1991 Apr 25;19(8):1951–1952. doi: 10.1093/nar/19.8.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Behavioral and molecular analyses suggest that circadian output is disrupted by disconnected mutants in D. melanogaster. EMBO J. 1992 Jan;11(1):1–6. doi: 10.1002/j.1460-2075.1992.tb05020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11711–11715. doi: 10.1073/pnas.89.24.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990 Feb 8;343(6258):536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991 May 17;252(5008):954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Huang Z. J., Edery I., Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993 Jul 15;364(6434):259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- Koelle M. R., Talbot W. S., Segraves W. A., Bender M. T., Cherbas P., Hogness D. S. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991 Oct 4;67(1):59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- Kondo T., Tsinoremas N. F., Golden S. S., Johnson C. H., Kutsuna S., Ishiura M. Circadian clock mutants of cyanobacteria. Science. 1994 Nov 18;266(5188):1233–1236. doi: 10.1126/science.7973706. [DOI] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zwiebel L. J., Hinton D., Benzer S., Hall J. C., Rosbash M. The period gene encodes a predominantly nuclear protein in adult Drosophila. J Neurosci. 1992 Jul;12(7):2735–2744. doi: 10.1523/JNEUROSCI.12-07-02735.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Tsinoremas N. F., Johnson C. H., Lebedeva N. V., Golden S. S., Ishiura M., Kondo T. Circadian orchestration of gene expression in cyanobacteria. Genes Dev. 1995 Jun 15;9(12):1469–1478. doi: 10.1101/gad.9.12.1469. [DOI] [PubMed] [Google Scholar]
- Loros J. J., Denome S. A., Dunlap J. C. Molecular cloning of genes under control of the circadian clock in Neurospora. Science. 1989 Jan 20;243(4889):385–388. doi: 10.1126/science.2563175. [DOI] [PubMed] [Google Scholar]
- McClung C. R., Fox B. A., Dunlap J. C. The Neurospora clock gene frequency shares a sequence element with the Drosophila clock gene period. Nature. 1989 Jun 15;339(6225):558–562. doi: 10.1038/339558a0. [DOI] [PubMed] [Google Scholar]
- Millar A. J., Carré I. A., Strayer C. A., Chua N. H., Kay S. A. Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science. 1995 Feb 24;267(5201):1161–1163. doi: 10.1126/science.7855595. [DOI] [PubMed] [Google Scholar]
- O'Connell P. O., Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984 Jul 11;12(13):5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T. L. Time is the essence: molecular analysis of the biological clock. Science. 1994 Mar 18;263(5153):1570–1572. doi: 10.1126/science.8128243. [DOI] [PubMed] [Google Scholar]
- Palazzolo M. J., Hyde D. R., VijayRaghavan K., Mecklenburg K., Benzer S., Meyerowitz E. Use of a new strategy to isolate and characterize 436 Drosophila cDNA clones corresponding to RNAs detected in adult heads but not in early embryos. Neuron. 1989 Oct;3(4):527–539. doi: 10.1016/0896-6273(89)90211-0. [DOI] [PubMed] [Google Scholar]
- Ralph M. R., Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988 Sep 2;241(4870):1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- Redding K., Holcomb C., Fuller R. S. Immunolocalization of Kex2 protease identifies a putative late Golgi compartment in the yeast Saccharomyces cerevisiae. J Cell Biol. 1991 May;113(3):527–538. doi: 10.1083/jcb.113.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A., Price J. L., Man B., Young M. W. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science. 1994 Mar 18;263(5153):1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- Strathmann M., Hamilton B. A., Mayeda C. A., Simon M. I., Meyerowitz E. M., Palazzolo M. J. Transposon-facilitated DNA sequencing. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1247–1250. doi: 10.1073/pnas.88.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi J. S. Circadian-clock regulation of gene expression. Curr Opin Genet Dev. 1993 Apr;3(2):301–309. doi: 10.1016/0959-437x(93)90038-q. [DOI] [PubMed] [Google Scholar]
- Van Gelder R. N., Bae H., Palazzolo M. J., Krasnow M. A. Extent and character of circadian gene expression in Drosophila melanogaster: identification of twenty oscillating mRNAs in the fly head. Curr Biol. 1995 Dec 1;5(12):1424–1436. doi: 10.1016/s0960-9822(95)00280-6. [DOI] [PubMed] [Google Scholar]
- Vitaterna M. H., King D. P., Chang A. M., Kornhauser J. M., Lowrey P. L., McDonald J. D., Dove W. F., Pinto L. H., Turek F. W., Takahashi J. S. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science. 1994 Apr 29;264(5159):719–725. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler D. A., Hamblen-Coyle M. J., Dushay M. S., Hall J. C. Behavior in light-dark cycles of Drosophila mutants that are arrhythmic, blind, or both. J Biol Rhythms. 1993 Spring;8(1):67–94. doi: 10.1177/074873049300800106. [DOI] [PubMed] [Google Scholar]
- Zeng H., Hardin P. E., Rosbash M. Constitutive overexpression of the Drosophila period protein inhibits period mRNA cycling. EMBO J. 1994 Aug 1;13(15):3590–3598. doi: 10.1002/j.1460-2075.1994.tb06666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerr D. M., Hall J. C., Rosbash M., Siwicki K. K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990 Aug;10(8):2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]